 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
We evaluated the expression of the neural markers, neuron-specific enolase, and synaptophysin, as a tool to confirm the diagnosis of retinoblastoma (RB) in undifferentiated and advanced tumors. Additionally, we determined whether the extent of RB-associated protein (pRb) expression is helpful in assessing the prognosis in RB patients.
Methods
Conventional whole tissue section and tissue microarray immunohistochemistry for neuron-specific enolase, synaptophysin, and pRb were carried out in a series of 22 RBs.
Results
Neuron-specific enolase and synaptophysin were expressed in 75%–100% of the tumor cells, and the staining intensity was strong. Two RBs expressed pRb in 75%–100% of the tumor cells, also with strong staining intensity. Concordance between the immunohistochemical outcomes for whole tissue staining and tissue microarray staining was 76.2% for neuron-specific enolase, 85.7% for synaptophysin, and 80.0% for pRb.
Conclusion
Neuron-specific enolase and synaptophysin have the potential to be useful markers for the diagnosis of RBs. Extensive and strong pRb staining is not associated with less aggressive tumor behavior according to the pathologic classification of RBs.
Introduction
Retinoblastoma (RB) is the most common primary intraocular malignant tumor in children.Citation1 The incidence ranges from 1:14,000 to 1:20,000 live births (7,000–8,000 new cases per year worldwide). Incidence rates in the USA are 2.9 cases per 100,000 per year in children younger than 1 year and 1.6 cases per 100,000 per year in 1-year-old children. More than 90% of these cases are diagnosed before the age of 5 years. The frequency is higher in Africa, India, and Latin America.
The current survival rate in the USA approaches 100%. However, in Africa, this rate is very low between 20% and 46%.Citation2 If survival rates were similar to the developed countries globally, RB deaths could be reduced by 88%. RB metastases occur in less than 5% of patients in developed countries but still represent a challenge in developing countries.Citation3
The RB gene (RB1) is localized to chromosome 13q14.2. This is the first tumor suppressor gene described and was isolated in 1986.Citation4 RB develops when biallelic inactivation or loss of both alleles occur. This is consistent with Knudson’s “two-hit” hypothesis in which two complementary events are necessary to convert a normal retinal cell into a tumor cell.Citation5 In 60% of cases, mutations appear locally in the developing retina. About 40% have germline mutations of the RB1 gene and are inherited in an autosomal dominant fashion. All bilateral cases are the result of germline mutations.Citation6
The RB1 gene encodes a protein, RB-associated protein (pRb), that acts as a tumor growth suppressor.Citation7,Citation8 pRb is a 928 amino acid nuclear phosphoprotein that plays a critical role in regulating cell cycle progression at the G1/S phase transition. During the G0/G1 phase, the hypophosphorylated active form interacts with the E2F family of transcription factors, inhibiting the transcription of E2F target genes. In response to mitogenic stimuli, pRb is phosphorylated and E2F transcription factors are released, inducing gene expression leading to progression through the cell cycle.
The histogenesis of RB is not well known. Recent findings indicate that RB cells derive from cone precursors.Citation9 Histological examination reveals that RB cells are undifferentiated, having scant cytoplasm and prominent, oval or round basophilic nuclei with finely granular chromatin and no nucleoli. Numerous mitotic figures are usually found. Formation of Flexner–Wintersteiner rosettes is a characteristic of RBs. These rosettes are composed of a ring of high cuboidal cells surrounding an apical lumen.Citation10–Citation13 Tumor growth rate is limited by the ability to induce the formation of new vessels; hence, large areas of coagulative necrosis and dystrophic calcification frequently appear.Citation14
Immunohistochemistry is widely used in histopathologic diagnosis and for research purposes.Citation15,Citation16 However, no specific marker is currently available for RB. The use of neuron-specific enolase and synaptophysin could improve the diagnostic accuracy in RB, which would be very useful in advanced tumors.
The practice of transferring multiple tissue samples to a single slide for immunohistochemical analysis was implemented by Battifora in 1986.Citation17 In 1998, Kononen et alCitation18 invented a mechanism that allowed the quick and precise construction of tissue microarrays that greatly facilitated analysis. It enabled the standardization of immunohistochemical studies because all specimens are processed at one time under identical conditions. The main drawback of tissue microarray analysis is the partial or total loss of one or more tissue core samples. Because ~10%–15% of the tissue cores can be lost,Citation19 the inclusion of two cores per tumor is usually sufficient and comparable to the study of whole tissue sections in more than 95% of cases.Citation20
Undifferentiated and advanced RB tumors, especially those masses with extensive necrosis where no ocular structures are identifiable, can pose a diagnostic challenge to pathologists. Therefore, the purpose of this study was to test the value of assessing the expression of neuron-specific enolase and synaptophysin, both of which are neural markers, as a tool to confirm the diagnosis of undifferentiated and advanced RB tumors in Angolan patients.
Patients and methods
Tissue samples
This study was performed in accordance with tenets of the Declaration of Helsinki, and all procedures were approved by the University of Valladolid ethics committee. Informed written consent was obtained from the parents of each of the patients.
Retinoblastoma patients (n = 21; 11 males and 10 females) were selected from the archives of IOBA Ocular Pathology Laboratory covering a 10-year period. In one RB patient, after the right eye was removed, metastatic masses were subsequently found in the right lower eyelid and cheek. Thus, tissue samples from 22 RB tumors (17 eye enucleations and 5 eviscerations) were analyzed by standard light microscopy and by immunohistochemistry of formalin-fixed and paraffin-embedded tissue blocks. Twenty-one patients were from Angola as arranged by an agreement between the University of Valladolid and the National Eye Institute of Angola. All eye specimens obtained from Angolan patients were sent to Spain for processing and diagnosis. Enucleation and evisceration specimens were fixed in 10% formalin and sent by express mail to Spain. The specimens arrived ~7–10 days after surgery. One case from a Spanish patient showing superficial invasion of the optic nerve was also included.
American Joint Committee on Cancer, Cancer Staging Manual guidelines for pathological staging were used.
The samples were processed in a Leica ASP-300 tissue processor (Leica Biosystems, Nussloch, Germany) using a 32-hour program for eye globes. The globes were subsequently embedded in paraffin, and 4-µm sections were obtained and collected on FLEX IHC Microscope Slides (Dako, Glostrup, Denmark).
The degree of necrosis in H&E sections was evaluated as focal (<25%), moderate (≥25%–50%), extensive (>50%–75%), or very extensive (>75%) of the tumor cells. The degree of apoptosis in H&E sections was evaluated as focal (<10%), moderate (≥10%–25%), or intense (>25%) of tumor cells.
A manual tissue microarray instrument (Beecher Instruments, Silver Spring, MD, USA) was used to construct each microarray. The most appropriate tumor areas were identified on H&E-stained sections from each tissue donor block. Two cores of 1.5-mm diameter were taken per case. A total of 44 cores were present in the tissue microarray block.
Immunohistochemistry
The immunohistochemical study was performed in a Dako Autostainer Link 48 instrument (Dako) for both whole tissue sections and tissue microarrays.
Pretreatment
Heat-induced epitope retrieval was performed in a Dako PT Link (Dako) using EnVision™ FLEX Target Retrieval Solution, High pH (50×) (Dako) for neuron-specific enolase and synaptophysin. EnVision™ FLEX Target Retrieval Solution, Low pH (50×) (Dako) was used for pRb. The slides were washed in Envision™ Flex Wash Buffer 20× (Dako) at room temperature (RT) for 5 minutes. Inhibition of endogenous peroxidases was achieved using EnVision™ FLEX Peroxidase-Blocking Reagent (Dako) for 5 min.
Incubation with primary antibodies
Ready-to-use primary monoclonal antibodies were employed for neuron-specific enolase and synaptophysin. pRb antibody was used at a 1:25 dilution (). Negative control slides were incubated with ready-to-use FLEX Negative Control Mouse (Dako). The slides were treated with EnVision™ FLEX/HRP for 20 min, followed by washing with EnVision™ FLEX Wash Buffer (20×). Substrate + chromogen (diaminobenzidine) were then applied, followed by another washing with EnVision™ FLEX Wash Buffer (20×). The tissue was then counterstained with EnVision™ FLEX Hematoxylin.
Table 1 Primary antibodies and incubation protocols
Assessment of immunohistochemical staining
A semiquantitative assessment of the immunohistochemical results was carried out by one of the authors according to the scheme used by The Swedish Human Protein Atlas Project.Citation21 All samples were examined under the microscope at low and high magnifications. The fraction of stained cells was scored as <25%, ≥25%–75%, or >75%. The staining intensity was scored as negative, weak, moderate, or strong. The subcellular localization was designated as nuclear, cytoplasmic, or membranous.
In the tissue microarray, two cores were used from each case to avoid tissue lost. Study cases were excluded only if both cylinders were lost. This occurred for one case each stained for neuron-specific enolase and synaptophysin, and for two cases of pRb. A map specifying the exact position of each tumor was prepared to facilitate the interpretation of the results. When the scores differed between two cores of the same case, the highest score was used.
The percentage of immunohistochemical staining outcomes that were concordant between the whole tissue sections and the core tissue sections was calculated as follows:
Results
In 13 cases (59%), the tumor was in the left eye, and in nine cases (41%), it was in the right eye. The median age at diagnosis was 2.9 years (range 1–8 years). The degree of necrosis was extensive in 13 tumors and very extensive in three of the tumors. The degree of apoptosis was moderate or intense in most of the tumors studied (19 cases). Calcifications were present in 18 of the RBs. The median number of mitotic cells per 10 high power fields (40×) was 12.76. Choroidal and scleral invasion were present in 17 tumors. The diameter of uveal invasion was greater than 3 mm in most of the cases. In many, malignant cells affected the internal fibers of the sclera; therefore, these infiltrations were classified as significant choroidal invasion according to the recommendations of the International Retinoblastoma Staging Working Group.Citation22 Anterior chamber invasion was identified in 18 cases. Sixteen cases (72.7%) were classified as pT4 tumors, which are characterized by tumor invasion of the optic nerve to the resection line or exhibited extraocular extension elsewhere.Citation23
Immunohistochemistry
Whole tissue sections
For neuron-specific enolase, 17 RBs (77%) had 75%–100% positive cells and five cases (23%) had 25%–75% positive cells. Twenty RBs (91%) were strongly positive () and two cases (9%) were moderately positive. Necrotic cells in all cases were strongly positive.
Figure 1 Whole tissue section immunohistochemistry.
Abbreviations: pRb, RB-associated protein; RB, retinoblastoma.
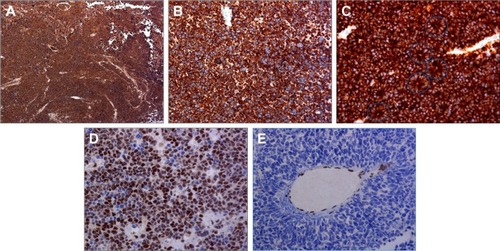
For synaptophysin, 20 tumors (91%) had 75%–100% positive cells () and two (9%) had 25%–75% positive cells. All 22 tumors were strongly positive. Greater staining intensity for synaptophysin was localized to apical cytoplasm of Flexner–Wintersteiner rosettes (). In all cases, necrotic cells were strongly positive.
For pRb, one case was positive in more than 75% of the tumor cells and had moderate expression in the nucleus (). Twenty-one tumors (95.5%) were negative for pRb ( and ).
Table 2 Immunohistochemistry: whole tissue sections
Table 3 Immunohistochemistry: whole tissue sections
Tissue microarrays
For neuron-specific enolase, in 14 tumors (67%), 75%–100% of the cells were positive and had strong staining intensity (). Six tumors (28%) had 25%–75% positive cells that were stained with moderate intensity. One tumor (5%) was focally positive with less than 25% of the cells stained. Both cylinders were lost in one case, which was excluded for evaluation. There was 76.2% concordance with the results obtained from conventional whole tissue sections ().
Table 4 Immunohistochemistry: neuron-specific enolase: whole tissue sections and tissue microarray
Figure 2 Tissue microarray immunohistochemistry.
Abbreviation: pRb, retinoblastoma-associated protein.
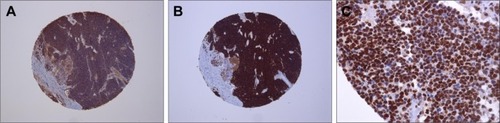
For synaptophysin, in 18 tumors (86%), 75%–100% of the cells were positive and had strong staining intensity (). Two cases (9%) had 25%–75% positive cells with moderate staining intensity, and one case (5%) had focal staining with less than 25% positive cells. Both cylinders were lost in one case so it could not be used for evaluation. There was 85.7% concordance with the results obtained from whole tissue sections ().
Table 5 Immunohistochemistry: synaptophysin: whole tissue sections and tissue microarray
For pRb, in two cases (10%), 75%–100% of the cells were positive and had strong staining intensity (). Three cases (15%) had focal staining with less than 25% positive cells, and 15 tumors (75%) were completely negative for pRb. In two cases, both cylinders were lost so only 20 RBs could be used for evaluation. There was 80.0% concordance with the results obtained from conventional whole tissue sections ().
Table 6 Immunohistochemistry: pRb: whole tissue sections and tissue microarray
Discussion
In developed countries, RBs are usually diagnosed at early stages, when tumors are small and confined to the eye. Eye-conservative therapy is the standard treatment in these cases; hence, the proportion of patients treated with enucleation is decreasing in high-income countries.Citation2 However, enucleation is required for more advanced tumors, and all enucleated eye globes must be submitted for a complete histopathological examination by an experienced ocular pathologist.
The grade of optic nerve involvement and the age at diagnosis of infiltration are the factors most significantly associated with survival in RB patients.Citation24 If tumor cells infiltrate the optic nerve beyond the lamina cribrosa and there is also involvement of the surgical margin, the mortality rate rises to 78%.
Invasion of the optic nerve beyond the lamina cribrosa, choroidal invasion, scleral invasion, and infiltration of the anterior segment are considered high-risk histopathologic factors. The presence of these high-risk factors is associated with an increased risk of local recurrence and metastases.Citation22,Citation25 Advanced tumors are often found in developing countries like Angola, and the diagnosis is made when extraocular invasion has already occurred. Conditions to be considered in the differential diagnosis of RBs include rhabdomyosarcoma, Burkitt’s lymphoma, primitive neuroectodermal tumor, and orbital metastases of a Wilms’ tumor.
Based on the histopathological findings, patients can be divided into three categories: low, medium, and high risk.Citation26 Those who fall into the medium- and high-risk categories receive chemotherapy. More aggressive treatment is given to the high-risk group. Sadly, in many cases, children living in developing countries may not have access to these therapies. Another issue is poor access to a complete histopathology report.Citation27
The presence of optic nerve infiltration of all the RBs in this study and the existence of choroidal, scleral, and anterior chamber invasion in most of the tumors are likely to be related to the delay in the diagnosis of Angolan patients. Unfortunately, when the diagnosis is delayed, RBs grow in an uncontrolled manner.
In 1984, Kyritsis et al used immunofluorescence microscopy to search for neuron-specific enolase and glial fibrillary acidic protein in Y-79 cells, a human RB cell line.Citation28 They found that undifferentiated cells expressed both markers while those differentiated to neurons expressed only neuron-specific enolase. Y-79 cells that differentiated to glial cells expressed glial fibrillary acidic protein. This differential expression was related to a change in cell morphology, and they suggested that RBs originate from primitive neuroectodermal cells with the ability to differentiate to neural or glial cell lines.Citation28
In the late 1980s and in the 1990s, the first immunohistochemical studies were performed on RB surgical specimens to identify the cell of origin. Antibodies against target proteins included the neuronal markers synaptophysin and neuron-specific enolase, glial markers S-100 and glial fibrillary acidic protein, and other markers including pRb, p53, and bcl-2.Citation29–Citation32 In the late 1990s, these efforts were abandoned, probably because it was assumed that the RB cell of origin was the one that expressed neural markers or neural and glial markers. In the present study, we sought a new approach to test the value of neuron-specific enolase and synaptophysin (neural markers) expression as a tool to confirm the diagnosis of RBs. Also, the degree of pRb expression would help to assess the prognosis in RB patients as shown in other tumors.Citation33
Molnar et al reported the immunohistochemical distribution of neuron-specific enolase, glial fibrillary acidic protein, myelin-associated glycoprotein, and S-100 on seven human RBs.Citation34 In that study, most of the tumor cells were positive for neuron-specific enolase, but Flexner–Wintersteiner rosettes were negative or weakly positive. In our study, we found intense and strong positivity for neuron-specific enolase in one case with abundant Flexner–Wintersteiner rosettes.
Garrido et alCitation35 reported that all differentiated RBs (n = 10) were positive for neuron-specific enolase, but only two of five poorly differentiated RBs were positive. This is consistent with other findings where the percentage of positive cases for neuron-specific enolase in poorly differentiated tumors was lower compared with well-differentiated RBs.Citation31 In our study, we found intense staining for neuron-specific enolase in most of the 22 RBs that we studied. Furthermore, 21 of the 22 tumors were poorly differentiated. This is consistent with Sun et al who found that in 27 undifferentiated tumors, the cells were strongly positive for neuron-specific enolase.Citation36 We believe that the different results obtained by Molnar et al,Citation34 Garrido et al,Citation35 and He et alCitation31 compared to our results may be due to the use of a neuron-specific enolase ready-to-use monoclonal antibody that is more specific than the polyclonal antibodies used previously.
Yuge et al detected synaptophysin by immunohistochemistry only in the apical cytoplasm of cells forming rosettes, and no reactivity was observed in any of the undifferentiated tumors.Citation29 Kivelä et al found that nine out of 27 poorly differentiated tumors were completely negative for synaptophysin, and He et al found similar results in 35 undifferentiated RBs.Citation30,Citation31 However, He et al also reported that synaptophysin was present in the rosettes of 41 differentiated tumors. In our series, synaptophysin was detected in all of the poorly differentiated RBs. We also found greater staining intensity for synaptophysin in the apical cytoplasm of Flexner–Wintersteiner rosettes.
Yuge et al did not find pRb expression in a series of 34 RBs.Citation29 In contrast, Orjuela et al found nuclear pRb in 11 of 86 RBs, and 4 cases had diffuse staining in more than 80% of the tumor cells.Citation37 They also concluded that tumors negative for pRb were those with extraocular extension. Low expression levels of pRb are also correlated with a worse prognosis in patients with prostate, breast, endometrial adenocarcinomas, and laryngeal carcinomas.Citation38–Citation41 Furthermore, transitional bladder cell carcinomas with decreased expression of pRb are more aggressive than tumors expressing pRb in most of the cells.Citation42
Two of the RBs in our study were strongly positive for pRb and were classified as pT4, ie, tumors with invasion of the surgical margin and extraocular extension. Even though there was strong and diffuse expression of pRb, this did not appear to be related to lower tumor aggressiveness. We believe that pRb could appear as a functionally inactive protein due to mutations that do not affect the expression. Alternatively, the RB protein could be present in a phosphorylated form that is not active, as documented in previous studies.Citation33,Citation43,Citation44
The concordance between whole tissue and tissue microarray immunohistochemical outcomes was greater than 76% for neuron-specific enolase, synaptophysin, and pRb. This greatly exceeds the minimal acceptable level of 50%. Thus, tissue microarray analysis of neuron-specific enolase and synaptophysin could be a valuable aid in RB diagnosis.
The main limitation of this study was the lack of follow-up and outcome data for the patients. Of the 21 patients, 20 were diagnosed and treated in Angola by local ophthalmologists; therefore, we were unable to access their medical records from Spain.
Conclusion
Retinoblastoma cells express the neural markers neuron-specific enolase and synaptophysin. Necrotic cells were also positive for neuron-specific enolase and synaptophysin. These results suggest that neuron-specific enolase and synaptophysin are useful markers for the diagnosis of RBs. This is especially so in those cases with extensive necrosis where no ocular structures are identifiable as is often the case in patients from developing countries. The extensive distribution and strong staining of pRb is not associated with a less aggressive behavior according to the pathologic classification of RBs.Citation23
To the best of our knowledge, this is the first tissue microarray study performed in RB samples. This technique is highly efficient, saving reagent costs and allowing all of the samples to be treated under the same conditions in a single experiment. Immunohistochemical analysis for neuron-specific enolase and synaptophysin could improve the diagnostic accuracy in RBs, which would be helpful in advanced and undifferentiated tumors.
Acknowledgments
The Spanish Agency for International Development Cooperation (Spain’s Ministry of Foreign Affairs and Cooperation) contributed to the delivery of the specimens from Angola to Valladolid.
Disclosure
The authors alone are responsible for the content and writing of the article and report no conflicts of interest in this work.
References
- National Cancer Institute [webpage on the Internet]Age-Specific Rates and Counts for the Top 5 Cancer Sites by Single Year of Age at Diagnosis SEER Cancer Incidence 2007–2011National Cancer Institute, Surveillance, Epidemiology, and End Results Program Available from: http://seer.cancer.gov/csr/1975_2011/browse_csr.php?sectionSEL=28&pageSEL=sect_28_table.13.html#aAccessed October 7, 2016
- HoustonSKMurrayTGWolfeSQFernandesCECurrent update on retinoblastomaInt Ophthalmol Clin20115117791
- KiveläTThe epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and deathBr J Ophthalmol20099391129113119704035
- FriendSHBernardsRRogeljSA human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcomaNature198632360896436462877398
- KnudsonAGJrMutation and cancer: statistical study of retinoblastomaProc Natl Acad Sci U S A19716848208235279523
- BuninGRMeadowsATEmanuelBSBuckleyJDWoodsWGHammondGDPre- and postconception factors associated with sporadic heritable and nonheritable retinoblastomaCancer Res19894920573057352790788
- LeeWHShewJYHongFDThe retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activityNature198732961406426453657987
- SachdevaUMO’BrienJMUnderstanding pRb: toward the necessary development of targeted treatments for retinoblastomaJ Clin Invest2012122242543422293180
- XuXLSinghHPWangLRb suppresses human cone-precursor-derived retinoblastoma tumoursNature2014514752238538825252974
- YanoffMSassaniJWRetinoblastoma and pseudogliomaYanoffMSassaniJWOcular PathologySt LouisMosby Elsevier2009733763
- McLeanIWRetinoblastomas, retinocytomas, and pseudoretinoblastomasSpencerWHOphthalmic Pathology: An Atlas and a TextbookPhiladelphiaW. B. Saunders Company199613321438
- McLeanIWBurnierMNZimmermanLEJakobiecFATumors of the retina Atlas of Tumor Pathology3rd edWashington, DCArmed Forces Institute of Pathology199497154
- HoganMJZimmermanLERetina: neoplasmsHoganMJZimmermanLEOphthalmic Pathology An Atlas and TextbookPhiladelphiaW. B. Saunders Company1962516525
- BurnierMNMcLeanIWZimmermanLERosenbergSHRetinoblastoma. The relationship of proliferating cells to blood vesselsInvest Ophthalmol Vis Sci19903110203720402211000
- The Human Protein Atlas [webpage on the Internet]Methods: ImmunohistochemistryThe Human Protein Atlas Available from: http://www.proteinatlas.org/learn/method/immunohistochemistryAccessed October 7, 2016
- CoonsAHCreechHJJonesRNImmunological properties of an antibody containing a fluorescent groupExp Biol Med1941472200202
- BattiforaHThe multitumor (sausage) tissue block: novel method for immunohistochemical antibody testingLab Invest19865522442483525985
- KononenJBubendorfLKallioniemiATissue microarrays for high-throughput molecular profiling of tumor specimensNat Med1998478448479662379
- IlyasMGrabschHEllisIONational Cancer Research Institute (UK) Biomarker and Imaging Clinical Studies GroupGuidelines and considerations for conducting experiments using tissue microarraysHistopathology201362282783923672312
- CampRLCharetteLARimmDLValidation of tissue microarray technology in breast carcinomaLab Invest200080121943194911140706
- The Human Protein Atlas [webpage on the Internet]Assays & AnnotationThe Human Protein Atlas Available from: http://www.proteinatlas.org/about/assays+annotation#ihAccessed October 7, 2016
- SastreXChantadaGLDozFInternational Retinoblastoma Staging Working GroupProceedings of the consensus meetings from the International Retinoblastoma Staging Working Group on the pathology guidelines for the examination of enucleated eyes and evaluation of prognostic risk factors in retinoblastomaArch Pathol Lab Med200913381199120219653709
- EdgeSByrdDRComptonCCFritzAGGreeneFLTrottiAAJCC Cancer Staging Manual7th edNew YorkSpringer-Verlag2010
- MagrammIAbramsonDHEllsworthRMOptic nerve involvement in retinoblastomaOphthalmology19899622172222704542
- EagleRCJrHigh-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic studyArch Pathol Lab Med200913381203120919653710
- SullivanEMWilsonMWBillupsCAPathologic risk-based adjuvant chemotherapy for unilateral retinoblastoma following enucleationJ Pediatr Hematol Oncol2014366e335e34024577551
- DimarasHKimaniKDimbaEARetinoblastomaLancet201237998241436144622414599
- KyritsisAPTsokosMTricheTJChaderGJRetinoblastoma – origin from a primitive neuroectodermal cell?Nature198430759504714736694739
- YugeKNakajimaMUemuraYMikiHUyamaMTsuburaAImmunohistochemical features of the human retina and retinoblastomaVirchows Arch199542665715757655737
- KiveläTTarkkanenAVirtanenISynaptophysin in the human retina and retinoblastoma. An immunohistochemical and Western blotting studyInvest Ophthalmol Vis Sci19893022122192464554
- HeWHashimotoHTsuneyoshiMEnjojiMInomataHA reassessment of histologic classification and an immunohistochemical study of 88 retinoblastomas. A special reference to the advent of bipolar-like cellsCancer19927012290129081451072
- XuKPLiuSLNiCImmunohistochemical evidence of neuronal and glial differentiation in retinoblastomaBr J Ophthalmol19957987717767547791
- CoteRJDunnMDChatterjeeSJElevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53Cancer Res1998586109010949515785
- MolnarMLStefanssonKMartonLSTripathiRSMolnarGKImmunohistochemistry of retinoblastomas in humansAm J Ophthalmol19849733013076199979
- GarridoCMArraAStudies of ocular retinoblastomas with immunoperoxidase techniquesOphthalmologica198619342422473295638
- SunXLYokoyamaTMinodaKSakumaAImmunohistochemical studies of retinoblastomaJpn J Ophthalmol19903421491571699015
- OrjuelaMOrlowIDudasMAlterations of cell cycle regulators affecting the RB pathway in nonfamilial retinoblastomaHum Pathol200132553754411381373
- SharmaAComstockCEKnudsenESRetinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cellsCancer Res200767136192620317616676
- DerenziniMMontanaroLViciMRelationship between the RB1 mRNA level and the expression of phosphorylated RB protein in human breast cancers: their relevance in cell proliferation activity and patient clinical outcomeHistol Histopathol200722550551317330805
- SemczukAMarzecBRoessnerAJakowickiJAWojcierowskiJSchneider-StockRLoss of heterozygosity of the retinoblastoma gene is correlated with the altered pRb expression in human endometrial cancerVirchows Arch2002441657758312461615
- PietruszewskaWKlatkaJBorzeckiARieskePLoss of heterozygosity for Rb locus and pRb immunostaining in laryngeal cancer: a clinicopathologic, molecular and immunohistochemical studyFolia Histochem Cytobiol200846447948519141402
- Cordon-CardoCWartingerDPetrylakDAltered expression of the retinoblastoma gene product: prognostic indicator in bladder cancerJ Natl Cancer Inst19928416125112561640484
- Rodríguez-CruzMSanchezRArenasDpRb detection as a common event in human retinoblastomas: an immunohistochemical studyActa Histochem2008110210911617963824
- NorkTMMillecchiaLLPoulsenGImmunolocalization of the retinoblastoma protein in the human eye and in retinoblastomaInvest Ophthalmol Vis Sci1994356268226928188462
