Abstract
Purpose
The purpose of this study was to report the functional and anatomical outcomes of a prospective study resulting from repeated dexamethasone intravitreal implants in patients with uveitic refractory macular edema.
Methods
Twelve eyes of 9 patients with intermediate and posterior noninfectious inflammatory uveitis complicated with refractory macular edema were regularly reviewed after a dexamethasone intravitreal implant. Patients were examined at baseline, 30, 90, 135, and 180 days with best-corrected visual acuity (BCVA), complete slit-lamp examination, intraocular pressure (IOP), optical coherence tomography, and fluorescein angiography. After 6 months of follow-up, eyes were reassessed to receive a second implant.
Results
BCVA significantly improved when comparing the baseline values after the first and second implant (16.2 and 25.8 letters, respectively, 9.6 letters improvements, p<0.05). BCVA was better after the second implant compared to the first one throughout the follow-up, but without statistical significance. Mean central macular thickness (CMT) was 446.3±129.9 μm at baseline and was significantly reduced until day 135 (p<0.05). CMT reductions after the second injection showed a similar pattern, though differences were not statistically significant. Cataract progression was observed in 4 of 8 phakic eyes (50%) after the first implant, and in 2 of 3 phakic eyes following the second implant, with 1 eye requiring cataract surgery. One eye developed an IOP >30 mmHg 30 days after the second implant, treated topically.
Conclusion
Repeated dexamethasone intravitreal implants in uveitic patients with refractory macular edema can be used effectively in a clinical setting with an acceptable safety profile.
Introduction
Uveitis is a group of inflammatory diseases of the eye accounting for 10%–15% of legally blind people in the Western world.Citation1–Citation3 Noninfectious uveitis is the most common cause of uveitis. The clinical presentation is often a chronic and/or recurrent inflammation, leading to the breakdown of the inner and outer blood–retinal barrier, to increased vessels permeability, swelling of the central retina, and cystoid macular edema (CME). Several factors are implicated, including increased levels of vascular endothelial growth factor, inflammatory mediators (prostaglandins, interleukin-6), as well as a reduction of the endothelial tight junctions.Citation4–Citation7 Corticosteroids were the mainstay in the treatment of uveitis, with considerable advances in the immunosuppressive therapy established in recent years.Citation8,Citation9 Steroids present important systemic and ocular side effects, the latter being increased intraocular pressure (IOP) with a varying range of 20.8%–52% and accelerated cataract formation in 29% of patients.Citation10–Citation15 The most effective route of administration of steroids is a matter of debate.Citation16,Citation17,Citation30 The Ozurdex® implant (Allergan, Inc, Irvine, CA, USA) injected into the vitreous chamber through a 22-gauge applicator has proved to be effective in treating macular edema from branch retinal vein occlusion/central retinal vein occlusion, as well as uveitis and other etiologies.Citation18–Citation22 The implant is a biodegradable copolymer composed of polylactic acid and polyglycolic acid that erodes into carbon dioxide and water, while dexamethasone is released into the eye.Citation23,Citation24
The purpose of this case series was to report our experience in a real-life setting of ≥1 dexamethasone intravitreal implants in patients affected by noninfectious intermediate uveitis, posterior uveitis, or panuveitis whose CME was refractory to other steroid therapies.
Methods
Study design
This is a prospective, single center, interventional study. All patients signed an informed written consent form before initiation of the procedures performed as per standard of care according to the Italian Ophthalmic Society guidelines. The study was approved by the local Ethic Committee (Comitato Etico A.O.U Città della Salute e della Scienza – A.O. Ordine Mauriziano – A.S.L Città di Torino, protocol no 0033070).
Study population
Patients of at least 18 years of age with a diagnosis of inter-mediate or posterior noninfectious inflammatory uveitis or panuveitis with persistent macular edema lasting for ≥90 days refractory to oral or peribulbar steroid treatment were offered long-acting dexamethasone intravitreal implant. Patients had steroid systemic treatment that had to be stable for ≥3 months at the time of the injection. After the dexamethasone intravitreal injection, no systemic treatment was added. All eyes received topical nonsteroidal anti-inflammatory drugs 3 times a day for 1 month following the injection.
Key exclusion criteria were moderate or severe glaucoma not controlled by medical therapy, steroid responder patients (defined as IOP ≥30 mmHg or rise of ≥15 mmHg after steroid administration), poorly controlled hypertension (defined as systolic pressure >160 mmHg and diastolic pressure >90 mmHg), and poorly controlled diabetes (with HbA1c >13%).
Study treatment
From November 2011 to January 2014, 12 eyes of 9 patients were included in the study. The dexamethasone intravitreal implant was inserted into the vitreous cavity through the pars plana using a single-use 22-gauge applicator by an experienced ophthalmologist (AMF). Prior to each treatment, the study eye was anesthetized with topical anesthetics and prepared according to standard guidelines of the Italian Ophthalmic Society for intravitreal injection of the long-acting dexamethasone implant. All patients were treated with a topical ophthalmic antibiotic 4 times a day starting 3 days prior to the day of their study procedure (day 0) and continuing for 5 days after the procedure.
Outcome measures and follow-up
Patients were evaluated at baseline, 7, 30, 90, 135, and 180 days after the study treatment. The primary outcome at 6 months was improvement of best-corrected visual acuity (BCVA) measured using the standardized Early Treatment Diabetic Retinopathy Study protocol. Secondary outcomes were reduction of central macular thickness (CMT) and improvement of ocular inflammation. Complete slit-lamp biomicroscopy was performed in order to detect nuclear, cortical, or posterior subcapsular lens opacities and signs of inflammation in the anterior chamber (Tyndall effect, cells, flare). IOP was measured with a Goldmann applanation tonometer using a cutoff of ≥25 mmHg or a rise of ≥20% compared to the patient’s baseline IOP to treat ocular hypertension. Posterior ocular inflammation was evaluated through indirect fundus examination for vitreous haze evaluation and examination of the posterior pole of the retina. Fluorangiography (HRA1; Heidelberg, Germany) was also performed with intravenous fluorescein and indocyanine green taking images of both the posterior pole and retinal periphery during early, mid, and late phases. Measurements of CMT were carried out using optical coherence tomography (OCT; SD-OCT RTV100; Optovue Inc, Fremont, CA, USA).
The primary outcome of BCVA improvement was evaluated using a cutoff of a 15-letter improvement (3 lines on standard visual acuity chart) and a second 10-letter improvement cutoff (2 lines on standard visual acuity chart). CMT thickness reduction was considered significant when it reduced >20% compared to the initial value.
Eyes were reassessed after 6 months of follow-up to receive a second implant. Eyes with a BCVA improvement of ≥10 letters and/or a decrease in CMT of ≥20% compared to baseline were considered to be responding to the treatment. Eyes with a BCVA improvement of <10 letters and those with a CMT reduction <20% at OCT were considered as non-responding to the treatment.
Lens status
The Lens Opacities Classification System (LOCS) III grading system was used to assess the severity of cataract. The threshold for referring a patient for cataract surgery was a progression of lens opacity according to the LOCS system associated with reduction of BCVA.
Data analysis and statistical methods
Mean values of BCVA, CMT, and IOP were recorded at each visit and compared with baseline values using Student’s t-test for paired samples. An α level of 0.05 was considered statistically significant. Baseline digital photos were taken to evaluate the reduction of ocular inflammation throughout the follow-up and were compared on a clinical basis.
Results
Patient demographics and baseline characteristics are listed in .
Table 1 Patient demographics and baseline characteristics
Mean age was 60.8 years (range 23–78 years), two-third of the patients were male, and the vast majority (8 of 9 patients, 88.9%) were Caucasians. Seven out of 12 eyes (58.3%) were phakic, 4 (33.3%) were pseudophakic, and 1 eye (8.7%) was aphakic. Epiretinal membranes (ERMs) were present at baseline in 4 eyes, 4 developed it during the follow-up, and in 4 eyes no membranes were observed throughout the whole period. None of the ERMs required surgical intervention. During the visit at day 180, 5 eyes of 5 patients were considered suitable to be reinjected: all of them presented an improvement of both central retinal thickness (CRT) and BCVA. The mean reinjection time was 8.8 months (range 7–10) (). Seven eyes of 4 patients did not show a significant improvement in BCVA or a reduction in CMT and were not reinjected. Three of these had stable visual acuity at day 180, whereas in 3 other eyes no significant improvement in BCVA was detected throughout the follow-up. Migration of the implant into the anterior chamber happened in 1 aphakic eye, and thus was not considered suitable for repeated injections.
Visual acuity
First implant
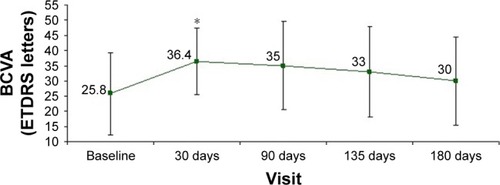
Mean BCVA changes after 1 dexamethasone intravitreal implant in the 12 eyes considered in this study are shown in . Pre-implant mean visual acuity was 20.3 letters (±15.9). It showed significant improvement at all follow-up visits (p<0.05), with a gain of 12 letters (32.3±18.1) at day 30, 11.9 letters (32.25±18.9) at day 90, 10.1 letters (30.4±18.7) at day 135, and 8 letters (28.3±17.9) at day 180. Fifty percent of the eyes (6 out of 12) had a 15-letter improvement at day 30 and 90, and 25% (4 out of 12) at day 135 and 180. Fifty percent of the patients obtained a 10-letter improvement at days 30, 90, and 135. This was maintained in 33% of the eyes at day 180. BCVA improvement maintained its statistical significance when analyzing only the worst eye of patients who received treatment in both eyes ().
Figure 1 Changes in BCVA following one dexamethasone intravitreal implant in 12 eyes affected by noninfectious inflammatory uveitis.
Abbreviations: BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study.
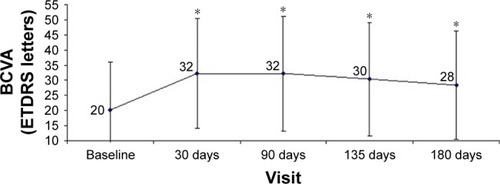
Second implant
Considering the reinjected eyes, mean visual acuity showed a significant increase of 10.6 letters (p<0.05) after the second implant at day 30 (36.4±10.9 letters compared to 26±13.6 letters at baseline). Although BCVA increased during the entire period with a gain of 9.2 letters (35±14.5 letters) at 90 days, 7.2 letters (33±14.8 letters) at 135 days, and 4.2 letters (30±14.5 letters) at 180 days, the difference with baseline values was not statistically significant (p>0.05) ().
CMT
First implant
Mean CMT was 446.3±129.9 μm at baseline and was significantly reduced throughout the 135th day of follow-up visit (p<0.05) ().
Table 2 Changes in mean CMT following one dexamethasone intravitreal implant, in 12 eyes diagnosed with noninfectious inflammatory uveitis
In all, 66.7% (8 out of 12) of the patients presented a CMT reduction ≥20% compared to mean baseline values until day 135. This percentage reduced to 33% at the 6 months follow-up visit. When analyzing only the worst eyes of those patients who received treatment in both eyes, the CMT was significantly reduced until day 135 (p<0.05) ().
Second implant
CMT at baseline was 493.2±134.6 μm. At day 30, CMT was reduced to 360.8±134.6 μm, to 371.6±102.3 μm at 90 days, to 445.8±157.7 μm at 135 days, to 462.8±187.5 μm at 180 days (p>0.05) (). CMT reductions after the second injection showed a similar pattern when compared to the first injection, though differences between the 2 groups were not statistically significant.
Table 3 Changes in mean CMT following a second dexamethasone intravitreal implant in 5 eyes diagnosed with noninfectious inflammatory uveitis
IOP
Following the first implant, mean IOP at baseline was 11.91±3.57 mmHg. It rose to 13.75±4.24 mmHg 30 days after the procedure, to 14.33±5.10 mmHg at day 90, to 13±3.41 mmHg at day 135, and to 13.91±2.06 mmHg at 6 months follow-up. These variations never appeared to be statistically significant (p>0.05). An IOP ≥25 mmHg was not observed during the first implant.
After the second implant, IOP was higher at 30 days (17±7.77 mmHg) compared to pre-implant values (14.6±5.4 mmHg). This was due to a single eye which developed an IOP of 30 mmHg at day 30 and was successfully treated with topical therapy (brinzolamide 10 mg/mL + timolol 5 mg/mL) until the following visit at day 90.
Lens status
Cataract progression was observed in 4 of 8 phakic eyes (50%) after the first dexamethasone intravitreal implant, and in 2 of 3 phakic eyes following the second implant, with 1 eye requiring cataract surgery with posterior chamber intraocular lens implant.
Anterior chamber migration
Migration into the anterior chamber of the device was observed 24 hours after the injection in the only aphakic patient. This complication was handled with pupil dilation and postural maneuvres, enabling repositioning of the implant in the vitreous chamber (Figure S1).
Repeat injections of dexamethasone
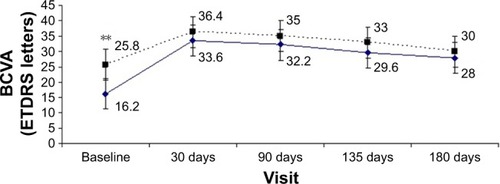
Mean BCVA values of the 5 patients who underwent the first and the second implant are plotted in . For those patients who received 2 dexamethasone intravitreal implants, mean values of BCVA and CMT were compared for the baseline and for each subsequent visit. Mean visual acuity at baseline was better before the second implant compared to initial visual acuity prior to the first implant. There was a 9.6-letter improvement (16.2 letters before first implant and 25.8 letters before second implant); this difference was statistically significant (p<0.05). All the following visits showed a greater BCVA after the second device implant compared to the first one (36.4 vs 33.6 letters at 30 days, 35 vs 32.2 letters at 90 days, 33.4 vs 30 letters at 135 days, 31.4 vs 28 letters at 180 days), but none were statistically significant. CMT of the first implant was 504.4 μm at baseline, 373 μm at 30 days, 319.4 μm at 90 days, 348.6 μm at 135 days, and 485 μm at 180 days, whereas during the second treatment it reached 493.2, 360.8, 371.6, 445.8, and 462.8 μm, respectively. These differences were not statistically significant.
Figure 3 Best-corrected mean visual acuity after 1 dexamethasone intravitreal implant in 5 eyes of 5 patients who responded to treatment.
Abbreviations: BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study.
Vitreoretinal interface
Six eyes out of 12 (50%) demonstrated abnormalities of the vitreo-macular interface, ranging from subtle thickening without traction (5 eyes, 41%) to ERMs. We did not notice a progression of the ERM during follow-up. In all these cases, after the implant the membranes followed the shape of the retina suggesting that the tractional role was minimal (Figures S2–S4).
Conclusion
Blindness is attributed to uveitis in 10%–15% of cases in the developed world.Citation1 One of the most common complications is CME, which may be particularly difficult to treat. Its chronicity accounts for severe visual loss, occurring in 25%–50% of patients.Citation2–Citation4
In this study, we examined the effect of repeated intravitreal injections of 0.7 mg long-acting dexamethasone in eyes with recalcitrant macular edema secondary to noninfectious inflammatory uveitis in a clinical setting. All eyes had undergone at least one line of therapy, with oral steroids and/or immunosuppressive medications, without any effect on the macular edema.
After the first implant, we found 1) a significant improvement of BCVA through the entire 6 month follow-up period, 2) a significant reduction of CMT until day 135, with a thickness increase observed at 180 days, 3) a safety profile consistent with the other studies, and 4) a case of anterior chamber dislocation in aphakic eye, successfully treated with postural maneuvres.
The HURON studyCitation22 examined the use of a single dexamethasone implant in cases of chronic noninfectious uveitis, showing an improvement in BCVA and vitritis accompanied by a decrease in CRT. In the 77 eyes receiving 0.7 mg long-acting dexamethasone, the mean visual acuity was 58 letters at baseline, with 46.2% of eyes achieving a ≥15-letter improvement at 6 months (p<0.001). In an observational case series on repeat dexamethasone implants in noninfectious uveitis by Tomkins-Netzer et al,Citation25 the mean baseline visual acuity was 0.47 and 0.43 at 6 months. Zarranz-Ventura et alCitation26 reported in another multicenter retrospective cohort study a visual acuity of 0.68 logMAR at baseline, significantly improving to 0.6 logMAR at 6 months. In our cohort, pre-implant BCVA, with a mean value of 20.3 (±15.9) letters, significantly improved to 28.3±17.9 letters (p<0.05) at 6 months, and 4 out of 12 eyes (25%) showed a 15-letter improvement at the same time point. These values seem to be more in line with retrospective studies,Citation25,Citation26 having patient inclusion criteria not as strict as those found in randomized trials.Citation22 CRT was significantly reduced initially, increasing subsequently at 6 months. A similar trend was noted by Nobre-Cardoso et al,Citation27 where the relapse of macular edema occurred at 3 months after the first injection.
Following our re-treatment criteria, 6 eyes were not treated: 3 eyes because the patients responded to the first implant without a recrudescence of the disease at 180 days; and 3 other eyes because the reduction of CMT was not associated with an improvement of visual acuity during the follow-up. Repeated injections achieved a BCVA gain similar to the first implant throughout the entire period, a reduction in CMT that was stable during the follow-up, although not statistically significant. Comparing the follow-up visits of the first and second implant, we noticed that functional improvement was similar, whereas the time trend of CMT differed. As the first treatment induced an initial CMT reduction followed by a relapse of macular edema, the second implant stabilized it at lower values during the whole follow-up. Other studies found a better functional and anatomical profile before the second implant in eyes receiving multiple injections.Citation27,Citation28
The risk of increased IOP is a well-known side effect after dexamethasone intravitreal implant,Citation10,Citation11,Citation18 and in nonsteroid responders it can be effectively managed with topical therapy.Citation22,Citation25–Citation27 In our cohort, 1 out of 12 eyes had an IOP >21 mmHg (8.3%) after the first injection and 1 out of 5 eyes had an IOP >25 mmHg (20%) after the second injection. Both were effectively treated with local IOP lowering medication. The rate of this complication was similar to that found by Tomkins-Netzer et al (7.9% after first implant, 17.4% after second implant), and lower compared with other studies.Citation26,Citation27 Cataract progression occurred in 4 out of 8 phakic eyes after the first implant and in 2 out of 3 phakic eyes after the second implant; in both cases, cataract surgery was required. The rates of cataract incidence vary by a considerable degree, with 50% of eyes developing cataract in the study published by Arcinue et al,Citation29 to 4.9% in the HURON study,Citation22 or 7% in the retrospective analysis of 47 eyes.Citation27 We estimate that the cohort of phakic patients in this study is too scarce to be comparable to other studies. Anterior chamber migration of the device is an adverse event reported in the literature and one of the risks underlined in the Food and Drug Administration Safety Report for aphakic and (anterior chamber intraocular lens) AC-IOL eyes.Citation31–Citation34 In our series, the anterior chamber displacement occurred in the only aphakic eye of our study and it was successfully managed with postural maneuvres. Evidence of complications in aphakic patients and AC-IOLs is stronger at present,Citation35 while this was not the case during the inclusion of patients in this study. Before the first injection, 8 of 12 eyes (66%) presented with an ERM, without metamorphopsia or traction. The implant proved effective in reducing the macular thickness even in the presence of ERM and determined a functional improvement. In a few cases reported in the literature, intravitreal dexamethasone implant in association with a vitreo-macular traction resulted in the formation of a macular hole or pseudohole.Citation36,Citation37 In our study, no macular hole presented over the 26 months study.
This paper had some limitations especially concerning the less number of patients and the absence of a control group to compare the results. Also, a longer follow-up with a higher reinjection frequency would allow to detect changes and side effects more accurately.
In conclusion, this single-arm interventional prospective study illustrates how dexamethasone intravitreal implant in uveitic patients with refractory macular edema can be used effectively in a clinical setting. It also shows that in patients with alterations of the vitro-macular interface, the implant can be effective in reducing the macular thickness before any surgical therapy is attempted. Patients with recurrent macular edema may benefit from a second implant although some of them can show an increase of IOP. Further randomized studies are needed to evaluate the role of dexamethasone multiple implant on cataract development.
Supplementary materials
Figure S1 Anterior chamber migration of the long-acting intravitreal dexamethasone implant with corneal edema in an aphakic patient.
Notes: Postural maneuvres with previous pupil dilation allowed correct repositioning of the implant with full resolution of the corneal edema and restoration of the VA before the complication. (A) Migration of the dexamethasone intravitreal implant into the anterior chamber. (B) Resolution of corneal edema after repositioning of the device into the vitreous chamber.
Abbreviation: VA, visual acuity.
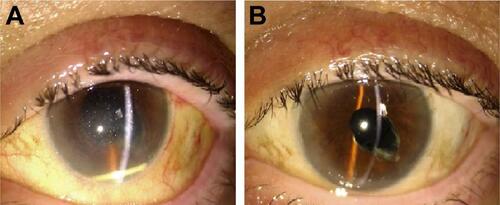
Figure S2 OCT image of an epiretinal membrane 30 days after intravitreal dexamethasone injection.
Abbreviation: OCT, optical coherence tomography.
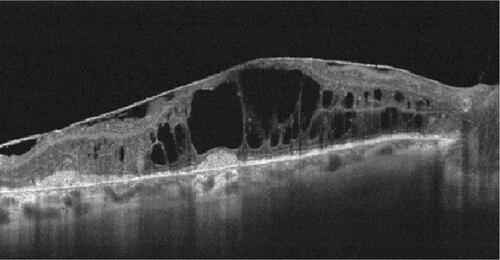
Figure S3 OCT image of an epiretinal membrane 90 days after intravitreal dexamethasone injection.
Abbreviation: OCT, optical coherence tomography.
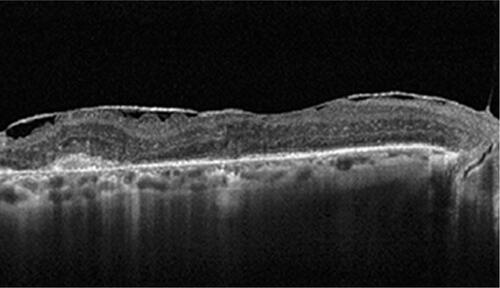
Figure S4 OCT image of an epiretinal membrane 180 days after intravitreal dexamethasone injection.
Abbreviation: OCT, optical coherence tomography.
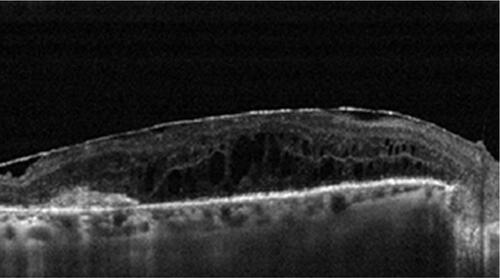
Table S1 Eyes receiving the first and second dexamethasone intravitreal implant, and the mean interval between injections
Table S2 Changes in BCVA and CMT following one dexamethasone intravitreal implant in 9 eyes affected by noninfectious inflammatory uveitis, considering only the worst eye for patients receiving treatment in both eyes
Disclosure
The authors report no conflicts of interest in this work.
References
- RothovaASuttorp-van SchultenMSFrits TreffersWKijlstraACauses and frequency of blindness in patients with intraocular inflammatory diseaseBr J Ophthalmol1996803323368703885
- NussenblattRBThe natural history of uveitisInt Ophthalmol1990145–63033082249907
- Thomkins-NetzerOTalatIBarALong-term clinical outcome and causes of visual loss in patients with uveitisOphthalmology20141212387239225178807
- de SmetMDTaylorSRBodaghiBUnderstanding uveitis: the impact of research on visual outcomesProg Retin Eye Res20113045247021807112
- JabsDANussenblattRBRosenbaumJTStandardization of uveitis nomenclature for reporting clinical data. Results of the First International WorkshopAm J Ophthalmol200514050951616196117
- KempenJHSugarEAJaffeGJFluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edemaOphthalmology20131201852185923706700
- FreemanGMatosKPavesioCECystoid macular oedema in uveitis: an unsolved problemEye (Lond)200115121711318279
- JapACheeSPImmunosuppressive therapy for ocular diseasesCurr Opin Ophthalmol200819653554018854699
- JabsDARosenbaumJTFosterCSGuidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panelAm J Ophthalmol200714481281718036872
- FardetDLKassarACabaneJFlahaultACorticosteroid-induced adverse events in adultsDrug Saf20073086188117867724
- ArmalyMFStatistical attributes of the steroid hypertensive response in the clinically normal eye. I. The demonstration of three levels of responseInvest Ophthalmol1965418719714283012
- ArmalyMFEffect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eyeArch Ophthalmol19637049249914078871
- BakriSJBeerPMThe effect of intravitreal triamcinolone acetonide on intraocular pressureOphthalmic Surg Lasers Imaging20033438639014509462
- KogureAOhkoshiKKogureSYamaguchiTKishiSEfficacy and retention times of intravitreal triamcinolone acetonide for macular edemaJpn J Ophthalmol20085212212618626735
- KonstantopoulosAWilliamsCPNewsomRSLuffAJOcular morbidity associated with intravitreal triamcinolone acetonideEye20072131732016710433
- NguyenQDCallanDDugelPGodfreyDGGoldsteinDAWilenskyJTTreating chronic non-infectious posterior segment uveitis: the impact of cumulative damage. Proceedings of an expert panel roundtable discussionRetina200626Suppl 81616395131
- KarimRSykakisELightmanSFraser-BellSInterventions for the treatment of uveitic macular edema: a systematic review and meta-analysisClin Ophthalmol201371109114423807831
- HallerJABandelloFBelfortRJrOZURDEX GENEVA Study GroupRandomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusionOphthalmology201011761134114620417567
- HallerJABandelloFBelfortRJrOZURDEX GENEVA Study GroupDexamethasone intravitreal implant in patients with macular edema related to branch or central vein occlusion: twelve-month study resultsOphthalmology2011118122453246021764136
- KuppermannBDBlumenkranzMSHallerJADexamethasone DDS Phase II Study GroupRandomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edemaArch Ophthalmol2007125330931717353400
- HunterRSLoboAMDexamethasone intravitreal implant for the treatment of noninfectious uveitisClin Ophthalmol201151613162122140307
- LowderCBelfortRLightmanSDexamethasone intravitreal implant for noninfectious intermediate or posterior uveitisArch Ophthalmol201112954555321220619
- HallerJADugelPWeinbergDVChouCWhitcupSMEvaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edemaRetina200929465118827732
- Chang-LinJEAttarMAcheampongAAPharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implantInvest Ophthalmol Vis Sci2011521808620702826
- Tomkins-NetzerOTaylorSRJBarATreatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitisOphthalmology20141211649165424650556
- Zarranz-VenturaJCarrenoEJohnstonRLMulticenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, and reinjection frequencyAm J Ophthalmol20141581136114525217856
- Nobre-CardosoJChampionEDarugarAFelALehoangPBodaghiBTreatment of non-infectious uveitic macular edema with the intravitreal dexamethasone implantOcul Immunol Inflamm Epub2016322
- CaoJHMulvalhillMZhangLJoondephBCDaceyMFDexamethasone intravitreal implant in the treatment of persistent uveitic macular edema in the absence of active inflammationOphthalmology20141211649165424650556
- ArcinueCACerònOMFosterCSA comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitisJ Ocul Pharmacol Ther20132950150723297752
- Multicenter Uveitis Steroid Treatment Research GroupThe multicenter uveitis steroid treatment (MUST) trial: rationale, design and baseline characteristicsAm J Ophthalmol2010149455055620097325
- AdeniranJMFJusufbegovicDSchaalSCommon and rare ocular side-effects of the dexamethasone implantOcul Immunol Imflamm Epub201675
- KishoreSASchaalSManagement of anterior chamber dislocation of dexamethasone implantOcul Immunol Inflamm2013211909123323589
- Pardo-LópezDFrancés-MuñozEGallego-PinazoRDíaz-LlopisMAnterior chamber migration of dexamethasone intravitreal implant (Ozurdex®)Graefes Arch Clin Exp Ophthalmol2012250111703170421861084
- Marín-LambíesCGallego-PinazoRGarcía-DelpechSDíaz-LlopisMOzurdex® y afaquia: una combinaciòn a evitar [Ozurdex(®) and aphakia: a combination to avoid]Arch Soc Esp Oftalmol2012876191192 Spanish22633612
- BurkholderBMMoradiAThorneJEDunnJPThe dexamethasone intravitreal implant for noninfectious uveitis: practice patterns among uveitis specialistsOcul Immunol Inflamm201523644445326517764
- Di AntonioLTotoLCarpinetoPMastropasquaADe NicolaCMastropasquaLMacular pseudohole development after sustained-release dexamethasone intravitreal implant for macular edema due to central retinal vein occlusionEur J Ophthalmol201424113413723709332
- De BenedettoUBattaglia ParodiMKnutssonKALattanzioRBandelloFIaconoPMacular hole after injection of intravitreal implant for macular oedema due to central retinal vein occlusionActa Ophthalmol2013911e75e7722863290