Abstract
Purpose
The purpose of this study was to evaluate the effect of optimally timed combination treatment with angiogenic and glycolytic inhibitors on tumor burden, hypoxia, and angiogenesis in advanced retinoblastoma tumors.
Methods
LHBETATAG mice (n =30) were evaluated. Mice were divided into 5 groups (n =6) and received injections at 16 weeks of age (advanced tumors) with a) saline, b) anecortave acetate (AA), c) 2-deoxyglucose (2-DG), d) AA +2-DG (1 day post-AA treatment), or e) AA +2-DG (1 week post-AA treatment). Eyes were enucleated at 21 weeks and tumor sections were analyzed for hypoxia, angiogenesis, and tumor burden.
Results
Eyes treated with 2-DG 1 day post-AA injection showed a 23% (P =0.03) reduction in tumor burden compared with 2-DG alone and a 61% (P < 0.001) reduction compared with saline-treated eyes. Eyes treated with 2-DG 1 week post-AA injection showed no significant decrease in tumor burden compared with 2-DG alone (P = 0.21) and a 56% (P < 0.001) decrease in comparison with saline-treated eyes. 2-DG significantly reduced the total density of new blood vessels in tumors by 44% compared to saline controls (P < 0.001), but did not affect the density of mature vasculature.
Conclusions
Combination therapy with angiogenic and glycolytic inhibitors significantly enhanced tumor control. Synergistic effects were shown to be dependent on the temporal course of treatment, emphasizing optimal timing. 2-DG was shown to reduce the density of neovessels, demonstrating an antiangiogenic effect in vivo. As a result, angiogenic and glycolytic inhibitors may have significant potential as alternative therapies for treating children with retinoblastoma.
Introduction
Retinoblastoma is the most common intraocular malignancy in children, affecting ~1 in 15,000 children, for an incidence of 250–300 new diagnoses a year in the United States.Citation1–Citation4 Treatment has evolved, with survival rates climbing to 99%, and a large percentage of children maintaining functional vision.Citation5 Current treatment includes chemoreduction (carboplatin, vincristine, and etoposide) with local consolidation (thermotherapy or cryotherapy). Success rates are reported as 100% for small tumors, decreasing to 47%–83% for tumors with diffuse vitreous or subretinal seeds.Citation6,Citation7 Options for tumors that fail therapy include additional chemotherapy with associated systemic toxicities,Citation8–Citation15 radiation with the risk of secondary malignancies,Citation16,Citation17 and ultimately enucleation.Citation8 As a result of the poor response of advanced retinoblastoma tumors to current therapy and the inherent toxicities of current chemotherapeutics, development of local adjuvant therapies is imperative.
A paradigm shift in cancer treatment has recently occurred as therapies target not only hyperproliferating tumor cells with chemotherapy, but also target the tumor microenvironment.Citation18,Citation19 Hypoxia has gained considerable attention, being correlated with tumor growth, progression, resistance, and metastasis.Citation20 Solid tumors are known to consist of hypoxic regions, a microenvironment consisting of slow-growing, malignant cells that prove resistant to chemotherapy and radiation.Citation21–Citation23 Under harsh, hypoxic conditions, tumor cells adapt and survive through control of gene expression, leading to altered cellular metabolism and angiogenesis.Citation24 Slowly proliferating cells in the hypoxic regions must rely on anaerobic glycolysis to utilize glucose for energy in the form of ATP. Our previous study was the first to show that regional hypoxia is present in advanced tumors of a transgenic retinoblastoma model, with hypoxia ranging between 20% and 26%. It was also shown that systemic administration of 2-deoxyglucose (2-DG) alone as well as combined with chemotherapy significantly decreased tumor hypoxia and tumor burden.Citation25
Angiogenesis has also been identified as a critical component of tumor growth and survival. As solid tumors proliferate, they are dependent on the development of a network of new vasculature to deliver blood and oxygen to the highly metabolic neoplastic cells. Angiogenesis consists of a complex and coordinated process that involves the development of new blood vessels from existing ones. Multiple stages of angiogenesis can be targeted with antiangiogenic agents, making blood vessels an attractive target in cancer therapy. We have shown that the vasculature in retinoblastoma tumors is heterogeneous, consisting of mature and immature neovessels, with proportions changing as more advanced tumors contain mature vessels. Vascular targeting therapies with angiogenic inhibitors alone or in combination with chemotherapeutic agents have been shown to effectively control tumor burden in the LHBETATAG mouse model for retinoblastoma.Citation26 In addition, our group has also shown that 2-DG effectively inhibits endothelial cell angiogenesis in vitro and in vivo.Citation27
The purpose of the present study was to evaluate the efficacy and demonstrate the importance of optimally timed combination therapy with angiogenic and glycolytic inhibitors in controlling advanced tumor burden and reducing intratumoral hypoxia in the LHBETATAG mouse model for retinoblastoma. Additionally, we evaluated the effect of 2-DG on angiogenesis in vivo.
Methods
LHBETATAG transgenic mice
The study protocol was approved by the University of Miami Institutional Animal Care and Use Committee. All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The LHBETATAG transgenic mouse model used in this study has been characterized previously.Citation28
Injections
LHBETATAG transgenic retinoblastoma mice were evaluated (n = 30). The mice were divided into 5 groups at 16 weeks of age and received injections of either saline (APP Pharmaceuticals, Schaumburg, IL), anecortave acetate (AA; 300 μg/20 μL; Alcon Pharmaceuticals, Fort Worth, TX), 2-DG (500 mg/kg; Sigma-Aldrich, St Louis, MO), AA and 2-DG (1 day post-AA injection), or AA and 2-DG (1 week post-AA injection). AA was administered through a single subconjunctival injection to the right eyes only, and did not demonstrate any significant deleterious ocular side effects that are associated with ocular glucocorticoid therapy.Citation26 2-DG was administered through intraperitoneal injection, 3 times a week for 5 weeks. Saline (20 μL) was administered through subconjunctival injections. Eyes were enucleated at 21 weeks of age and were assessed for hypoxia, tumor burden, and angiogenesis.
Tumor burden measurements
Eyes were enucleated, snap frozen, serially sectioned, and processed for standard hematoxylin and eosin (H&E) staining. Microscopic images of H&E-stained sections (50 8 μm sections/eye) were obtained with a digital camera at a magnification of ×40. The section of the eye containing the largest cross-sectional tumor area was chosen for analysis. Tumor boundaries were traced (Image Pro Express Software; Media Cybernetics, Silver Spring, MD). Tumor areas for all eyes were averaged, yielding an average area for each group. Tumor burden was expressed as the tumor/globe ratio by dividing the tumor area by the area of the globe to normalize the data as previously described.Citation26
Measuring hypoxic regions
To assess tumor hypoxia after treatment, LHBETATAG mice 21 weeks of age (n = 6 per group) were injected intraperitoneally with a 0.16 mL suspension of pimonidazole (a drug used to detect hypoxia that penetrates all tissues, including the brain). This suspension consisted of 10 mg of pimonidazole hydrochloride (Chemicon, Temecula, CA) in 1 mL saline. Pimonidazole is known to bind to thiol-containing proteins in cells under low oxygen tension.Citation29 These adducts can be detected with specific antibodies and stained using immunohistochemical techniques. Animals were euthanized 2 h after pimonidazole injection, and eyes were harvested and sectioned for histopathologic examination. Eyes were fixed with cold methanol for 10 min and immunostained with a directly labeled antibody recognizing pimonidazole adducts (Hypoxyprobe 1-Mab-1-FITC, clone 4.3.11.3; Chemicon) or the same concentration of a directly labeled isotype control antibody (mouse IgG1-FITC; Caltag, Burlingame, CA). Background signal intensities were minimal. All samples were normalized to intensities from isotype controls. The values reported indicate the percentage of pimonidazole-stained areas in the tumors.
Serial cross sections of eyes were examined for the presence of the described markers with a laser confocal microscope (TCP SP5; Leica Microsystems, CMS GmbH, Mannheim, Germany). All images were digitally acquired and recompiled (Photoshop CS; Adobe, San Jose, CA). Sections were viewed at ×200 magnification.
Blood vessel measurements
2-DG (500 mg/kg) was administered through intraperitoneal injection into LHBETATAG retinoblastoma tumor-bearing mice starting at 16 weeks of age, 3 times a week for 5 weeks (n = 5). Saline was administered using the same method (n = 4). Mice were euthanized at 21 weeks of age. Tumor samples were frozen in OCT immediately after enucleation and serially sectioned (8 μm). Slides were fixed with methanol for 10 min (−20°C) and immunohistochemical analyses were performed. Total vessels were detected with Alexa Fluor 568 conjugated lectin (Bandeira simplicifolia, a pan-endothelial binding agent; 1:1000; Invitrogen, Carlsbad, CA).Citation30 Mature vessels were detected with α-smooth muscle actin (α-sma) Cy3 conjugate (1:3000; Sigma-Aldrich), which specifically binds to pericytes.Citation31 Neovessels were detected with anti-endoglin (CD105 Wi, 1:500; Abcam, Cambridge, MA), which has been shown to have specificity for endothelial cells (ECs) under-going angiogenesis.Citation32 Alexa Fluor 568 goat anti-mouse and 488 donkey anti-mouse were used as secondary antibodies for anti-collagen type IV and endoglin, respectively (1:500; Invitrogen). Omission of the primary antibody (secondary only) was used as a negative control for nonspecific binding. Cell nuclei were stained for 5 min with 4′,6′ diamidino-2-phenylindole (DAPI, 1:5000; Invitrogen).
Analyses were performed on digitized fluorescence microscopic images (×200) using Adobe Photoshop (Photoshop CS; Adobe). Tumors were analyzed for vascular hotspots (four or five hotspots per tumor sample, depending on the tumor size). Areas of each marker within a hotspot were measured and given as a fraction of the magnification field.
These densities were averaged for each marker within each tumor.
Statistical methods
Pimonidazole fluorescence in tumors and tumor burden analyses were investigated with two-way analysis of variance (ANOVA). Post hoc least-significant different tests were used to evaluate differences between treatment groups. Tumor burden differences between groups were evaluated by two-sample t-test. Differences in means among three or more groups were analyzed by ANOVA. Two-sided, two-sample t-tests were used to compare levels of new and mature blood vessels between controls and animals receiving 2-DG treatment. Means in between two groups were compared by t-test analysis. Differences were considered statistically significant at P < 0.05. Data are presented as means and standard deviation.
Results
Tumor burden
Tumor burden was analyzed for all groups ( and ). In the AA-treated group (), there was no change in tumor area in comparison with the saline-treated group (P = 0.43) (). There was a significant decrease in tumor burden (61%) following combination treatment with 2-DG administered 1 day post-AA treatment () in comparison with the saline-treated group (P < 0.001) and a 59% decrease in tumor burden in comparison with the AA-treated group (P < 0.001). Notably, there was a 23% decrease in tumor burden in comparison with the eyes treated with 2-DG alone (P = 0.03) (). There was a significant decrease in tumor burden (56%) following combination treatment with 2-DG administered 1 week post-AA () in comparison with the saline-treated group (P < 0.001), and a 54% decrease in tumor burden in comparison with the AA-treated group (P < 0.001). In contrast to 2-DG administered 1 day post-AA, 2-DG given 1 week post-AA showed no significant decrease in tumor burden in comparison with the eyes treated with 2-DG alone (P = 0.21). Finally, there was no significant difference in tumor burden between the two combination treatment groups (2-DG +AA) (P = 0.31).
Figure 1 Tumor area in anecortave acetate (AA) and 2-deoxyglucose (2-DG) groups in LHBETATAG retinal tumors. LHBETATAG mice 16 weeks of age (advanced tumors) were treated with saline, AA (300 μg/20 μL), 2-DG (500 mg/kg), AA (300 μg/20 μL) +2-DG (500 mg/kg 1 day post-AA injection), or AA (300 μg/20 μL) +2-DG (1 week post-AA injection). Eyes were analyzed 5 weeks post-initial treatment for residual tumor burden. There was a significant reduction in tumor area with 2-DG alone, as well as after combination treatment with AA and 2-DG compared to the saline control group (P < 0.001). Notably, there was a significant difference in tumor burden (23% reduction) between groups treated with 2-DG 1 day post-AA compared to 2-DG alone (P = 0.03).
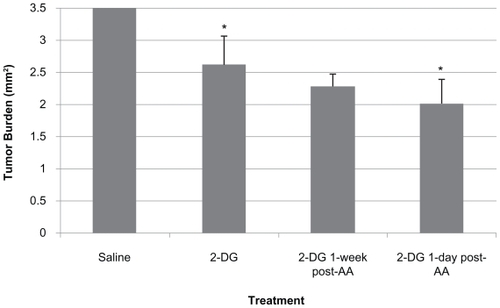
Figure 2 Tumor area in LHBETATAG retinal tumors. The uninvolved retina appears normal, and no signs of retinal toxicity are evident. A) Saline treated, B) anecortave acetate (AA) treated, C) 2-deoxyglucose (2-DG) treated, D) 2-DG 1 day post- AA, and E) 2-DG 1 week post-AA. There was a significant reduction in tumor burden in the group treated with 2-DG 1 day post-AA treatment in comparison with saline (61% reduction) as well as 2-DG alone (23% reduction) (P < 0.001 and P < 0.05, respectively). Original magnifications, ×40 for each group; representative H&E images.
Percent hypoxia
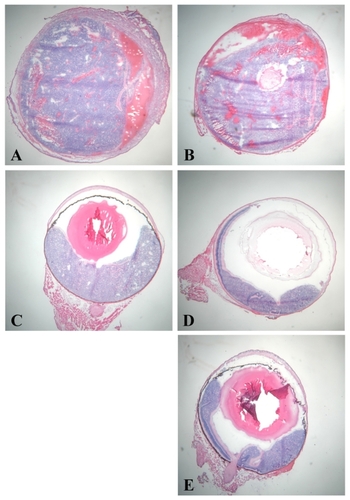
LHBETATAG retinal tumors treated with saline were used as controls and were analyzed for hypoxic regions ( and ). The tumors were found to be 19% hypoxic (P < 0.001). In the AA-treated group, 18% of the tumor was hypoxic (P < 0.001) (). In the group treated with 2-DG 1 day post-AA injection, the tumor was 0.24% hypoxic (P < 0.001) (). In the group treated with 2-DG 1 week post-AA acetate injection, the tumor was 0.3% hypoxic (P < 0.001) (). There was no significant change in hypoxic area in the AA-treated group in comparison with the saline-treated group (P = 0.19). There was a significant decrease (98%) in hypoxia in the groups treated with 2-DG 1 day and 1 week post-AA injection in comparison with AA alone (P < 0.001 for both groups). There was no significant difference in hypoxic areas between the groups treated with 2-DG 1 day and 1 week post-AA injection in comparison with the 2-DG-treated group (P = 0.98 and P = 0.95, respectively). There was no significant difference in hypoxic areas between the two groups treated with combination treatment (AA +2-DG) and 2-DG therapy (P = 0.93) ().
Figure 3 Percentage hypoxia in anecortave acetate (AA) and 2-deoxyglucose (2-DG) combination treatment groups in 16-week-old LHBETATAG retinal tumors. 2-DG and 2-DG +AA combination therapy significantly decreases hypoxic regions in LHBETATAG tumors.
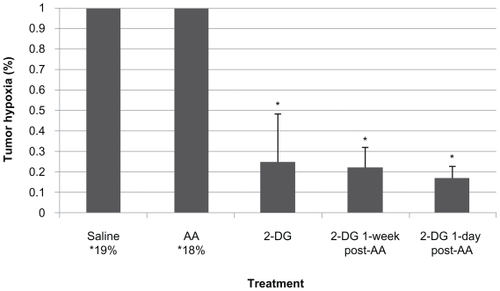
Figure 4 Post-treatment hypoxia in LHBETATAG retinal tumors. A) Saline treated, B) anecortave acetate (AA) treated, C) 2-deoxyglucose (2-DG) treated, D) 2-DG 1 day post-AA, and E) 2-DG 1 week post-AA. There is a significant decrease in the levels of hypoxia in eyes treated with 2-DG alone as well as combination treatment (AA +2-DG) compared with the saline controls (P < 0.001, for both). Representative composite images of pimonidazole (green)-stained and DAPI (blue, nuclear stain)- stained ocular sections of LHBETATAG mice treated with saline, AA, 2-DG, 2-DG 1 day post-AA injection, or 2-DG 1 week post-AA injection at ×200 magnification. Significant areas of hypoxia can be seen in the A) saline-treated group and the B) AA-treated group.
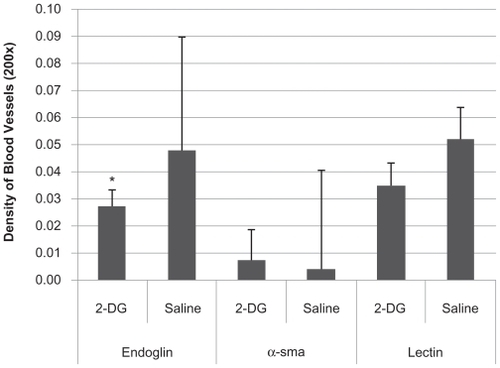
Effect of 2-DG on tumor vasculature
To assess the effects of the administration of 2-DG on the vasculature of LHBETATAG retinal tumors, the densities of new, mature, and total blood were measured (). In advanced disease (21 weeks of age), a significant reduction (44%) in the average density of new blood vessels (endoglin) was found in the retinal tumors that received 2-DG treatment when compared with controls (P < 0.001, ANOVA). However, there was no significant difference in the average densities of both mature (α-sma) and total vasculature (lectin) in the retinal tumors that received 2-DG treatment when compared with controls (P >0.05, ANOVA).
Figure 5 Effect of 2-deoxyglucose (2-DG) on vasculature. Sixteen-week-old LHBETATAG mice were treated with 2-DG 3 times/week for 5 weeks. Eyes were enucleated at 21 weeks, corresponding to advanced tumor ages. Vasculature was assessed for total (lectin), mature (α-sma), and immature, new (endoglin) vessels. 2-DG led to a 44% reduction in immature vasculature compared to saline controls, a significant difference (P < 0.001). However, 2-DG did not have a significant effect on mature vasculature and reduced total vasculature by 35% compared to saline controls (both not statistically significant, P >0.05).
Notes: *Statistically significant difference (P < 0.001).
Discussion
Our current study is the first to demonstrate the combined treatment of advanced retinoblastoma tumors in the LHBETATAG mouse model with angiogenic inhibitors and glycolytic inhibitors avoiding chemotherapy. We have previously demonstrated the effectiveness of 2-DG alone in decreasing tumor hypoxia and in tumor control. However, when 2-DG was combined with the angiogenic inhibitor, AA, advanced tumor control was significantly increased, as the combined treatment resulted in a 23% decrease in tumor burden compared to 2-DG alone. Importantly, the enhanced response of tumors to the combination treatment occurred in the group that received 2-DG 1 day after AA treatment, whereas the group treated 1 week after AA did not differ significantly from 2-DG alone. Mice were treated at 16 weeks of age, when eyes harbor advanced tumors. As shown in prior studies, these advanced tumors consist of heterogeneous vasculature, with a greater proportion of mature versus immature vessels. As a result, AA would be expected to have a more robust effect the earlier tumors were treated, as these earlier tumors have a greater proportion of immature vessels. Moreover, advanced tumors have a greater degree of hypoxia, thus maximizing the effect of 2-DG treatment, and potentially diminishing the observed effect of the AA. Despite these limitations, combination treatment with AA and 2-DG proved to significantly enhance tumor control compared to 2-DG alone.
Solid tumors are known to consist of hypoxic regions composed of slow-growing, malignant populations of tumor cells. The cells in the hypoxic microenvironment must adapt to these harsh conditions in order to survive. Adaptations may be mediated through a variety of mechanisms, with cells altering the genetic expression of certain key pathways. Several O2-sensitive pathways have been identified, including the mammalian target of rapamycin (mTOR), hypoxia-inducible factor (HIF), unfolded protein response (UPR), and the urokinase plasminogen activator (uPA) systems, that potentially contribute to adaptation and survival.Citation24 Reduced oxygen tensions have been shown to upregulate the transcription factor HIF, leading to increased production of genes for glucose transporters (GLUT), glycolytic enzymes, and angiogenesis.Citation33–Citation35 Additionally, the uPA system may activate enzymes, notably gelatinases such as matrix metalloproteinases (MMP) that break down the extracellular matrix, facilitating angiogenesis and tumor invasion.Citation36 As a result, adjuvant therapies that target key aspects of these adaptations are needed.
Glycolytic inhibitors, such as 2-DG, exploit the altered cellular metabolism that hypoxic cells utilize for survival. Unlike cells in normoxic conditions, the cells in the hypoxic regions must use anaerobic glycolysis as the sole source of energy. 2-DG competes with glucose for cellular transporters (ie, GLUT-1) and key glycolytic enzymes, such as hexokinase. As a result, 2-DG inhibits the metabolic machinery of the cells, preventing the production of energy in the form of ATP, and effectively causing cell death. Our previous studies have demonstrated that targeting hypoxic cells with 2-DG alone, as well as combining this treatment with chemotherapy, leads to a significant reduction in tumor hypoxia and tumor burden.Citation25
2-DG has been shown to be effective at targeting hypoxic cells, as well as inhibiting endothelial cell angiogenesis.Citation27 The spatial distribution of blood vessels and the effects of antiangiogenic agents in LHBETATAG retinal tumors have been characterized previously.Citation37 In the current study, we have shown that 2-DG significantly reduces the density of immature neovessels by 43% compared to controls. Conversely, 2-DG does not have an effect on mature tumor vasculature. Overall, total vasculature in tumors was reduced by 34%. Angiogenesis, or the formation of new vessels from existing vessels, is imperative for tumor growth and progression as neoplastic cells require nutrient delivery and waste removal. As a result of hypoxia and other factors in the tumor microenvironment, angiogenesis is stimulated, forming a haphazard network of new vessels that require stabilization by cytokines and growth factors, such as vascular endothelial growth factor. New vessels consist of proliferating ECs, while mature vessels are supported by pericytes that provide necessary stabilization. ECs have been shown to rely on glycolysis for metabolism even under aerobic conditions.Citation38–Citation40 It has been hypothesized that proliferating cells may utilize glycolysis as an adaptation to oxidative stress secondary to oxygen free radicals.Citation39,Citation40 With a greater reliance on glycolysis for endothelial proliferation, 2-DG as a glycolytic inhibitor may target ECs, effectively serving as an antiangiogenic agent.
The use of glycolytic inhibitors is a promising alternative for advanced disease. More importantly, the current study provides additional support to the mechanistic effect of 2-DG on angiogenesis. Because retinoblastoma tumors promote angiogenesis and are highly dependent on their vascular supply, a dual mechanistic effect of 2-DG targeting both the slowly proliferating hypoxic cells as well as the proliferating ECs is demonstrated.
Antiangiogenic agents, such as AA, inhibit vascular growth, effectively rendering a tumor without sufficient blood supply, leading to hypoxia and cell death. Prior studies have shown the efficacy of antiangiogenic agents in retinoblastoma tumor control in the LHBETATAG animal model.Citation26 As tumors undergo proliferation, an ‘angiogenic switch’ occurs, where the tumor must stimulate angiogenesis to support further development. As tumors become more advanced, these neovessels are remodeled and transformed into mature vessels. The ECs of new vessels rely on growth factors produced by gelatinase degradation of the extracellular matrix to support growth and development, while mature vessels rely on pericytes for support. Recently, we have shown that AA affects gelatinase activity, leading to a decrease in MMP-2 and MMP-9.Citation41 Prior studies have correlated AA treatment with increased levels of plasminogen activator inhibitor 1, effectively impeding uPA activity.Citation42,Citation43 As a result, the dynamic tumor microenvironment consisting of a heterogeneous network of blood vessels can be targeted by angiogenic inhibitors. However, further strategies to modulate or target the formation of mature vessels are needed to potentially maximize tumor control.
The results of our study emphasize a key concept regarding adjuvant treatments and their effectiveness in tumor control, specifically, optimized timing. We have shown in prior studies that when AA is combined with chemotherapeutic agents, the administration of AA after completion of the chemotherapy cycle yielded more effective tumor control than treatment during the cycle.Citation26 It is hypothesized that angiogenic inhibitors alter blood flow through vessels, potentially reducing the delivery of chemotherapeutic agents to the tumor. Additionally, we have shown that when 2-DG is combined with carboplatin, tumor control is enhanced when 2-DG is given prior to chemotherapy.Citation25 Further, with the current study, timing of 2-DG following AA treatment led to significant differences in advanced tumor control. AA has been shown to significantly reduce total vessel density in tumors, primarily immature neovessels, as well as increase hypoxia in tumors by 28% and 17% 1 day and 1 week post-treatment, respectively.Citation44 With reduction in blood vessels, there is a decrease in blood flow resulting in less delivery of oxygen and nutrients to highly metabolic neoplastic cells. The tumor microenvironment is altered, leading to a greater degree of hypoxia. With the increase in hypoxia following AA treatment, this is an optimal time for treatment with 2-DG which targets hypoxic cells. As a result, with optimally timed treatment using angiogenic and glycolytic inhibitors, advanced tumor control is enhanced more than with single-agent treatment. These studies emphasize that as adjuvant therapies are developed to target various aspects of the tumor microenvironment and associated genetic and molecular pathways, optimally timed delivery must be considered to not only enhance tumor control, but also to avoid antagonism between various treatment modalities.
Current therapy for retinoblastoma has improved survival and globe salvage, with chemotherapy and focal consolidation providing tumor control in 100% of tumors without seeding, to 47%–83% in more advanced tumors with diffuse retinal and vitreous seeds.Citation6,Citation7 Despite treatment success, current chemotherapy exposes children to highly toxic medications with severe side effects. Additionally, advanced disease responds poorly to treatment, often necessitating enucleation for disease control. Adjuvant therapies, such as glycolytic inhibitors, angiogenic inhibitors, and agents that target molecular and genetic pathways involved in tumor proliferation could potentially be combined with current treatment regimens. The current study shows that tumor control can be achieved with combination therapy without the need for chemotherapy. Adjuvant therapies may eliminate the need for chemotherapy or allow reduced doses or cycles of toxic agents. Finally, combined treatment may provide enhanced control of advanced tumors that previously were resistant to therapy. Although 2-DG was administered systemically through intraperitoneal injections, we are not advocating systemic delivery, secondary to potential effects on organ and vascular development. Rather, we have shown in prior studies that 2-DG and AA can be effectively delivered locally through subconjunctival injections. Additionally, with new protocols utilizing supraselective ophthalmic artery cannulation, adjuvant therapies may potentially be delivered focally by selective intra-arterial delivery.
From a recent interview with Dr Watson, a cofounder of the DNA double helix, he had this to say about the future of cancer treatment: ‘there are often many types of cancer-causing genetic “drivers” within single cancer cells … given the inherent genetic instability of most cancer cells, the use of drugs acting against single drivers would all too soon lead to the emergence of genetic variants driven by increasingly destructive second, if not third, drivers. Most anticancer drugs, then, will probably never reach their full potential unless they are given in combination with other drugs developed against second or even third drivers’.Citation45 We feel that the future of retinoblastoma treatment echoes these sentiments, as we continue to show that adjuvant therapies that target components of the tumor microenvironment can enhance tumor control and potentially decrease the reliance on toxic chemotherapeutic agents.
In the present study, we have characterized the effect of the combination treatment with 2-DG and AA on tumor growth, hypoxia, angiogenesis, and blood vessel maturation in LHBETATAG retinal tumors. We have demonstrated that advanced tumor control is enhanced with combination therapy utilizing glycolytic and angiogenic inhibitors compared to single agents alone. The effects were significantly influenced by the temporal relationship of therapies, thus emphasizing the importance of optimally timed treatments. The current findings suggest that angiogenic and glycolytic inhibitors can be combined with a synergistic effect on tumor control. We also demonstrated the dual mechanistic action of 2-DG by targeting hypoxic cells as well as ECs, effectively reducing angiogenesis. With a greater understanding of the mechanism of adjuvant therapies such as glycolytic inhibitors and angiogenic inhibitors, treatments may be effectively combined with or without current chemotherapeutic agents. As a result, this combination may have potential value as adjuvant therapy for children with retinoblastomas.
Acknowledgments
This study is supported by NIH center grants (R01 EY013629, R01 EY12651, and P30 EY014801), the American Cancer Society, Sylvester Comprehensive Cancer Center, and by an unrestricted grant to the University of Miami from Research to Prevent Blindness, Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
- TamboliAPodgorMJHormJWThe incidence of retinoblastoma in the United States: 1974 through 1985Arch Ophthalmol199010811281322288550
- PendergrassTWDavisSIncidence of retinoblastoma in the United StatesArch Ophthalmol1980987120412107396771
- AbramsonDHRetinoblastoma 1990: diagnosis, treatment, and implicationsPediatr Ann19901963873952201000
- DevesaSSThe incidence of retinoblastomaAm J Ophthalmol19758022632651155565
- AbramsonDHRetinoblastoma in the 20th century: past success and future challenges the Weisenfeld lectureInvest Ophthalmol Vis Sci20054682683269116043839
- ShieldsCLMashayekhiAAuAKThe international classification of retinoblastoma predicts chemoreduction successOphthalmology2006113122276228016996605
- ScheflerACCicciarelliNFeuerWToledanoSMurrayTGMacular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablationOphthalmology2007114116216917070578
- ChanHSGallieBLMunierFLBeck PopovicMChemotherapy for retinoblastomaOphthalmol Clin North Am20051815563viii15763191
- WhiteLThe role of chemotherapy in the treatment of retinoblastomaRetina1983331941996356260
- WhiteLChemotherapy in retinoblastoma: current status and future directionsAm J Pediatr Hematol Oncol19911321892012069230
- EttingerLJGaynonPSKrailoMDA phase II study of carboplatin in children with recurrent or progressive solid tumors. A report from the Childrens Cancer GroupCancer1994734129713018313334
- DozFPinkertonRWhat is the place of carboplatin in paediatric oncology?Eur J Cancer199430A21942018155394
- TuckerMAD’AngioGJBoiceJDJrBone sarcomas linked to radiotherapy and chemotherapy in childrenN Engl J Med1987317105885933475572
- BenzMSScottIUMurrayTGKramerDToledanoSComplications of systemic chemotherapy as treatment of retinoblastomaArch Ophthalmol2000118457757810766148
- GombosDSHungerfordJAbramsonDHSecondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor?Ophthalmology200711471378138317613328
- ScottIUMurrayTGFeuerWJExternal beam radiotherapy in retinoblastoma: tumor control and comparison of 2 techniquesArch Ophthalmol1999117676677010369587
- EngCLiFPAbramsonDHMortality from second tumors among long-term survivors of retinoblastomaJ Natl Cancer Inst19938514112111288320741
- BissellMJRadiskyDCRizkiAWeaverVMPetersenOWThe organizing principle: microenvironmental influences in the normal and malignant breastDifferentiation2002709–1053754612492495
- HanahanDWeinbergRAThe hallmarks of cancerCell20001001577010647931
- DewhirstMWCaoYMoellerBCycling hypoxia and free radicals regulate angiogenesis and radiotherapy responseNat Rev Cancer20088642543718500244
- BushRSJenkinRDAlltWEDef initive evidence for hypoxic cells influencing cure in cancer therapyBr J Cancer Suppl19783302306277250
- ShannonAMBouchier-HayesDJCondronCMToomeyDTumour hypoxia, chemotherapeutic resistance and hypoxia-related therapiesCancer Treat Rev200329429730712927570
- TeicherBAHypoxia and drug resistanceCancer Metastasis Rev19941321391687923547
- WoutersBGKoritzinskyMHypoxia signalling through mTOR and the unfolded protein response in cancerNat Rev Cancer200881185186418846101
- BoutridHJockovichMEMurrayTGTargeting hypoxia, a novel treatment for advanced retinoblastomaInvest Ophthalmol Vis Sci20084972799280518326690
- JockovichMEMurrayTGEscalona-BenzEHernandezEFeuerWAnecortave acetate as single and adjuvant therapy in the treatment of retinal tumors of LH(BETA)T(AG) miceInvest Ophthalmol Vis Sci20064741264126816565356
- MerchanJRKovacsKRailsbackJWAntiangiogenic activity of 2-deoxy-D-glucosePLOSone2010510e13699
- WindleJJAlbertDMO’BrienJMRetinoblastoma in transgenic miceNature199034362596656691689463
- VariaMACalkins-AdamsDPRinkerLHPimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinomaGynecol Oncol19987122702779826471
- LaitinenLGriffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissuesHistochem J19871942252343597137
- NehlsVDrenckhahnDHeterogeneity of microvascular pericytes for smooth muscle type alpha-actinJ Cell Biol199111311471542007619
- TanakaFOtakeYYanagiharaKEvaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibodyClin Cancer Res20017113410341511705856
- SemenzaGLWangGLA nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activationMol Cell Biol19921212544754541448077
- WangGLJiangBHRueEASemenzaGLHypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tensionProc Natl Acad Sci U S A19959212551055147539918
- MaxwellPHPughCWRatcliffePJActivation of the HIF pathway in cancerCurr Opin Genet Dev200111329329911377966
- RademakersSESpanPNKaandersJHSweepFCvan der KogelAJBussinkJMolecular aspects of tumour hypoxiaMol Oncol200821415319383328
- JockovichMEBajenaruMLPinaYRetinoblastoma tumor vessel maturation impacts efficacy of vessel targeting in the LH(BETA)T(AG) mouse modelInvest Ophthalmol Vis Sci20074862476248217525173
- CulicOGruwelMLSchraderJEnergy turnover of vascular endothelial cellsAm J Physiol19972731 Pt 1C205C2139252458
- DobrinaASoranzoMRRossiFIsolation of metabolically active endothelial cells in high yield from bovine cavernous bodies. A model for functional studies on freshly isolated microvascular endothelial cellsCell Tissue Res198323235795916883458
- PetersKKampGBerzAChanges in human endothelial cell energy metabolic capacities during in vitro cultivation. The role of “aerobic glycolysis” and proliferationCell Physiol Biochem2009245–648349219910688
- BajenaruMLPinaYMurrayTGGelatinase expression in retinoblastoma: modulation of LH(BETA)T(AG) retinal tumor development by anecortave acetateInvest Ophthalmol Vis Sci20105162860286420107171
- DeryuginaEIQuigleyJPMatrix metalloproteinases and tumor metastasisCancer Metastasis Rev200625193416680569
- PennJSRajaratnamVSCollierRJClarkAFThe effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurityInvest Ophthalmol Vis Sci200142128329011133880
- BoutridHPinaYCebullaCMIncreased hypoxia following vessel targeting in a murine model of retinoblastomaInvest Ophthalmol Vis Sci200950125537554319578014
- WatsonJDTo fight cancer, know the enemyNew York Times2009Aug 5