Abstract
Purpose
To assess alterations of retinal layers in healthy subjects over 60 years old.
Methods
Retinal layers of 160 healthy subjects (aged 60–100 years) without any retinal pathology were imaged using spectral domain optical coherence tomography. Mean thickness of retinal nerve fiber layer, ganglion cell/inner plexiform layer (GCLIPL), inner nuclear layer, outer plexiform layer/outer nuclear layer, photoreceptor complex (PR) and retinal thickness (RT) were measured in a 3.45 mm grid. Correlations between age and layers were estimated and linear regression equations were calculated. Different age-groups (60–69, 70–79, 80–89 years and nonagenarians, each group with 40 participants) were compared.
Results
Significant age-thickness correlations were observed for GCLIPL (P<0.001, r=−0.394), PR (P<0.001, r=−0.370) and RT (P<0.001, r=−0.290). A comparison between age groups 60–69 years and nonagenarians showed no significant thickness alteration of retinal nerve fiber layer (21.80±2.18 μm vs 22.82±2.97 μm, P=0.163), inner nuclear layer (37.23±3.02 μm vs 36.01±3.24 μm, P=0.07) and outer plexiform layer/outer nuclear layer (104.95±6.56 μm vs 104.23±7.59 μm, P=0.567), while GCLIPL (83.35±7.35 μm vs 74.38±9.09 μm), PR (83.03±3.31 μm vs 79.34±2.09 μm) and RT (330.64±12.63 μm vs 316.83±18.35 μm) showed a significant decrease (P<0.001 for all).
Conclusion
Our study provides normative data of alterations of retinal layers for persons aged 60 years to nonagenarians and indicates a continuous decrease of RT, PR, and GCLIPL. This data may be useful for clinical trials investigating macular diseases in older patients.
Introduction
Spectral domain optical coherence tomography (SDOCT) is a cornerstone of posterior segment imaging of the eye, providing non-invasive and reproducible measurements of different retinal layers. It is widely used in clinical practice but also in clinical trials for retinal diseases such as age-related macular degeneration (AMD) and diabetic macular edema. For all these applications, it is important to understand the effect of aging on the various retinal layers. So far, normative SDOCT data is limited to subjects aged approximately 70 years.Citation1 However, due to increased life expectancy, it will be necessary to provide normative data for clinical routine and clinical trials for the elderly.
The focus of this study was to assess alterations in SDOCT of individual retinal layers in the eyes of healthy subjects aged from 60 to 100 years.
Methods
One hundred and sixty eyes from 160 healthy subjects aged between 60 to 100 years without AMD and without other retinal or optic disc pathology (high myopia ≥−6.00 diopters, myopic fundus degeneration, diabetic retinopathy/maculopathy, uveitis, macular hole, epiretinal membrane, vitreomacular traction, retinal vascular disease, glaucoma, etc.) were included in this study (four age groups: 60–69, 70–79, 80–89 years and nonagenarians, each with 40 participants). All subjects were healthy control participants from The European Genetic Database (www.eugenda.org) who fulfilled the inclusion criteria and were randomized before the start of the study.
Grading of retinal images included stereo fundus photographies (stereo technique is performed with slightly shifting of the camera and sequential images of the same subject can be obtained for a stereo-pair) and SDOCTs (Spectralis HRA, Heidelberg Engineering, Heidelberg, Germany). The study was performed in accordance with the tenets of the Declaration of Helsinki, and the Medical Research Involving Human Subjects Act (WMO) and was approved by the local ethics committee of the University Hospitals in Cologne and Nijmegen. Written informed consent was obtained from all participants.
The nonagenarians (90–100 year olds) could have only small drusen or pigmentary abnormalities with not more than nine small drusen in the Early Treatment of Diabetic Retinopathy Study grid, while participants aged <90 years were not permitted to have any drusen or any other qualitative abnormalities in the whole SDOCT volume scan of both eyes.
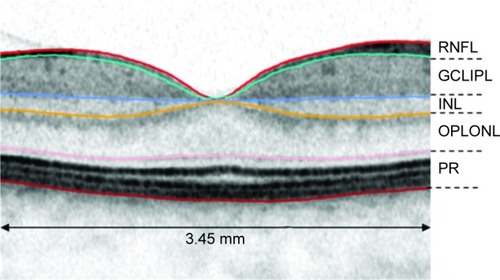
Calculations of mean thickness values of standardized SDOCT scans (protocol of 37 B-scans) were performed in a 3.45 mm grid that was manually centered on the fovea. Automatic delineation was performed by Spectralis software (Heidelberg Eye Explorer Software Version 2014, Version 1.9.10.0, Heidelberg Engineering GmbH, Germany) and misalignments were manually corrected. The calculations were performed for retinal nerve fiber layer (RNFL), ganglion cell layer/inner plexiform layer (GCLIPL), inner nuclear layer (INL), outer plexiform layer/outer nuclear layer (OPLONL) and for photoreceptor (PR) complex (external limiting membrane until Bruch’s membrane) (). The combined thickness of all retinal layers was referred to as retinal thickness (RT). These layers were chosen because of their good visibility on SDOCT, as reported in a previous study.Citation2
Figure 1 Schematic illustration of 3.45 mm diameter grid and chosen retinal layers for segmentation.
Abbreviatons: RNFL, retinal nerve fiber layer; GCLIPL, ganglion cell/inner plexiform layer; INL, inner nuclear layer; OPLONL, outer plexiform layer/outer nuclear layer; PR, photoreceptor complex.
Due to previously reported moderate-to-high concordance in retinal layer thicknesses between the right and the left eye,Citation2,Citation3 we subsequently decided to use the right eye for segmentation in all eyes, except in 15 eyes where poor image quality precluded the use of right eye images.
To avoid gender influence, groups included equal numbers of male and female subjects (20 males and 20 females in each age-group), only the nonagenarian group consisted of 12 males and 28 females due to a shortage of male nonagenarians.
Statistical analysis
All analyses were conducted using SPSS software version 22.0 (IBM Corporation, Armonk, NY, USA). Linear regression analysis was performed for all patients. Age-thickness correlations were performed using Spearman-Rho coefficient. Nonparametric Mann–Whitney U-test was used for thickness comparison of different retinal layers between 60–69 years age group vs ≥90 years age group. P-values <0.05 were accepted as significant.
Results
In this study, a total of 160 healthy eyes (145 right eyes, 15 left eyes) were evaluated. Mean age of participants was 79.3±10.9 years (range 60–100 years). Demographics and mean thickness values of each evaluated layer are outlined in for the different age groups.
Table 1 Demographics, mean ± SD of each evaluated layer for each age-group
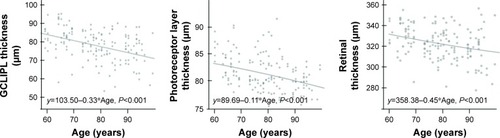
Thickness of retinal layer and age correlated significantly for GCLIPL (P<0.001, r=−0.394), PR (P<0.001, r=−0.370) and RT (P<0.001, r=−0.290) but not for RNFL, INL, and OPLONL. Regression equations for these layers with each scatterplot are shown in .
Figure 2 Regression equations for age-related retinal thickness changes in subjects aged 60–100 years.
Abbreviaton: GCLIPL, ganglion cell layer/inner plexiform layer.
Comparison of subjects aged 60–69 years to nonagenarians showed no significant thickness alterations for RNFL (mean 21.80±2.18 μm vs 22.82±2.97 μm, P=0.163), INL (mean 37.23±3.02 μm vs 36.01±3.24 μm, P=0.07) and OPLONL (mean 104.95±6.56 μm vs 104.23±7.59 μm, P=0.567), where alteration of GCLIPL (83.35±7.35 μm vs 74.38±9.09 μm), PR (83.03±3.31 μm vs 79.34±2.09 μm) and RT (330.64±12.63 μm vs 316.83±18.35 μm) were significant (P<0.001 for all) ().
Discussion
In this study, we provided normative data of thickness of different retinal layers measured with SDOCT in healthy subjects aged 60 to 100 years.
Previous studies report an age-dependent reduction of macular RNFL thickness within middle-age persons.Citation4–Citation8 However macular RNFL in SDOCT in elderly has not been investigated. In our study, we observed that macular RNFL did not alter after the sixth decade, whereas GCLIPL decreased significantly with increasing age. In concordance, histological studies investigating postmortem human donor eyes also reported a decrease of GCL with increasing age.Citation9 Along with this finding, a highly significant cell density decrease of GCL from 20–40 year olds and a slower rate of GCL loss in the following decades was reported.Citation9 However, with the SDOCT, GCL cannot reliably be distinguished from the INL layer, therefore a one-to one comparison with histological methods is very difficult.
In this study, we observed a continuous age-dependent decrease of macular retinal thickness. Our results may partly explain the controversial results of several previously published studies; while some studies reported no association of macular thickness with age in healthy eyes,Citation10,Citation11 others report a decrease of retinal thickness and volume with increasing age.Citation3,Citation5,Citation12–Citation15
In agreement with previous studies,Citation4 we found no association of OPLONL thickness alteration with age, although, a reorganization of cells in OPLONL during normal aging cannot be excluded.Citation4,Citation16
From all evaluated layers, PR-complex seemed to have the most significant alterations after the age of 80 years. This is partly in concordance with previous macular electroretinogram reports of healthy elderly individuals, that showed a significant age-related decrease of amplitudes that is possibly related to decreased recovery of pigment regeneration in the elderly.Citation17 Also, studies that investigated histological sections of human donor eyes reported a photoreceptor and retinal pigment epithelium (RPE) loss with increasing age, and an elevated RPE lipofuscin accumulation over years with the highest values between 73–88 years.Citation18
Our study included a large nonagenarian group, who primarily came from a small area in Germany. The recruitment of this special cohort of nonagenarians, who were able to come to attend and comprehend the study’s procedures, caused an unavoidable selection bias of the “healthy and mobile” in our study. However, as a consequence of this selection bias, the number of nonagenarians with Alzheimer Disease, dementia or large cerebral infarction, which may affect RNFL thickness,Citation19,Citation20 was reduced to a minimum. Another limitation is that RNFL thickness changes should be monitored ideally with additional SDOCT scan around optic nerve head.Citation5 Also, thickness changes of all retinal layers can be measured in a 1, 3, and 6 mm diameter grid, although there is a high concordance in measured mean macular thickness in the central areas by the shorter (3.45 mm) and the longer (6 mm) scan length mapping protocols.Citation21 A further limitation in our study was using neither a longitudinal nor epidemiological design.
Our study also has several strengths. Concerning our purpose, no studies were found to include an age group of ≥90 years. As there is a rising life expectancy, it is important for future clinical trials to introduce a new age-category of very old patients with corresponding controls. Our study included a large group of nonagenarians with a maximum age of 100 years. Nonagenarian participants were carefully chosen and showed neither AMD nor other macular or optic disc co-pathologies in fundus photographies and in SDOCT. All other subjects except nonagenarians were randomly chosen from a large group of healthy participants (without macular or optic disc pathology) of The European Genetic Database (count >600).
In summary, in this study we intended to provide normative SDOCT data of different retinal layers using SDOCT from 60 year olds to nonagenarians. As there is a growing number of highly aged persons due to rising life expectancy, our data may be useful for clinical trials investigating the macular diseases for an upcoming age class in society.
Disclosure
The authors report no conflicts of interest in this work.
References
- MyersCEKleinBEMeuerSMRetinal thickness measured by spectral-domain optical coherence tomography in eyes without retinal abnormalities: the Beaver Dam Eye StudyAm J Ophthalmol20151593445.e1456.e125461295
- CaramoyADroegeKMKirchhofBFauserSRetinal layers measurements in healthy eyes and in eyes receiving silicone oil-based endotamponadeActa Ophthalmol2014924e292e29724238324
- CaramoyAFoersterJAllakhiarovaESpectral-domain optical coherence tomography in subjects over 60 years of age, and its implications for designing clinical trialsBr J Ophthalmol201296101325133022863948
- OotoSHangaiMTomidokoroAEffects of age, sex, and axial length on the three-dimensional profile of normal macular layer structuresInvest Ophthalmol Vis Sci201152128769877921989721
- DemirkayaNvan DijkHWvan SchuppenSMEffect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomographyInvest Ophthalmol Vis Sci20135474934494023761080
- RougierMBKorobelnikJFMaletFRetinal nerve fibre layer thickness measured with SD-OCT in a population-based study of French elderly subjects: the Alienor studyActa Ophthalmol201593653954525586172
- AlasilTWangKKeanePAAnalysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomographyJ Glaucoma201322753254122549477
- LeungCKYuMWeinrebRNRetinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related lossOphthalmology2012119473173722264886
- GaoHHollyfieldJGAging of the human retina. Differential loss of neurons and retinal pigment epithelial cellsInvest Ophthalmol Vis Sci19923311171730530
- AdhiMAzizSMuhammadKAdhiMIMacular thickness by age and gender in healthy eyes using spectral domain optical coherence tomographyPLoS One201275e3763822629435
- WongACChanCWHuiSPRelationship of gender, body mass index, and axial length with central retinal thickness using optical coherence tomographyEye (Lond)200519329229715258609
- AlamoutiBFunkJRetinal thickness decreases with age: an OCT studyBri J Ophthalmol2003877899901
- SongWKLeeSCLeeESKimCYKimSSMacular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain-optical coherence tomography studyInvest Ophthalmol Vis Sci20105183913391820357206
- ErikssonUAlmAMacular thickness decreases with age in normal eyes: a study on the macular thickness map protocol in the Stratus OCTB J Ophthal2009931114481452
- RaoHLKumarAUBabuJGKumarASenthilSGarudadriCSPredictors of normal optic nerve head, retinal nerve fiber layer, and macular parameters measured by spectral domain optical coherence tomographyInvest Ophthalmol Vis Sci20115221103111021087966
- EliasiehKLietsLCChalupaLMCellular reorganization in the human retina during normal agingInvest Ophthalmol Vis Sci20074862824283017525218
- MessenioDMaranoGGerosaSIannelliFBiganzoliEMThe influence of age on the recovery of the ERG photostress testDoc Ophthalmol20131262879723184310
- DoreyCKWuGEbensteinDGarsdAWeiterJJCell loss in the aging retina. Relationship to lipofuscin accumulation and macular degenerationInvest Ophthalmol Vis Sci1989308169116992759786
- MoschosMMMarkopoulosIChatziralliIStructural and functional impairment of the retina and optic nerve in Alzheimer’s diseaseCurr Alzheimer Res20129778278822698074
- ParkHYParkYGChoAHParkCKTransneuronal retrograde degeneration of the retinal ganglion cells in patients with cerebral infarctionOphthalmology201312061292129923395544
- WexlerASandTElsasTBMacular thickness measurements in healthy Norwegian volunteers: an optical coherence tomography studyBMC Ophthalmol2010101320465801
