Abstract
A 42-year-old man has been under long-term follow-up since he was a child for congenital glaucoma and buphthalmos in both eyes. His left eye best corrected visual acuity (BCVA) was counting fingers, due to end-stage glaucoma. He was on maximal medical therapy with an intraocular pressure (IOP) maintained at mid to low twenties. His right eye, the only seeing eye, had a BCVA of 6/9. This eye had undergone multiple glaucoma laser and surgical procedures, including an initial first Molteno drainage device inserted superonasally that failed in April 2003 due to fibrotic membrane over the tube opening. As a result, he subsequently had a second Molteno drainage device inserted inferotemporally. To further maximize his vision he had an uncomplicated cataract extraction and intraocular lens implant in December 2004, after which he developed postoperative cystoid macular edema and corneal endothelial failure. He underwent a penetrating keratoplasty in the right eye thereafter in March 2007. After approximately a year, the second Molteno device developed drainage tube retraction, which was managed surgically to maintain optimum IOP in the right eye. His right eye vision to date is maintained at 6/12.
Case report
Congenital glaucoma and resultant buphthalmos are very difficult conditions to manage. If intraocular pressure (IOP) is not controlled in early childhood, this can result in devastating consequences for the patient’s vision. This was the case with our patient, such that his left vision was very poor. As a result of coexisting left amblyopia and glaucoma, the eye had poor vision at best corrected visual acuity (BCVA) of hand movements. His left eye IOP was well controlled in the low twenties with maximal medical therapy, namely cosopt bd, brimonidine bd, latanoprost nocte, and pilocarpine 2% qds. As a child in the 1970s, the patient had had a trabeculectomy in this eye that failed. The main aim for the eye was to keep it comfortable with IOP control.
The right eye had a small island of central vision remaining. As a result, the right eye underwent interventional management to try to control the IOP when pressures were high/spiking, as it was the only eye with useful vision at BCVA 6/9 (see ). Target IOP was 10–11 mm Hg.
Figure 1 Graph showing IOP (mm Hg) variance over time in the right eye with points of intervention when there was a spike in IOP.
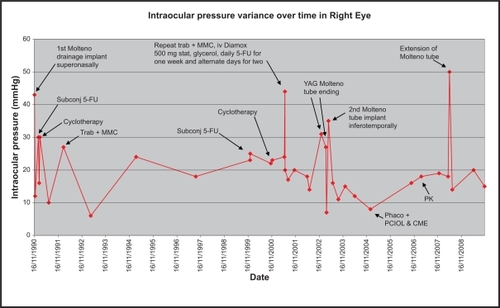
In addition to medical therapy, the patient had iridencleisis procedures as a child in his right eye. He subsequently went on to have a Molteno drainage device implant superonasally for uncontrolled IOP at 43 mm Hg in 1990. Over the course of 1 year, the IOP remained unsettled despite subconjunctival 5-fluorouracil (5-FU) and cyclocryotherapy. This was noticed by the patient as repeated intermittent haloes throughout the day. This was likely due to pressure spikes. The right eye underwent a trabeculectomy + mitomycin-C (MMC) in 1992 when the IOP was successfully reduced to low twenties with additional medical treatment. In 2001, he had a repeat trabeculectomy + MMC and subsequent repeated 5-FU injections over 3 weeks to reduce fibrotic drive. This proved to be successful for only 1 year. Thereafter, similar symptoms of haloes returned. A fibrotic membrane over the Molteno tube opening was treated with neodymium-doped yttrium aluminium garnet laser twice unsuccessfully.
The Molteno tube had then retracted and needed repositioning, but unfortunately the pressure reduction was short lived and therefore a second Molteno drainage implant was positioned inferotemporally. This proved to be by far the most lasting success with an IOP reduction to the mid-teens for 2 years on no medical therapy. The situation was much more settled, but without the haloes the patient started to notice a “haze” in front of his vision. This was due to a posterior subcapsular cataract that developed over a long period of time secondary to frequent use of topical steroid drops for keeping inflammation and fibrosis at bay postsurgery. He underwent an uncomplicated phacoemulsification with intraocular lens implant in December 2004, after which he developed postoperative cystoid macular edema confirmed on ocular coherence tomography. This was appropriately managed, but subsequently the cornea decompensated with epithelial edema in 2006. The patient underwent a penetrating keratoplasty in 2007 with a clear graft to date. The pressure remained controlled in the mid-teens until 2008, when he developed tube retraction in the second functioning Molteno drainage implant. Aggressive scarring response leads to a higher incidence of tube retraction.Citation1–Citation3
On discussion of the risks and benefits, it was decided that he undergo tube extension (see Surgical technique). Thereafter, on timoptol 0.5% bd, his pressure remained in the mid-teens (see , last measured March 2010: 16 mm Hg), which to date has settled with preservation of the central island of vision and BCVA of 6/12.
A pre-existing angle anomaly that is further compounded due to persistent raised IOP and chronic inflammatory response secondary to previous multiple surgeries is often the cause of poor long-term structural and functional outcome in this case. Delayed implantation of drainage devices at a later age of 22 years might also in part have contributed to the poor surgical outcome.
Glaucoma drainage device (GDD) surgery is now common in complex cases such as infantile glaucoma. The outcome of these procedures is encouraging and increasingly supports their use as first-line surgical options for the treatment of high-risk adult and juvenile glaucoma.Citation4
This surgical intervention is contraindicated in patients who have borderline endothelial function and are unable to comply with self-care and close follow-up. Complications include hypotony, suboptimal raised IOP, tube retraction/obstruction, diplopia, corneal decompensation/corneal graft failure, suprachoroidal hemorrhage, endophthalmitis, and resultant loss of vision.
Surgical technique
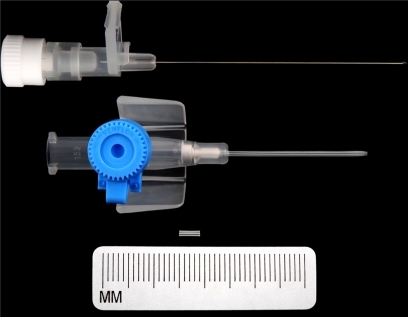
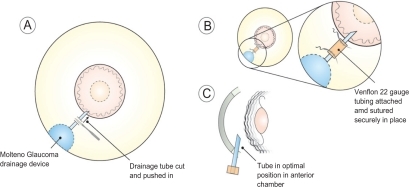
GDD tube retraction is a well recognized but uncommon complication. A variety of surgical devices has been used to extend drainage device tubes, namely 22-gauge angiocatheter, Crawford tubing, and Tube Extender® (New World Medical, Inc., Rancho Cucamonga, CA, USA).Citation5 In this patient, a 4 mm, 22-gauge angiocatheter (Venflon) cannula (see ) was used to connect the proximal and distal ends of the cut drainage tube where the distal end was advanced 4 mm into the anterior chamber with optimum positioning (see and ).
Figure 4 Postoperative appearance of optimal glaucoma drainage device tube position in anterior chamber.
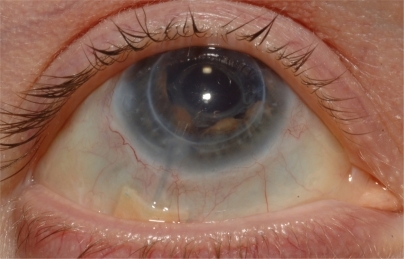
The tube portion of the GDD was adequately exposed by careful conjunctival dissection. A 4 mm extending segment of 22-gauge intravenous catheter tubing was cut using a sharp blade. The distal end of the GDD tube was advanced 4 mm into the anterior chamber with optimal positioning (see ). The extending segment fit very securely over the cut ends of the tube proximally and distally. The segment was further secured with 10-0 nylon at each end (see ). An appropriately sized donor scleral patch graft was then placed over the tube, and 8-0 absorbable sutures were used to close the conjunctiva. Anterior chamber paracentesis was then performed and balanced salt solution injected to ensure patency of the tube. Viscoelastic was injected into the anterior chamber to prevent hypotony. This allowed for re-establishment of aqueous humor drainage and resultant control of IOP postoperatively. Great care must be taken that corneal endothelial touch due to the persisting shallow anterior chamber during initial phases of valve surgery is prevented. If not prevented or managed properly, intrusion of uveal tissue in the tube osteum can ensue, and subsequent fibrous ingrowth can occur within the tube.
The results of the successful use of alternative modality using an angiocatheter segment to extend GDD tubes were first described by Smith and Doyle.Citation1 The technique used in this patient was similar to the one described by Bansal et al.Citation6 They described two complex pediatric cases who required extension of retracted GDD tube. One case had satisfactory reduction in IOP, whereas the second case had only 25% reduction in IOP due to scarring over the plate.Citation6
The Venflon cannula used to make the extension segment is biocompatible, as it is made of polytetrafluoroethylene (Teflon), which is an inert material and is used for making extensions for extraocular musclesCitation7 and vascular prosthesis.Citation8 It is also used in other parts of the body without any known long- term complications.Citation9
The commercially available Tube Extender has been used to extend retracted GDD tubes.Citation5 It is relatively expensive ($200 per Tube Extender, compared with about $4 per intravenous catheter).Citation6 The main problem with its use is its distal end, which is relatively bulky and does not seem to be ideal for placement near the limbus.Citation5 Further, it may be difficult to suture the relatively larger distal end if the sclera is already thin due to multiple glaucoma procedures, as in this case.
An alternative to tube retraction is repositioning the GDD anteriorly. This requires extensive dissection of conjunctiva and tenon’s capsule and can lead to postoperative scarring, especially in this case. Implantation of a new GDD would be a major undertaking for this patient. Due to multiple glaucoma procedures, it would be technically challenging to inplant a third Molteno device.
Conclusion
Undoubtedly, management of congenital glaucoma and buphthalmos remains a challenging task. GDD provide an effective option for long-term IOP reduction in complex glaucoma cases. Nevertheless, they are not without complications.
In situations of tube retraction, the use of a 22-gauge angiocatheter allowed for restoration of adequate IOP control and was the best surgical option available due to the complexity associated with previous multiple glaucoma procedures.
Disclosure
The authors report no conflicts of interest in this work.
References
- SmithMFDoyleJWResults of another modality for extending glaucoma drainage tubesJ Glaucoma1999831031410529931
- NetlandPAWaltonDSGlaucoma drainage implant in pediatric patientsOphthalmic Surg1993247237298290209
- MunozMTomeyKFTraversoCClinical experience with the Molteno implant in advanced infantile glaucomaJ Pediatr Ophthalmol Strabismus19912868722051291
- GeddeSJThe Tube Versus Trabeculectomy Study GroupResults from the Tube Versus Trabeculectomy StudyMiddle East Afr J Ophthalmol200916310711120142972
- FechterHPSarkisianSRBudenzDLHow Do I Extend a Tube?Glaucoma TodayJan-Feb20093740
- BansalAFenertyCHExtension of retracted glaucoma drainage tube using 22-gauge intravenous catheter in complex pediatric glaucoma: surgical techniquesJ Glaucoma201019424825119661827
- LangmannALindnerSWackernagelWPolytetrafluoroethylene (Goretex) for muscle elongation in the surgical treatment of strabismus with restricted motilityActa Ophthalmol Scand20068425025316637846
- ParanTSCorballyMTGross-RomEExperience with aortic grafting during excision of large abdominal neuroblastomas in childrenJ Pediatr Surg20084333534018280285
- HurstBSPermanent implantation of expanded polytetrafluoroethylene is safe for pelvic surgery. United States Expanded Polytetrafluoroethylene Reproductive Surgery Study GroupHum Reprod19991492592710221220