Abstract
There is a clear association between dry eye disease (DED) and skin inflammatory diseases occurring in close proximity to the eyelids, such as facial skin rosacea. Intense pulsed light (IPL) is widely accepted as a treatment for skin rosacea. A number of recent studies demonstrated that, in patients suffering from meibomian gland dysfunction (MGD), IPL therapy also reduces signs and symptoms of DED. Despite these encouraging results, in the context of DED and MGD, the mechanisms of action of IPL are not well understood. The purpose of this review was to raise the potential mechanisms of action and to discuss their plausibility.
Introduction
Dry eye disease (DED) is “a multifactorial disease of the tears and ocular surface…” that afflicts hundreds of millions around the world.Citation1 In the US alone, 40 million people are estimated to suffer from, or to be predisposed to, this debilitating condition.Citation2 DED is mostly age related,Citation1 but can also be triggered by refractiveCitation3,Citation4 or cataract surgery.Citation5–Citation7 In addition, preexisting DED significantly increases the risk of prolonged or severe post-op signs and symptoms of dry eye.Citation8,Citation9 Refractive and cataract surgery patients have high visual expectations, and increasingly sophisticated intraocular lens and corneal ablation designs heighten the importance of good ocular surface health. Success of refractive and cataract surgeries is therefore, in many cases, fundamentally dependent on effectively addressing preexisting or iatrogenic DED. The most common form of DED is evaporative, which is mainly due to meibomian gland dysfunction (MGD).Citation10 Current standard of care of MGD includes anti-inflammatory drugs, warm compresses, and meibomian gland expression.Citation11–Citation13
There is a clear association between MGD and skin inflammatory diseases occurring in close proximity to the eyelids. A common example is facial skin rosacea. One in ten people are affected by this skin condition, with >80% of these patients having concomitant MGD.Citation14 In 20% of the cases, ocular signs precede skin rosaceaCitation15 – possibly suggesting that skin rosacea could already exist in a subclinical form.
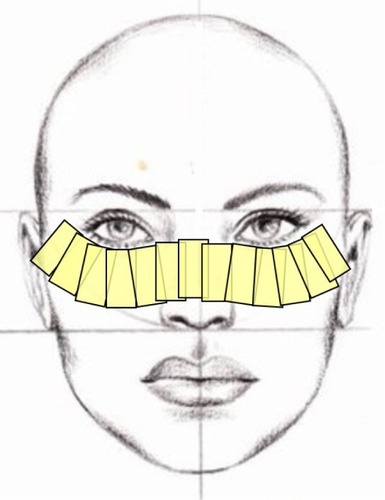
Intense pulsed light (IPL) is widely accepted as a treatment for skin rosacea.Citation16 More than a decade ago, Toyos et al noticed that facial skin rosacea patients treated with IPL reported a significant improvement in their dry eye symptoms.Citation17 Since then, a number of studies confirmed that IPL therapy reduces both signs and symptoms of dry eye.Citation18–Citation23 In these studies, IPL therapy comprised several sessions given several weeks apart. Each session consisted of IPL pulses applied from tragus to tragus, just below the lower eyelids and including the nose, as illustrated in .
Figure 1 Treatment area in IPL therapy of MGD.
Abbreviations: IPL, intense pulsed light; MGD, meibomian gland dysfunction.
Despite these encouraging results, the mechanism of action is not well understood. The purpose of this review is to raise the potential mechanisms of action and to discuss their plausibility.
Thrombosis of abnormal blood vessels
Facial skin rosacea is a chronic disorder presenting with vascular and inflammatory signs. The overwhelming majority of patients afflicted with this condition also suffer from MGD.Citation14 Although the causal relationship is not entirely clear, it seems reasonable that MGD patients might benefit from treatment of their concomitant skin condition. One of the primary features of rosacea is skin erythema and telangiectasia. It has been proposed that these abnormal blood vessels release inflammatory mediators.Citation18 Via the facial artery and orbital vasculature, these molecules could easily propagate to the eyelids, subsequently triggering the inflammation of meibomian glands and leading to their dysfunction and atrophy.
The beneficial effect of IPL on erythema and telangiectasia has been extensively studied and reported.Citation16 Light energy absorbed by hemoglobin transforms to heat and causes the localized destruction of superficial blood vessels (thrombosis). In the case of patients affected with MGD, destruction of abnormal erythematous blood vessels reduces a key reservoir of inflammatory mediators, thus removing a major source of inflammation from the eyelids and meibomian glands.
Heating and liquefying the meibum
Eyelid temperature significantly influences the physical properties of meibomian gland secretions, also known as meibum.Citation24 At higher temperatures, meibum becomes less viscous, which more easily allows its normal distribution over the cornea. At room temperature, the temperature at the eyelids is ~33°C.Citation25 In patients with MGD, lipid composition may be altered, reflecting changes in the configuration of hydrocarbon chain and lipid–lipid interaction strength. As a result, the phase-transition temperature (the temperature at which the meibomian lipids switch from an ordered and gel-like phase to a disordered and fluid-like phase) may increase, compared to healthy subjects.
In a study that analyzed the physical properties of meibum, the phase-transition temperature was ~28°C for meibum from healthy donors (below eyelid temperature), and just above 32°C for meibum from donors afflicted with MGD (above eyelid temperature).Citation26 Because the phase-transition temperature of human meibum is near physiological body temperature, a small increase of 4°C is sufficient to change the meibum from gel like to fluid.
Indeed, warming the eyelids (with warm compresses or more sophisticated and automated devices) has some therapeutic value, as it facilitates meibomian gland expression.Citation27 Craig et alCitation19 noted that IPL application could induce an increase in skin temperature. However, these authors argued that any increase is modest and short lived: immediately after IPL application, the skin temperature increased by <1°C.Citation19 However, it should be noted that in their study, skin temperature was measured with infrared thermography a few seconds after treatment and only after removal of the conducting gel. During these few seconds, the skin could cool down considerably and lose heat. It is therefore difficult to infer from this measurement what the temperature of the eyelids would be during IPL treatment itself.
However, whether or not IPL energy is sufficient to warm the skin is less important than its thermal effect on blood vessels under the surface. The eyelids are extensively fed by capillaries and arterioles branching off the facial artery. A mathematical model demonstrates that in medium and large blood vessels (>150 μm), a single IPL pulse of 30 ms duration raises the temperature at the center of the vessel to 80°C–90°C, above the temperature required to cause coagulation and thrombosis as discussed above.Citation28 In contrast, in small (60 μm) blood vessels, the temperature may reach only 45°C–70°C, depending on fluence.Citation28 This temperature elevation is insufficient to cause the destruction of blood vessels, but it is probably enough to raise the temperature of eyelid skin (and meibomian glands) by a few degrees, possibly above the phase-transition temperature. Even if brief, this thermal response could be enough to unclog the meibomian glands and restore their ability to excrete meibum during blinking.
Reducing the epithelial turnover and decreasing the risk for gland obstruction
As often occurs in skin diseases, cutaneous rosacea is accompanied by a dramatic increase in epithelial skin turnover. In a mechanism similar to dandruff production, large amounts of dead epithelial skin cells detach from the epidermal surface and create debris. Since the ducts of meibomian glands are paved with the same type of epithelial cells, accumulation of debris on the lid margin is likely to occur. This, in combination with poor lid hygiene, could potentially clog the orifices of meibomian glands.Citation29 IPL treatment of rosacea could, thus, decrease the epithelial turnover and reduce the risk factor for obstruction.
Photomodulation
Photomodulation is a process by which light in the visible and infrared portions of the electromagnetic spectrum induces intracellular changes at the gene and protein levels. The biological basis of this process is not well understood. According to the Karu model, red (~630 nm) photons are absorbed in cytochrome C oxidase (Cox), a key enzyme in the electron transport chain embedded within the membrane of mitochondria. Photoexcitation of Cox prompts a photochemical cascade, inducing changes in the redox properties of components along this mitochondrial respiratory chain, leading to quickened electron transfer and, hence, to an increase in ATP production.Citation30,Citation31 The cytoplasmic rise of ATP activates various intracellular/extracellular exchange mechanisms (pumps and transporters), resulting in an increase in intracellular free calcium concentration.
Smith proposes a complementary model, by which the absorption of infrared photons (~810 nm) induces molecular rotations and vibrations of various molecules.Citation32 When such physical forces are exerted on calcium channels, the permeability of these channels is altered such that the influx of calcium ions increases. Here as well, the end result is an abrupt surge in intracellular calcium concentration.
This calcium signal activates cellular responses in a variety of ways. In the case of fibroblasts, cell proliferation is enhanced and collagen synthesis is increased;Citation33 skin-homing T cells are recruited;Citation34 local blood flow is increased; macrophages cells are activated;Citation35 epidermal keratinocytes increase the secretion of proinflammatory or anti-inflammatory cytokines and chemokines, depending on the context.
Activating fibroblasts and enhancing collagen synthesis
The extracellular matrix comprises three types of fibers: collagen, reticular, and elastin.Citation36 With age, all the three types of fibers relax to some extent, thus compromising the natural rigidity and elasticity of tissues. At the eyelid skin level, this process can lead to poor apposition of the lid margins and incomplete blinks, resulting in reduced meibum pumping out of the meibomian glands. This can lead in turn to increased tear evaporation.
Fibroblast cells are responsible for the production of collagen fibers in wound healing and tissue repair. As mentioned earlier, photomodulation can prompt the proliferation of fibroblasts and upregulate the synthesis of collagen fibers.Citation33 An in vitro study showed that a pulsed 660 nm (LED) light enhanced collagen production in a tissue-engineered reconstructed skin model.Citation37 In another in vitro study, irradiation of skin fibroblasts with IPL (800–1,200 nm) increased the proliferation rate of fibroblasts and increased the expression of collagen genes.Citation38 These results are also supported by clinical studies.Citation39
Eradicating Demodex
One of the potential mediators of blepharitis and MGD are Demodex folliculum mites, a type of ectoparasite that normally burrows deep into sebaceous and meibomian glands to feed on their sebum/meibum secretions.Citation40 In healthy skin, the degree of infestation with Demodex mites is controlled. Demodex mites are normally colonized with Bacillus olerinus.Citation41,Citation42 Rosacea patients present with increased Demodex population on the face, high serum reactivity to B. olerinus proteins, and reduced levels of sebum.Citation43
The causal relationship between rosacea and Demodex is not clear. Some researchers argue that rosacea is fundamentally an infectious disease resulting from Demodex thriving on skin damaged by a combination of age, adverse weathering, and changes in sebum composition.Citation44 Others claim that erythema and superficial telangiectasia (which are characteristics of rosacea) induce edema of the dermis, which in turn increases skin colonization of Demodex.Citation45
A direct consequence of Demodex proliferation is the dramatic increase in bacterial load on the eyelids,Citation46 particularly B. olerinus. The excessive presence of B. olerinus near the eyelids triggers a cascade of events that may degenerate into chronic inflammation of the ocular surface. First, the immune system responds by orchestrating an army of proinflammatory agents, including antimicrobial peptides, toll-like receptors, cytokines, chemokines, and matrix metal-loproteinases (MMPs).Citation47,Citation48 In small quantities, these agents may perform well. But an acute inflammatory response may turn into a chronic, self-perpetuating condition. Second, B. olerinus releases toxic substances, including lipases which enzymatically alter lipid composition. A change in the ratio of saturated to unsaturated fats of the meibum could raise its melting point, increase its viscosity, and impede its secretion. In addition, one by-product of lipase activity on sebum/meibum is oleic acid, which could play a role in the keratinization of the lid margin, and plugging of the meibomian gland orifices.Citation13 All of these events could aggravate and perpetuate inflammation inside the meibomian glands.
The pigmented exoskeleton of Demodex contains chromophore that absorbs IPL energy. Histologic analysis demonstrated that IPL treatment induces coagulation and necrosis of Demodex.Citation49,Citation50 By eradication of Demodex, IPL could decrease the microbial load on eyelids and potentially break the vicious cycle of inflammation.
Modulating the secretion of pro- and anti-inflammatory molecules
Inflammation has a pivotal role in the development and propagation of evaporative DED in early as well as advanced phases of the disease.Citation51 Factors that adversely affect tear film stability and osmolarity can induce ocular damage and initiate an inflammatory cascade that generates a powerful immunological response which, in turn, may cause further damage at the ocular surface, creating a self-perpetuating inflammatory cycle. Clinical studies consistently report elevated levels of inflammatory molecules in the tears and ocular surface of patients with DED.Citation52 The levels of these cytokines/chemokines are often correlated with pain, tear instability, tear production, and/or ocular surface integrity.Citation51
IPL has the potential to interfere with this inflammatory cycle, by upregulation of anti-inflammatory cytokines, or downregulation of proinflammatory cytokines, or both. A few examples are noteworthy:
In cultured keratinocytes, IPL treatment led to a fivefold increase in the levels of interleukin-10 (IL-10), an anti-inflammatory protein that inhibits cytokine production in T cells.Citation53 In fibroblasts, IPL has a bidirectional effect on the secretion of transforming growth factor-β1 (TGF-β1): inhibition at low fluences, but enhancement at high fluences.Citation54 TFG-β is an interesting example, because it has both pro- and anti-inflammatory effects, depending on the context and the cellular environment. As an anti-inflammatory agent, TGF-β modulates the proliferation of T cells after encountering ocular surface epithelium, prevents their migration to the conjunctiva,Citation55 and suppresses natural killer (NK) cells.
A third example is the proinflammatory cytokine IL-6, which is downregulated subsequent to LED phototherapy.Citation56
Yet another example is the effect of IPL on the skin of acne patients: IPL significantly reduces inflammatory lesions, presumably by downregulation of tumor necrosis factor-α (TNF-α) (one of the cytokines which make up the acute phase of inflammation).Citation57
The inflammatory cascade in dry eye is extremely complex and incompletely understood. However, it is plausible that at least part of the beneficial effect of IPL on DED patients occurs by interfering with the positive feedback loop underlying the inflammatory cycle of this pathology.
Suppressing MMPs
Another type of proteins involved in the pathogenesis of dry eye are MMPs. These enzymes participate in extracellular matrix remodeling and are both directly and indirectly affected by IPL. For example, in skin fibroblasts, IPL treatment decreases the concentration of MMPs, by downregulation at the mRNA level.Citation58 In corneal epithelia cells, TNF-α and IL-1 upregulate several types of MMPs.Citation59 Recall that TNF-α is downregulated by IPL.Citation57 Therefore, IPL indirectly diminishes the levels of these MMPs. It is interesting to note that corticosteroids relieve dry eye symptoms by similar pathways: they interfere with the inflammatory cycle by lowering the cellular levels of cytokines, chemokines, and MMPs.Citation60–Citation62
Reactive oxidative species (ROS)
In rosacea, inflammation is associated with the generation of ROS released by neutrophils and other inflammatory cells.Citation63 ROS are highly reactive molecules containing oxygen, also widely referred to as free radicals. Examples of ROS include superoxide anions (O2–) and hydroxyl radicals (OH–). Abnormally high levels of ROS may result in oxidative stress, as was identified in the tear film of dry eye patients.Citation64
There are conflicting reports regarding the effect of visible light irradiation on the levels of ROS. For example, absorption of visible light in mitochondrial and cell membrane cytochromes generate ROS and thus could induce oxidative stress.Citation65 One report shows that application of light results in reduced levels of ROS.Citation66 Several researchers have proposed that the effect of light on ROS levels follows a biphasic dose response, also known as the Arndt–Schultz curve.Citation67,Citation68
Separately, either one of these contradictory effects could have a beneficial effect on dry eye patients. Following low-level light irradiation, an increase in ROS is described by the ascending part of the Arndt–Schultz curve. In this situation, light irradiation would result in excessive production of ROS and antimicrobial activity, thus reducing the bacterial load on eyelids. At higher doses, the descending part of the Arndt–Schultz curve could describe the antioxidant roles of light irradiation. In this part of the dose–response curve, light irradiation would result in the attenuation of ROS levels, thus diminishing oxidative stress and inflammation.
Conclusion
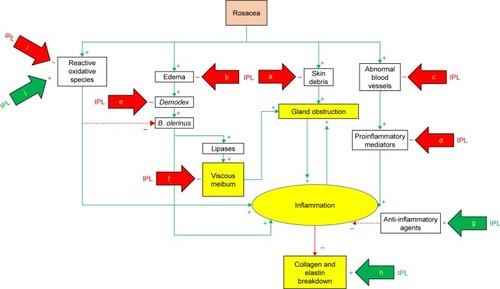
Dry eye is a multifactorial disease. Potential mechanisms whereby IPL could achieve clinical improvement include thrombosis of abnormal blood vessels below the skin surrounding the eyes, heating the meibomian glands and liquefying the meibum, activation of fibroblasts and enhancing the synthesis of new collagen fibers, eradication of Demodex and decreasing the bacterial load on the eyelids, interference with the inflammatory cycle by regulation of anti-inflammatory agents and MMPs, reducing the turnover of skin epithelial cells and decreasing the risk of physical obstruction of the meibomian glands, and changes in the levels of ROS (). While any one of these mechanisms of action has the potential to explain the effect of IPL on DED, it is also possible that multiple mechanisms of action are at play. As IPL becomes more commonly used in the treatment of DED, the specific contribution of each of these modes of action will be further elucidated.
Figure 2 Mechanisms of action of IPL (simplified model).
Abbreviations: IPL, intense pulsed light; a, skin rejuvenation; b, rosacea treatment; c, thrombosis; d, down-regulation; e, coagulation; f, warming and liquefying; g, up-regulation; h, fibroblasts activation; i, attenuation; j, production.
Disclosure
SJD is a consultant for Lumenis Ltd. The author reports no other conflicts of interest in this work.
References
- DEWSThe definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkshopOcul Surf200752759217508116
- DingJSullivanDAging and dry eye diseaseExp Gerontol201247748349022569356
- LevittAGalorAWeissJChronic dry eye symptoms after LASIK: parallels and lessons to be learned from other persistent post-operative pain disordersMol Pain2015112125896684
- ShojaMBesharatiMDry eye after LASIK for myopia: incidence and risk factorsEur J Ophthalmol20071711617294376
- LiXHuLHuJWangWInvestigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgeryCornea2007269 Suppl 1S16S2017881910
- AngRDarttDTsubotaKDry eye after refractive surgeryCurr Opin Ophthalmol200112431832211507347
- RobertsCElieEDry eye symptoms following cataract surgeryInsight20073211421
- ShteinRPost-LASIK dry eyeExpert Rev Ophthalmol20116557558222174730
- CetinkayaSMestanEAcirNCetinkayaYDadaciZYenerHThe course of dry eye after phacoemulsification surgeryBMC Ophthalmol201515681525571769
- LempMCrewsLBronAFoulksGSullivanBDistribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohortCornea2008271142114719034129
- ThodeALatkanyRCurrent and emerging therapeutic strategies for the treatment of meibomian gland dysfunction (MGD)Drugs201575111177118526130187
- EzuddinNAlawaKGalorATherapeutic strategies to treat dry eye in an aging populationDrugs Aging201532750551326123947
- GeerlingGTauberJBaudouinCThe international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunctionInvest Ophthalmol Vis Sci20115242050206421450919
- VisoERodríguez-AresMDOubiñaBGudeFPrevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of SpainIOVS201253626012606
- GhanemVMehraNWongSMannisMThe prevalence of ocular signs in acne rosacea: comparing patients from ophthalmology and dermatology clinicsCornea200322323023312658088
- PapageorgiouPClaytonWNorwoodSChopraSRustinMTreatment of rosacea with intense pulsed light: significant improvement and long-lasting resultsBr J Dermatol2008159362863218565174
- ToyosRBuffaCYoungermanSCase report: dry–eye symptoms improve with intense pulsed light treatment Available from: www.eyeworld.org/article.php?sid=2698EyeWorld (ASCRS)92005Accessed February 15, 2015
- ToyosRMcGillWBriscoeDIntense pulsed light treatment for dry eye disease due to meibomian gland dysfunction: a 3-year retrospective studyPhotomed Laser Surg2015331414625594770
- CraigJChenYTurnbullPProspective trial of intense pulsed Light for the treatment of meibomian gland dysfunctionInvest Ophthalmol Vis Sci20155631965197025678687
- VeguntaSPatelDShenJCombination therapy of intense pulsed light therapy and meibomian gland expression (IPL/MGX) can improve dry eye symptoms and meibomian gland function in patients with refractory dry eye: a retrospective analysisCornea201635331832226785301
- VoraGGuptaPIntense pulsed light therapy for the treatment of evaporative dry eye diseaseCurr Opin Ophthalmol20152631431826058031
- JiangXLvHSongHEvaluation of the Safety and Effectiveness of Intense Pulsed Light in the Treatment of Meibomian Gland DysfunctionJ Ophthalmol20162016191069427413540
- DellSGasterRBarbarinoSCunninghamDProspective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunctionClin Ophthalmol20171181782728496300
- NagymihályiADiksteinSTiffanyJThe influence of eyelid temperature on the delivery of meibomian oilExp Eye Res200478336737015106914
- ButovichIMillarTHamBUnderstanding and analyzing meibomian lipids–a reviewCurr Eye Res200833540542018568877
- BorchmanDFoulksGYappertMHuman meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunctionIOVS201152638053817
- FinisDHayajnehJKönigCBorrelliMSchraderSGeerlingGEvaluation of an automated thermodynamic treatment (LipiFlow®) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trialOcul Surf20141214615424725326
- BäumlerWVuralELandthalerMMuzziFShafirsteinGThe effects of intense pulsed light (IPL) on blood vessels investigated by mathematical modelingLasers Surg Med200739213213917066482
- HenriquezAKorbDMeibomian glands and contact lens wearBr J Ophthalmol19816521081117459311
- KaruTPrimary and secondary mechanisms of action of visible to near- IR radiation on cellsJ Photochem Photobiol B199949111710365442
- FarivarSMalekshahabiTShiariRBiological effects of low level laser therapyJ Lasers Med Sci201452586225653800
- SmithKThe photobiological basis of low level laser radiation therapyLaser Ther199131924
- TakezakiSOmiTSatoSKawanaSUltrastructural observations of human skin following irradiation with visible red light-emitting diodes (LEDs): a preliminary in vivo reportLaser Ther2005144153160
- TakezakiSOmiTSatoSKawanaSLight-emitting diode phototherapy at 630 +/− 3 nm increases local levels of skin-homing T-cells in human subjectsJ Nippon Med Sch2006732758116641531
- YoungSBoltonPDysonMHarveyWDiamantopoulosCMacrophage responsiveness to light therapyLasers Surg Med1989954975052811573
- UshikiTCollagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpointArch Histol Cytol200265210912612164335
- BaroletDRobergeCAugerFBoucherAGermainLRegulation of skin collagen metabolism in vitro using a pulsed 660 nm LED light source: clinical correlation with a single-blinded studyJ Invest Dermatol2009129122751275919587693
- Cuerda-GalindoEDíaz-GilGPalomar-GallegoMLinares- GarcíaValdecasasRIncreased fibroblast proliferation and activity after applying intense pulsed light 800–1200 nmAnn Anat2015198667225547460
- GoldbergDCurrent trends in intense pulsed lightJ Clin Aesthet Dermatol201256455322768357
- LiuJShehaHTsengSPathogenic role of Demodex mites in blepharitisCurr Opin Allergy Clin Immunol201010550551020689407
- SzkaradkiewiczAChudzicka-StrugałaIKarpińskiTBacillus oleronius and Demodex mite infestation in patients with chronic blepharitisClin Microbiol Infect201218101020102522114987
- LiJO’ReillyNShehaHCorrelation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosaceaOphthalmology2010117587087720079929
- JarmudaSMcMahonFZabaRCorrelation between serum reactivity to Demodex-associated Bacillus oleronius proteins, and altered sebum levels and Demodex populations in erythematotelangiectatic rosacea patientsJ Med Microbiol201463Pt 225826224248990
- JarmudaSO’ReillyNZabaRJakubowiczOSzkaradkiewiczAKavanaghKPotential role of Demodex mites and bacteria in the induction of rosaceaJ Med Microbiol201261111504151022933353
- CribierBPathophysiology of rosacea: redness, telangiectasia, and rosaceaAnn Dermatol Venereol2011138Suppl 3184191
- O’ReillyNMenezesNKavanaghKPositive correlation between serum immunoreactivity to Demodex-associated Bacillus proteins and erythematotelangiectatic rosaceaBr J Dermatol201216751032103622709541
- MargalitAKowalczykMŻabaRKavanaghKThe role of altered cutaneous immune responses in the induction and persistence of rosaceaJ Dermatol Sci20168213826747056
- LaceyNDelaneySKavanaghKPowellFMite-related bacterial antigens stimulate inflammatory cells in rosaceaBr J Dermatol2007157347448117596156
- PrietoVSadickNLloretaJNicholsonJSheaCEffects of intense pulsed light on sun-damaged human skin, routine, and ultrastructural analysisLasers Surg Med2002302828511870785
- KirnTIntense pulsed light eradicates Demodex mitesSkin Allergy News200233137
- Enríquez-de-SalamancaACastellanosESternMTear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye diseaseMol Vis20101686287320508732
- StevensonWChauhanSDanaRDry eye disease: an immune-mediated ocular surface disorderArch Ophthalmol201213019010022232476
- ByunJChoiHMyungKChoiYExpression of IL-10, TGF-β1 and TNF-α in cultured keratinocytes (HaCaT Cells) after IPL treatment or ALA-IPL photodynamic treatmentAnn Dermatol2009211121720548849
- HuangJLuoXLuJIPL irradiation rejuvenates skin collagen via the bidirectional regulation of MMP-1 and TGF-β1 mediated by MAPKs in fibroblastsLasers Med Sci201126338138721161310
- De PaivaCVolpeEGandhiNDisruption of TGF-β signaling improves ocular surface epithelial disease in experimental autoimmune keratoconjunctivitis siccaPlos One2011612e2901722194977
- LeeSParkKChoiJA prospective, randomized, placebo-controlled, double-blinded, and split-face clinical study on LED phototherapy for skin rejuvenation: clinical, profilometric, histologic, ultrastructural, and biochemical evaluations and comparison of three different treatment settingsJ Photochem Photobiol B2007881516717566756
- TaylorMPorterRGonzalezMIntense pulsed light may improve inflammatory acne through TNF-α down-regulationJ Cosmet Laser Ther20141629610324245979
- WongWRShyuWLTsaiJWHsuKHLeeHYPangJHIntense pulsed light modulates the expressions of MMP-2, MMP-14 and TIMP-2 in skin dermal fibroblasts cultured within contracted collagen latticesJ Dermatol Sci2008511707318424078
- LiDShangTKimHSolomonALokeshwarBPflugfelderSRegulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cellsInvest Ophthalmol Vis Sci2003442928293512824233
- AragonaPAguennouzMRaniaLMatrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye diseaseOphthalmology20151221627125240629
- ByunYKimTKwonSEfficacy of combined 0.05% cyclosporine and 1% methylprednisolone treatment for chronic dry eyeCornea201231550951319730097
- De PaivaCCorralesRVillarrealACorticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eyeExp Eye Res200683352653516643899
- JonesDReactive oxygen species and rosaceaCutis200974Suppl 317203234
- AugustinASpitznasMKavianiNOxidative reactions in the tear fluid of patients suffering from dry eyesGraefes Arch Clin Exp Ophthalmol1995233116946988566825
- LubartREichlerMLaviRFriedmanHShainbergALow-energy laser irradiation promotes cellular redox activityPhotomed Laser Surg20052313915782024
- LanCHoPWuCYangRYuHLED 590 nm photomodulation reduces UVA-induced metalloproteinase-1 expression via upregulation of antioxidant enzyme catalaseJ Dermatol Sci201578212513225816722
- LubartRLaviRFriedmannHRochkindSPhotochemistry and photobiology of light absorption by living cellsPhotomed Laser Surg200624217918516706696
- HuangYYChenAHCarrollJMRHBiphasic dose response in low level light therapyDose Response20097435838320011653
