Abstract
Purpose
To evaluate the effect of a single vectored thermal pulsation (VTP) treatment in contact lens wearers with meibomian gland dysfunction (MGD) and dry eye symptoms.
Methods
The prospective, nonsignificant risk, open-label, randomized, multi-center clinical trial included 55 soft contact lens (SCL) wearers with MGD and evaporative dry eye. Subjects were randomized to the single VTP treatment group or an untreated control. The controls received a crossover VTP treatment at 3 months (crossover treatment group). Primary effectiveness measures were meibomian gland secretion (MGS) score and Standard Patient Evaluation of Eye Dryness (SPEED) that were evaluated at baseline, at 1 and 3 months post-VTP treatment, and at 1 month post-VTP treatment in the crossover treatment group. Exploratory variables included fluorescein tear break-up time (TBUT), lid wiper epitheliopathy (LWE), lid parallel conjunctival folds (LIPCOF), ocular surface staining, frequency of over-the-counter (OTC) drop use, and hours of comfortable contact lens wear.
Results
At 3 months, the treatment group showed significantly greater mean change from baseline in MGS (12.4±9.1 vs 1.4±6.4, p<0.0001), SPEED (−8.4±4.7 vs −0.7±4.4, p<0.0001) and significantly greater improvement in exploratory variables (TBUT, LWE, and frequency of OTC drop use) relative to the controls. Mean comfortable contact lens wearing time increased by 4.0±3.9 hours at 1 month. This was sustained for 3 months with no change in the control group. The crossover treatment group demonstrated similar results to the treatment group at 1 month post-VTP.
Conclusion
In SCL wearers with MGD, a single VTP treatment significantly improved mean meibomian gland function and significantly reduced dry eye signs and symptoms compared to an untreated control. The treatment increased mean comfortable lens wearing time by 4 hours (approximately doubling the pretreatment findings). This was sustained for up to 3 months post-treatment on average.
Introduction
Symptoms of dryness and discomfort are highly prevalent (up to 50%) among contact lens wearers and are the most commonly cited reason for the discontinuation of contact lens wear.Citation1–Citation4 Despite decades of advances in contact lens design and materials used to manufacture the lenses, the contact lens dropout rate remains consistently high at 16%–34% per year.Citation4,Citation5 The improvements in contact lens design serve, in part, to minimize the discomfort caused by the physical changes, which occur to a contact lens within minutes and hours of placement on the eye (eg, lens dehydration, pre-lens tear film stagnation, and post-lens debris).Citation6–Citation8 However, regardless of lens design optimization, there are ocular tissue changes that occur due to the disruptive presence of any contact lens on the eye.Citation3,Citation6–Citation8 Examples of ocular tissue changes that have been reported as strongly correlated with contact lens wear are lid wiper epitheliopathy (LWE), lid parallel conjunctival folds (LIPCOF), and meibomian gland dysfunction (MGD).Citation9–Citation11 The mechanisms responsible for these tissue changes can be largely categorized as the result of increased evaporative stress on the tear film, friction between the lid wiper and the ocular surface, and the resulting inflammatory cascades that predictably ensue.Citation12 Generally speaking, even with optimized contact lens design and materials, the importance of a stable and robust tear film and homeostatic ocular surface environment cannot be overstated, if successful contact lens wear is to be achieved.Citation6,Citation13,Citation14
While the volume of literature regarding the need for tear film stability in order to achieve comfortable contact lens wear is significant, the full extent of the impact of a contact lens on the ocular surface is not fully understood or characterized. A recent publication hypothesizes the adverse effect of a contact lens on the eye through the mechanism of chronic desiccating stress.Citation15 Chronic exposure to desiccating stress has been shown to result in protracted overstimulation of mouse meibomian gland meiboctyes. The unrelenting meibocyte upregulation accelerated the aging of the meibocytes, altered gland secretion quality, and ultimately led to gland atrophy in what were once healthy meibomian glands.Citation15 While the direct link between MGD and chronic exposure to desiccating stress, alone or in contact lens wear, has not been demonstrated in the human eye, the reported association between contact lens wear and MGD, now spanning several decades, is well established.Citation11,Citation16,Citation18–Citation20
The multiple studies noting the high prevalence and increased severity of MGD in contact lens wearersCitation11,Citation16,Citation18–Citation20 significantly outnumber reports to the contrary.Citation21 Regardless, even if no causal relationship between contact lens wear and MGD has been demonstrated, MGD will have a predictable negative impact on contact lens wear over both the short and long terms.Citation18,Citation19 In addition, any contact lens wearer with MGD is at risk for ocular discomfort and dry eye due to the MGD and evaporative dry eye.Citation19 Recently, MGD has been shown to negatively impact tear film host defense,Citation22 corneal epithelial cell and stromal nerve health,Citation23 conjunctival health,Citation24 and various other measures of ocular surface health.Citation25 These studies indicate that MGD compromises ocular surface health in multiple, foreseeable, and measurable ways, none of which would be advantageous for contact lens wear.
Previous studies have shown that when MGD is treated, contact lens wear is positively impacted in the form of improved tear film stability, increased comfortable contact lens wearing time, and reduced LWE.Citation16,Citation17,Citation26 The expanding body of evidence demonstrating the negative impact of MGD on ocular surface health in general and on contact lens wear, in particular, has led others to recommend that contact lens wearers should be routinely evaluated and treated for MGD.Citation18
Vectored thermal pulsation (VTP) treatment (LipiFlow®; TearScience Inc., Morrisville, NC, USA) is designed to directly treat MGD by evacuating the contents of both the upper and lower meibomian glands simultaneously during a single treatment.Citation27 While the success of the LipiFlow in treating MGD has been reported extensively,Citation27–Citation33 there is only one report of a small pilot study treating contact lens wearers with MGD with the LipiFlow System.Citation26 The results of this pilot study showed that a single VTP treatment increased mean daily contact lens wearing time by 3.6 hours as well as significantly reducing LWE. However, the study had several limitations, such as being a retrospective study at a single site. The purpose of this study was to prospectively assess the effect of a single VTP treatment in contact lens wearers with MGD on contact lens-related dry eye signs and symptoms in a randomized, multi-center clinical trial.
Methods
This prospective, multi-center clinical trial was conducted in compliance with US Code of Federal Regulations (CFR): 21 CFR Parts 50, 54, 56, and 812 and the Canadian Medical Device Regulations. The study was performed under the approval of three Institutional Review Boards (Schulman Associates IRB, Cincinnati, OH, USA; Western IRB, Puyallup, WA, USA; and University of Waterloo Office of Research Ethics, Waterloo, ON, Canada), and all tenets of the Declaration of Helsinki for the protection of human subjects in medical research were strictly observed (including obtaining written informed consent from all patients). The study involved postmarket use of the LipiFlow System and commercially available contact lenses. A sub-study involved the investigational use of the TearScience Meibographer as a nonsignificant risk device study at US sites only. This study was registered at the US National Institutes of Health (ClinicalTrials.gov; #NCT02102464). Between May 20, 2014, and February 5, 2015, 55 adult subjects with MGD and dry eye symptoms were randomized in the study at six sites in the USA and Canada. Subjects were not required to pay for their designated treatment.
Study design
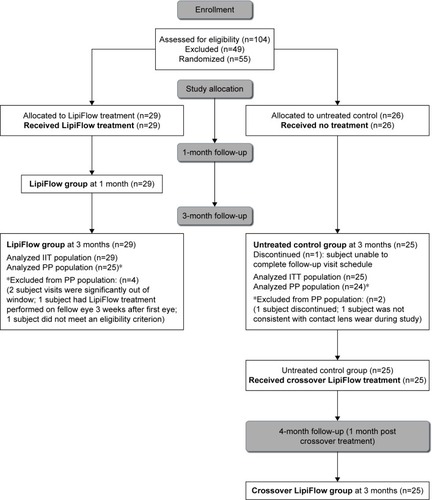
This study was an open-label, randomized, multi-center, clinical trial of a single VTP treatment vs an untreated control in contact lens wearers with MGD and evaporative dry eye. Subjects were randomized to receive a single VTP treatment in both eyes (treatment group) or no VTP treatment (untreated control group). Study endpoints were evaluated at 3 months by comparing the mean change from baseline to 3 months for treatment group with the untreated group. To facilitate subject recruitment, the untreated control group received a crossover VTP treatment (crossover treatment group) at 3 months. Both the treatment and crossover treatment groups were evaluated 1 month after receiving VTP treatment ().
Inclusion criteria
Patients were required to be aged at least 18 years and willing and able to comply with the study protocol, have MGD (a meibomian gland secretion [MGS] score of ≤15 for 15 glands of the lower lid) in both eyes; have a Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire score of ≥6;Citation34 have a minimum lipid layer thickness of ≤100 nm in both eyes (LipiView); have a desire to wear contact lenses habitually and not for intentional occasional or recreational wear only; wear soft daily contact lenses in both eyes with a total wearing time of 2–18 hours a day and 4–7 days a week on average (the original protocol, Protocol Revision 1, included a range of 2–12 hours of wearing time; this was increased in Protocol Revision, 2–18 hours, to facilitate recruitment); classified as having dry eye while wearing contact lenses based on composite score on Contact Lens Dry Eye Questionnaire (CLDEQ) – Long Form;Citation35 have worn the same type of commercially available soft contact lens (SCL) (brand, material, and dimensions) for at least the past 3 months (the original protocol, Protocol Revision 1, stated 6 months, which was reduced to 3 months in Protocol Revision 2 to facilitate recruitment); and have an assessment based on the clinician’s experience and judgment that contact lens fit, lens material, and compatible disinfecting solution were acceptable and not the reasons for contact lens discomfort. An additional criterion in Protocol Revision 1 was a comfortable contact lens wearing time of ≤4 hours per day on average; this criterion was removed in Protocol Revision 2 to facilitate recruitment.
Exclusion criteria
Conditions that could potentially interfere with the evaluation of or compromise treatment effectiveness or increase the risk of a procedure-related injury were listed as exclusion criteria. These conditions included moderate-to-severe allergic, vernal, or giant papillary conjunctivitis; active ocular infection or inflammation; ocular surface abnormalities compromising corneal integrity; eyelid abnormalities that could compromise lid function; recent (within the past 3 months) ocular surgery, herpes, or recurrent inflammation; systemic disease associated with dry eye; systemic medications known to cause dryness; current use of prescription medications and other treatments for MGD or dry eye (excluding over-the-counter [OTC] artificial tears, dietary supplements, and ocular lubricants); unwillingness to discontinue the use of these medications/treatments for the study duration (Protocol Revision 1 excluded the current use of these medications and treatments for the past 90 days, which was reduced to the past 30 days in Protocol Revision 2 to facilitate recruitment); and participation in another ophthalmic clinical trial involving a device or therapeutic drug within the past 30 days and employee, associate, or relative of an employee at a clinical study site.
Randomization and subject disposition
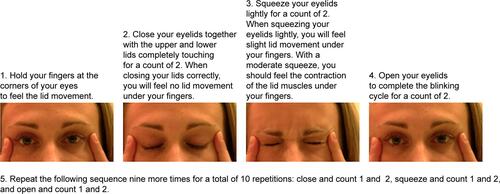
A total of 29 subjects (58 eyes) were randomized to the treatment group and 26 subjects (52 eyes) to the untreated control group. The control group received no treatment, while the treatment group received a single 12-minute VTP treatment with the LipiFlow System. The treatment group received manual eyelid margin cleaning prior to the VTP treatmentCitation36 and were instructed to perform blinking exercises (Figure S1) for 1 month after the treatment visit to foster healthy blinking habits.Citation37
Subjects in the control group were evaluated at 3 months post-baseline visit. After the examination at the 3 months post-baseline visit, the control subjects received a single 12-minute crossover VTP treatment and were evaluated again 1 month post-VTP treatment. Subjects in the treatment group were evaluated at 1 and 3 months post-VTP treatment. The complete subject disposition is given in the form of an abbreviated CONSORT flow diagram in .
The LipiFlow System
The LipiFlow System has been described in detail elsewhere.Citation27 Briefly, it is a prescription device for in-office use by a physician. The LipiFlow System is indicated the treatment of MGD and lipid deficiency or evaporative dry eye. The device applies controlled therapeutic heat to the inner eyelid surface with simultaneous intermittent directional pressure to the outer eyelid to facilitate evacuation of the gland contents during the heating phase of the treatment.Citation27
Study parameters
Study endpoint parameters were meibomian gland assessment and the SPEED questionnaire. These parameters were assessed at all study visits except the treatment visit. Meibomian gland assessment was performed using a previously described handheld instrument, Meibomian Gland Evaluator (MGE), to apply standardized pressure to the eyelid margin, which approximates the force of a deliberate blink over the glands.Citation38 A total of 15 glands were evaluated along the lower eyelid margin. This is achieved by evaluating five glands in each of the temporal, central, and nasal regions of the eyelid. Gland secretion characteristics were graded as 3 (clear liquid secretion), 2 (cloudy liquid secretion), 1 (inspissated/toothpaste consistency), and 0 (no secretion). The total MGS score for each eye was calculated based on the sum of the secretion grades (range of 0–45) for all evaluated glands.Citation38 In addition, the total number of functional meibomian glands, defined as having liquid secretion with a grade of 2 or 3, was counted with a range of 0–15.Citation27
The frequency and severity of dry eye symptoms were assessed with the SPEED questionnaire. The total SPEED score (range from 0 to 28) was calculated as the sum of frequency and severity scores for all questions answered.Citation34
Dynamic Meibomian Imaging (DMI)
Meibomian gland structure was captured on a subset of subjects (n=37, US sites only) using an investigational device (TearScience Meibographer; TearScience Inc.) with DMI. Meibomian gland structure was imaged at all study visits except the treatment visit. After data collection, an independent reader graded the gland structure according to a previously accepted method where the amount of atrophy in each eyelid (upper and lower) is semiquantitatively assessed and recorded as Grades 0–3: Grade 0, no atrophy; Grade 1, 1%–33% atrophy; Grade 2, 34%–66% atrophy; Grade 3, 67%–100% atrophy.Citation39–Citation41 The upper and lower eyelid atrophy scores were then combined to form a total combined meiboscore for each eye (maximum score =6) and an average eyelid meiboscore (maximum score =3).Citation39–Citation41
Tear break-up time (TBUT)
The exploratory endpoint, TBUT, was measured at all study visits except the treatment visit. The dry eye test (DET) (Amcon Laboratories, St Louis, MO, USA) was used to instill fluorescein dye.Citation42 Three separate measurements of TBUT were taken for each eye using a stopwatch to record the time. If breakup was not observed within 20 seconds, the measurement was to be terminated to prevent possible corneal drying. For data analysis, the three measurements were averaged to represent the mean TBUT for each eye.
Ocular surface staining
At all study visits except the treatment visit, corneal staining and conjunctival staining were evaluated with fluorescein and lissamine green, respectively, and recorded using standard methods on a scale of 0–3 reported in Lemp et al.Citation43 The total staining grade for the cornea was the sum of the grades for all five corneal regions (0–15). The total staining grade for the conjunctiva was the sum of the grades for all six conjunctival regions (0–18). The respective dyes were instilled using commercially available saline and standard fluorescein (Akorn, Inc., Farmington, CT, USA) and lissamine green strips (Bernell Corporation, Mishawaka, IN, USA). Staining was evaluated 90 seconds after the instillation of each respective dye. The corneal staining with fluorescein was completed and recorded prior to the instillation of lissamine green for the conjunctival staining procedure.
LWE
The full length and width of the wiper were examined for staining and graded using both standard fluorescein and lissamine green dyeCitation9 at all study visits except the treatment visit. Both dyes were instilled at the same time, twice, 90 seconds apart, using commercially available saline and standard fluorescein and lissamine green strips. Staining was evaluated 90 seconds after the instillation of the second combined dye instillation. Staining of the horizontal length was graded on a scale of 0 (<2 mm), 1 (2–4 mm), 2 (5–9 mm) and 3 (≥10 mm). Staining of the sagittal height was graded on a scale of 0 (<25%), 1 (25 to <50%), 2 (50 to <75%), and 3 (≥75%).Citation9 For data analysis, individual grades for length and width were averaged for a final grade for each staining method. The higher of the final fluorescein or lissamine green staining grades was used as the LWE severity grade.
LIPCOF
At all study visits except the treatment visit, the area perpendicular to the temporal and nasal limbus on the bulbar conjunctiva above the lower lid was assessed for LIPCOF under 18–24× slit lamp biomicroscope magnification and graded on a scale of 0–3 according to a previously accepted method:Citation10 Grade 0, no conjunctival folds visible; Grade 1, one permanent and clear parallel conjunctival fold (~0.05 mm thick or thicker); Grade 2, two permanent and clear parallel conjunctival folds; Grade 3, more than two permanent and clear parallel conjunctival folds.Citation10
Frequency of OTC drop use
The study permitted concomitant use of pre-existing OTC medications for MGD or dry eye, including artificial tears, ocular lubricants, and dietary supplements. However, subjects were instructed not to start any new OTC medications for the study duration. To control the concomitant use, subjects were required to report on the dosage and frequency of use at the initial visit to document the pre-existing OTC medications and, at each subsequent visit, to assess for any change in use during the study. To quantify the change in usage over time for those subjects taking topical OTC medications, the monthly frequency of topical OTC use was calculated assuming 30 days and 4 weeks in a month.
Total and comfortable contact lens wearing time
Total contact lens wearing time and comfortable contact lens wearing time in hours per day were reported by each subject at all study visits except the treatment visit. The information was captured in response to the following questions: 1) How many hours a day does the subject wear SCLs on average? And 2) How many hours a day does the subject report comfortable contact lens wear on average?Citation44,Citation45
Other questionnaire metrics
In addition to the SPEED questionnaire, subjects were self-administered the CLDEQ – Long FormCitation36,Citation46 – and Ocular Surface Disease Index (OSDI) questionnaire.Citation47,Citation48 CLDEQ was used to determine study eligibility based on having a diagnosis of dry eye while wearing contact lenses and to evaluate contact lens comfort related to dry eye. The OSDI questionnaire assessed the subjects’ frequency of dry eye symptoms in specific contexts. Total OSDI score was calculated as the sum of frequency scores for all symptoms multiplied by 25 and divided by the number of questions answered with a range from 0 to 100. Sub-scores for ocular symptoms, vision-related functioning, and environmental conditions were similarly calculated.Citation47
As a part of the questionnaire following VTP treatment or crossover VTP treatment, subjects were asked to report if the treatment improved their overall dry eye symptoms and, if yes, the percentage improvement on a scale from 10 to 100 in 10% increments. In addition, subjects were asked to report if they were able to perform any task better or longer as a result of treatment and, if yes, to list the task(s).
Study endpoints
The primary and secondary endpoints were the mean change in MGS and SPEED scores, respectively, from baseline to 3 months between the treated and untreated control groups. The primary study endpoint was used in the power analysis to determine study sample size. Adverse event data were collected at all study visits, but there were no safety endpoints in this study. The LipiFlow System is a nonsignificant risk device, and safety has been previously demonstrated.Citation27
Additional exploratory analyses
Additional exploratory analyses included TBUT, analysis of meibography images, and comparison between the treatment and untreated control groups in the mean change from baseline to 3 months in TBUT, LWE, LIPCOF, frequency of OTC drop use, total contact lens wearing time, and comfortable contact lens wearing time.
Statistical analysis
Statistical analysis was performed using the SAS (SAS Institute Inc., Cary, NC, USA) software Version 9.4. A two-sample t-test (one-sided alpha 0.025) was used to test the primary and secondary endpoints for the change from baseline to 3 months in meibomian gland score and SPEED score, respectively, between the treatment and untreated control groups. Descriptive statistics was provided for age, gender, race, ethnicity baseline measurements, and study examination findings summarized by group.
A comparability analysis was done by group and by protocol revisions (1 and 2) to assess the poolability of the data for demographic and baseline measurements. For quantitative variables, the comparison was done using two-sample t-tests or Wilcoxon rank sum tests, as appropriate. For qualitative variables, a Fisher’s exact test was used. Contact lens wear analyses were stratified by protocol revision and group because of the difference in inclusion criteria for total and comfortable contact lens wearing times between protocol revisions and to control for associated statistically significant baseline differences in wearing times.
Subject instructions and compliance
Subjects were provided with written instructions for contact lens wear, medication use during the study, and blinking exercises. Subject compliance with these instructions was reviewed at follow-up visits. At each visit, the subjects were reminded not to change their brand or type of contact lenses or disinfecting solution during the study period unless so advised by their eye doctor and to report any changes at subsequent visits as needed.
The medication instructions included a subject reminder to abstain from using any prescribed medication that can cause dry eye or prescribed medication or treatment intended to treat dry eye or MGD. Except for medical management of an adverse event, investigators were not permitted to prescribe or administer any new treatments for dry eye or MGD (including prescription medications, warm compresses, eyelid warming, eyelid massage, eyelid hygiene, meibomian gland expression, meibomian gland probing, punctal plug insertion, punctal occlusion, intense pulse light treatment of the face or eyelids, and additional LipiFlow treatment) for the study duration. For those subjects who were using pre-existing OTC products (eg, contact lens wetting lubricants, artificial tears, ocular lubricants, ointments, emollients, liposomal spray, or ω-3 dietary supplements), continued use was permitted during the study. The dosage and frequency of use was assessed at each visit. Subjects were instructed not to start using any new OTC products during the study.
After receiving either the VTP treatment or the crossover VTP treatment, subjects were provided with instructions (Figure S1) on how to perform daily blinking exercises 10 times a day for 1 month post-treatment along with tips for how to incorporate the exercises into their day. The actual frequency of blinking exercises performed was assessed at the 1 month post-treatment visit.
Study groups
For analysis purposes, there were two study groups: 1) the intent-to-treat (ITT) population (all randomized subjects) and 2) the per protocol (PP) population (all subjects who completed the study with no protocol deviations significantly affecting the integrity of the data). Safety analyses were performed with the ITT population. The primary and secondary endpoint analyses and the additional exploratory analyses were performed with both the ITT and the PP populations.
Results
There were no statistically significant (p>0.05) differences between groups or protocol revisions in demographics or baseline measurements of MGS score, SPEED score, LWE, LIPCOF, TBUT, or frequency in OTC drop use between the treatment and untreated control groups, supporting that subjects can be pooled across protocol revisions for these analyses. Furthermore, for the sub-study subjects, there was no statistically significant (p>0.05) difference in the baseline degree of meibomian gland atrophy (meiboscore) between groups. Although there was a statistically significant difference (p<0.05) between groups in baseline total contact lens wear time, there was no significant (p>0.05) difference between groups in baseline comfortable contact lens wear time. As expected because of the difference in inclusion criteria between protocol revisions, there was a statistically significant (p<0.05) difference between revisions in baseline total and comfortable contact lens wear times. To control for these significant baseline differences, the exploratory contact lens wearing time analyses were stratified by protocol revisions (1 and 2) and groups.
contains the demographics for the study subjects. The retention of subjects over the study duration was excellent. Subject accountability was 100% at 1 month, 98% at 3 months, and 96% at 4 months. One untreated control subject was discontinued prior to the 3-month visit because they were unable to complete the follow-up visit schedule.
Table 1 Demographics for treatment (VTP) and untreated control groups: ITT population
Primary and secondary effectiveness endpoints
shows the mean MGS and SPEED scores for the treatment and untreated control groups at baseline and 3 months. Between baseline and 3 months, the treatment group had a statistically significant greater mean change (improvement) in MGS score (p<0.0001), the number of functional meibomian glands (, p<0.0001), and SPEED score (p<0.0001) compared to the untreated control group. Similar improvements were observed in the crossover treatment group assessed 1 month post-VTP treatment.
Table 2 Summary of primary and secondary endpoints: ITT population
Table 3 Summary of statistically significant additional exploratory analyses
Additional exploratory analyses
Comfortable contact lens wearing time
Contact lens wear analyses were stratified by protocol revisions and groups because of the difference in inclusion criteria for contact lens wear time and to control associated statistically significant baseline differences in CL wear times. Contact lens wear analyses by groups and protocol revisions are summarized in and .
Figure 2 The mean change in comfortable contact lens wear time from baseline to 3 months post-single VTP treatment.
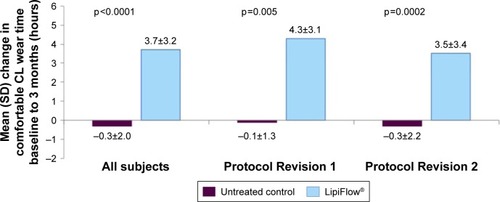
Table 4 Summary of contact lens wear analyses by protocol revision: ITT population
On average, subjects wore contact lenses 6.4±1.0 days per week in both the treatment and untreated control groups at baseline, which remained consistent at 3 months. Although the mean baseline total contact lens wear time per day was significantly longer for the treatment group than for the untreated control group (12.0 vs 10.2 hours, respectively; p=0.038), there was no significant mean change in total wear time at 3 months for either group. Furthermore, there was no statistically significant difference (p>0.05) in the mean change in total wear time from baseline to 3 months between groups overall or within each protocol revision.
However, the treatment group had a statistically significant greater mean increase from baseline to 3 months in the comfortable contact lens wear time than the untreated control group overall (3.7 vs −0.3 hours; p<0.0001) for Protocol Revision 1 (4.3 vs −0.1 hours; p=0.005) and Protocol Revision 2 (3.5 vs −0.3 hours; p=0.0002). In addition, the percentage of comfortable wear time out of total wear time per day was analyzed to control the baseline difference in total wear time between groups. From baseline to 3 months, the treatment group had a mean increase in the percentage of time contact lenses felt comfortable out of the total time worn per day from 45.0% (±22.0%) at baseline to 74.4% (±23.4%) at 3 months. The mean change in the percentage was significantly greater for the treatment group as compared to the untreated control group overall (29.4 vs −2.1%, p<0.0001) for Protocol Revision 1 (33.2 vs −4.0%, p=0.008) and Protocol Revision 2 (28.1 vs −1.4%, p=0.0002).
DMI
At US sites only where DMI was used to assess gland structure, the mean baseline average eyelid meiboscore was 1.8 (±0.7) for the treatment group (n=18 subjects) and 1.6 (±0.8) for the untreated control group (n=19 subjects), reflecting evidence of gland atrophy in both groups. There was no statistically significant difference (p>0.05) in the mean baseline average eyelid meiboscore between the treatment and untreated control groups. Furthermore, there was no statistically significant difference (p>0.05) in the average eyelid meiboscore between baseline and 1 month post-VTP treatment for all treated eyes. The mean baseline average eyelid meiboscore for all subjects was 1.67 (95% CI 1.4–1.9, n=37 subjects) (refer for examples of images captured using DMI).
Figure 3 DMI images from four different study subjects.
Abbreviation: DMI, Dynamic Meibomian Imaging.
TBUT
The treatment group demonstrated a statistically significant greater mean change in TBUT from baseline to 3 months than in the untreated control group (1.7 vs −0.2 seconds, respectively; p=0.0005). The crossover treatment group showed a similar mean increase in TBUT at 4 months as the treatment group at 1 month ().
Ocular surface staining
There was no statistically significant difference (p>0.05) between the treatment and untreated control groups in the change in total corneal staining grade from baseline to 3 months. The treatment group showed significantly (p=0.04) less increase in the total conjunctival staining grade from baseline to 3 months than the untreated control group in the PP population (); however, the difference between groups was not statistically significant (p>0.05) in the ITT population.
LWE
The treatment group demonstrated statistically significantly (p=0.048) greater reduction in the LWE severity grade from baseline to 3 months than the untreated control group ().
Lid margin parallel conjunctival folds (LIPCOF)
The treatment group had a statistically significantly (p=0.048) greater decrease in the temporal LIPCOF grade from baseline to 3 months than the untreated control group in the PP population (); however, the difference between groups was not significant (p>0.05) in the ITT population. For the nasal LIPCOF grade, there was no statistically significant (p>0.05) difference in the change in grade from baseline to 3 months between groups.
Frequency of OTC drop use
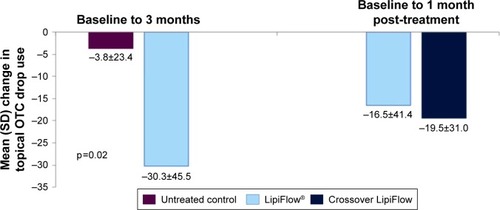
At baseline, 69.0% of treatment subjects and 73.1% of untreated control subjects used at least one topical OTC medication; most of these subjects used only artificial tears. For only those subjects using topical OTC medications at baseline, 60.0% of the treatment group and 27.8% of the untreated control group had a decrease in the frequency of use from baseline to 3 months. The treatment group had a statistically significant greater mean decrease in the monthly frequency of topical OTC use from baseline to 3 months than the untreated control group (−30.3 vs −3.8 times per month; p=0.029) ( and ).
Subject report of overall dry eye symptoms
The vast majority of subjects reported an improvement in their overall dry eye symptoms: 93.1% of subjects at 1 month post-VTP and 86.2% of subjects at 3 months post-VTP in the treatment group reported this improvement and 100% of patients in crossover treatment group reported an improvement in their overall dry eye symptoms at 1 month post-VTP treatment. Furthermore, for those subjects who reported subjective improvement in overall dry eye symptoms after VTP treatment, the mean percentage improvement was ~55% (specifically 56.3% at 1 month post-VTP and 59.6% at 3 months post-VTP in the treatment group and 55.6% at 1 month post-VTP in the crossover treatment group).
Subject report of improvement in tasks
The vast majority of subjects reported an improvement in ability to perform a task better or longer, such as visual tasks (eg, reading, computer use, and driving) and wearing contact lenses longer or more comfortably: 82.8% of subjects at 1 month post-VTP and 72.4% of subjects at 3 months post-VTP in the treatment group and 80.0% of subjects at 1 month post-VTP treatment in the crossover treatment group.
Other questionnaire metrics
All subjects had a dry eye diagnosis based on the CLDEQ at baseline. The treatment group had a statistically significant higher percentage of subjects who no longer had a dry eye diagnosis at 3 months based on the CLDEQ as compared to the untreated control group (44.8 vs 4.0%, respectively; p=0.0006).
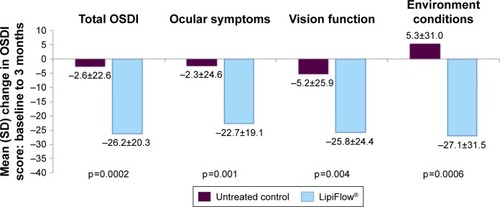
The treatment group had a significantly greater reduction in dry eye symptoms than the untreated control group based on the mean change total OSDI score from baseline to 3 months (−26.2 vs −2.6, respectively; p=0.0002). Correspondingly, the treatment group also demonstrated a statistically significant (p<0.05) greater mean change from baseline to 3 months in the OSDI sub-scores for ocular symptoms, vision-related functioning, and environmental conditions than the untreated control group, as shown in . The crossover treatment group displayed a similar reduction in symptoms based on the mean total OSDI score and sub-scores at 4 months and 1 month post-VTP treatment.
Adverse events
There were no adverse events reported as related to a device or a study procedure, and no serious adverse event or unanticipated adverse device effects were reported. These data validate the low-risk safety profile of the LipiFlow System, reported in prior studies. Eleven adverse events unrelated to a device were reported including five ocular events and six systemic events.
Furthermore, slit lamp findings observed immediately after VTP treatment in the treatment and crossover treatment groups, including eyelid edema, conjunctival edema, conjunctival hyperemia/injection, petechiae, and superficial punctate keratitis (SPK), were consistent with slit lamp findings noted post-treatment in prior studies.Citation27–Citation31 All immediate post-treatment slit lamp findings were transient and did not require medical treatment.
Changes in eyelid appearance and signs of obvious MGD after VTP treatment were not expected because MGD is a chronic condition resulting in long-standing morphological changes that can be permanent. However, a trend was observed in the treatment group for improvement from baseline to 3 months after VTP treatment with an increase from 41.4% to 58.6% of subjects with normal eyelid appearance and a decrease from 63.8% to 46.6% of subjects with signs of obvious MGD.
Discussion
The efficacy of a single VTP treatment in patients with MGD and dry eye is well established,Citation27–Citation33,Citation49 and VTP treatment has been shown to deliver a sustained treatment effect in multiple studies.Citation28,Citation30,Citation32,Citation49 The majority of prior VTP studies have focused on treating MGD in dry eye populations, including Sjögren’s patientsCitation32 and patients with refractory dry eye postrefractive surgery.Citation49,Citation50 However, contact lens wearers with MGD are a natural study population for the VTP treatment of MGD due to 1) the demands that a contact lens places on the tear film,Citation1–Citation13 2) the significant correlation of both severity and prevalence of MGD in contact lens wearers,Citation11,Citation16–Citation20 and 3) the consistently high contact lens dropout rate due to dry eye symptoms.Citation1,Citation5,Citation51–Citation54
The primary and secondary endpoints in this study were met with the treatment group demonstrating a statistically significant greater mean improvement meibomian gland score and a mean reduction in SPEED score from baseline to 3 months compared to the untreated control group. Similar mean improvements in meibomian gland and SPEED scores were observed at 1 month after crossover VTP treatment of the control group, which further supports the effectiveness of the device. These study findings are consistent with the effectiveness results of multiple prior studies using the LipiFlow System for the treatment of MGD. On average, prior studies have shown that a single VTP treatment increases gland function approximately threefold and reduces dry eye symptoms by ~50%.Citation28 In this study, measured 3 months post-VTP treatment, the mean meibomian gland function score had increased by 2.6 times from baseline and the mean dry eye symptom score decreased by 58%.
With regard to the imaging analysis of meibomian gland structure using a novel technology known as DMI, our data are consistent with the findings of prior experts.Citation41 Arita et al reported an average meiboscore for SCL wearers as 1.52 (95% CI 1.17–1.86, n=121 subjects) compared to the average meiboscore of noncontact lens wearers, which was 0.48 (95% CI 0.37–0.59, n=137 subjects).Citation39,Citation41 Our data showed an average meiboscore for contact lens wearers as 1.67 (95% CI 1.4–1.9, n=37 subjects).
The additional exploratory analyses reveal other benefits of VTP treatment for contact lens wearers with MGD. The treatment group showed statistically significantly greater mean improvements in the following parameters from baseline to 3 months as compared to the untreated control group: percentage of comfortable contact lens wear time out of the total contact lens wear time per day; monthly frequency of topical OTC medication use for MGD or dry eye; total OSDI score and sub-scores for ocular symptoms, vision-related functioning, and environmental conditions; percentage of subjects who no longer had a dry eye diagnosis based on the CLDEQ; number of functional meibomian glands; TBUT; and LWE severity. Furthermore, these findings were substantiated by similar mean improvements in the above parameters 1 month after crossover VTP treatment of the untreated control group.
This prospective, randomized, multicenter pilot clinical trial demonstrates the benefits of treating MGD in contact lens wearers. These results validate that a single VTP treatment can significantly improve meibomian gland function and tear film stability, decrease dependence on OTC medications, and reduce known contact lens-related ocular surface indicators of dryness and inflammation, such as LWE as early as 1 month following treatment. The most significant new finding in this study is that mean comfortable contact lens wear time increased, on average, by 4 hours per day at 1 month after VTP treatment, and the findings persisted over the entire study, approximately doubling the mean comfortable contact lens wearing time measured at baseline. This study also confirms that a single VTP treatment results in tangible improvement in the quality of life for most subjects, as evidenced by being able to perform a patient-reported task (such as using computers or digital devices, reading, and wearing contact lenses) significantly better or longer after treatment.
The timing of re-treatment was not addressed in this study. Prior studies on the use of VTP for MGD in noncontact lens wearing dry eye patients indicate that the VTP treatment effect for gland function and dry eye symptoms can be sustained for up to ≥12 months.Citation29,Citation31 These studies also highlight that early intervention optimizes treatment efficacyCitation29 and that patients with more severe disease are likely to benefit from a combination of in-office treatment to address gland obstruction along with patient-specific at-home adjunctive therapies.Citation31 How these findings relate to contact lens wearers with MGD remains to be tested.
The absence of device-related adverse events in this study further confirms the low-risk safety profile of the LipiFlow System, as demonstrated in prior studies. In addition, slit lamp findings observed immediately after VTP treatment were transient, required no medical treatment, and were consistent with prior studies.
While changes in eyelid appearance and signs of obvious MGD after VTP treatment were not expected because of chronic, long-standing nature of these morphological changes, improvement in eyelid appearance from baseline to 3 months after VTP treatment was observed and noted. These findings suggest that MGD-related eyelid and gland orifice changes can be improved through clearing of gland obstruction with the LipiFlow System.
The importance of blinking as a core mechanism for maintaining meibomian gland health is known;Citation37,Citation55–Citation57 however, there is no accepted standard for how to communicate this to patients in a clinical setting. In this study, all subjects were provided very specific blinking instructions and told to perform them daily for a month after VTP treatment. Compliance with blinking was assessed at the 1 month post-treatment visit and more than half of the subjects reported completing 10 repetitions of the exercises 5–10 times a day for 20–30 days of the month. However, compliance with blinking exercises was not a focus of this study and documentation of daily compliance with blinking exercises was not included in the study protocol. The impact of blinking exercises on the efficacy of VTP treatment has not been directly tested. This relationship should be investigated in future studies.
There are limitations to every study, and this study is no exception. This was a small study intended to assess the value of performing a larger clinical study in contact lens wearing patients with MGD. The study was open label, and the control group was untreated. We cannot rule out investigator bias or the placebo effect. However, the safety and efficacy of the treatment have been well documented in both randomized controlled multicenter trials and retrospective studies and case series analyses over several years.Citation27–Citation33,Citation49,Citation50
The purpose of this study was to assess the benefit of treating MGD in a contact lens wearing population with a single VTP treatment. The results clearly indicate that identifying and treating MGD in contact lens wearers have the potential to significantly improve meibomian gland function, reduce dry eye signs and symptoms, and dramatically increase comfortable contact lens wearing time. The data strongly support a prior conclusion that contact lens wearers should be routinely evaluated and treated for MGD.Citation18
Conclusion
The study results establish that there are multiple benefits of VTP treatment for MGD in contact lens wearers with discomfort and that these benefits far outweigh the risks of the VTP device. Of particular interest is that a single VTP treatment increased mean comfortable wearing time by 4 hours, on average, approximately doubling the number of comfortable contact lens wearing hours. This result was sustained out to 3 months post-treatment on average.
Acknowledgments
This study was supported by TearScience Inc., Morrisville, NC, USA.
Supplementary material
Disclosure
CAB and CAC are employees of TearScience, Inc. The other authors report no conflicts of interest in this work.
References
- YoungGWhy one million contact lens wearers dropped outCont Lens Anterior Eye2004272838516303533
- YoungGChalmersRNapierLKernJHuntCDumbletonKSoft contact lens-related dryness with and without clinical signsOptom Vis Sci20128981125113222820475
- CafferyBDogruMJonesLWContact lens comfortOptom Vis Sci201693879079227454831
- RahMJMercheaMMDoktorMQReducing dropout of contact lens wear with Biotrue multipurpose solutionClin Ophthalmol2014829329924493920
- RumpakisJ webpage on the InternetNew data on contact lens dropouts: an international perspectiveReview of Optometry2010 Available from: http://www.revoptom.com/content/d/contact_lenses/i/990/c/18929/Accessed October 2, 2017
- CraigJPWillcoxMDArgüesoPThe TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommitteeInvest Ophthalmol Vis Sci20135411TFOS123TFOS15624058139
- JonesLBrennanNAGonzález-MéijomeJThe TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommitteeInvest Ophthalmol Vis Sci20135411TFOS37TFOS7024058138
- Navascues-CornagoMMorganPBMaldonado-CodinaCEffect of Three Interventions on Contact Lens Comfort in symptomatic wearers: a randomized clinical trialPLoS One2015108e013532326267853
- KorbDRGreinerJVHermanJPLid-wiper epitheliopathy and dry-eye symptoms in contact lens wearersCLAO J200228421121612394549
- PultHPurslowCBerryMMurphyPJClinical tests for successful contact lens wear: relationship and predictive potentialOptom Vis Sci20088510E924E92918832967
- AritaRFukuokaSMorishigeNMeibomian gland dysfunction and contact lens discomfortEye Contact Lens2017431172228002224
- EfronNJonesLBronAJThe TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the ocular surface and adnexa subcommitteeInvest Ophthalmol Vis Sci201354TFOS98Y12224058133
- CraigJPNicholsKKAkpekEKTFOS DEWS II definition and classification reportOcul Surf201715327628328736335
- NicholsKKRedfernRLJacobJTThe TFOS International Workshop on Contact Lens Discomfort: report of the definition and classification subcommitteeInvest Ophthalmol Vis Sci20135411TFOS14TFOS1924058134
- SuhalimJLParfittGJXieYEffect of desiccating stress on mouse meibomian gland functionOcul Surf2014121596824439047
- HenriquezASKorbDRMeibomian glands and contact lens wearBr J Ophthalmol19816521081117459311
- PaughJRKnappLLMartinsonJRHomMMMeibomian therapy in problematic contact lens wearOptom Vis Sci199067118038062250886
- MachalińskaAZakrzewskaAAdamekBComparison of morphological and functional meibomian gland characteristics between daily contact lens wearers and nonwearersCornea20153491098110426114822
- CoxSMBerntsenDAChatterjeeNPerformance of Contact Lens Solutions Study GroupEyelid margin and meibomian gland characteristics and symptoms in lens wearersOptom Vis Sci201693890190827273272
- AlghamdiWMMarkoulliMHoldenBAPapasEBImpact of duration of contact lens wear on the structure and function of the meibomian glandsOphthalmic Physiol Opt201636212013126890701
- OngBLRelation between contact lens wear and meibomian gland dysfunctionOptom Vis Sci1996732082108725025
- MudgilPAntimicrobial role of human meibomian lipids at the ocular surfaceInvest Ophthalmol Vis Sci201455117272727725316725
- AziziSUçakTYaşarIKarakurtYErdoganESalmanIEvaluation of the corneal layers in meibomian-gland-dysfunction-related dry eye by in vivo slit-scanning confocal microscopySemin Ophthalmol201732337738327082732
- LiangQPanZZhouMEvaluation of optical coherence tomography meibography in patients with obstructive meibomian gland dysfunctionCornea201534101193119926226467
- BaudouinCMessmerEMAragonaPRevisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunctionBr J Ophthalmol2016100330030626781133
- KorbDRBlackieCAA single LipiFlow treatment increases soft contact lens wearing time and reduces lid wiper epitheliopathy and dry eye symptomsAbstract presented at the AAODenver, CO2014
- LaneSSDuBinerHBEpsteinRJA new system, the LipiFlow, for the treatment of meibomian gland dysfunctionCornea201231439640422222996
- BlackieCACarlsonANKorbDRTreatment for meibomian gland dysfunction and dry eye symptoms with a single-dose vectored thermal pulsation: a reviewCurr Opin Ophthalmol201526430631326058030
- BlackieCAColemanCAHollandEJThe sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eyeClin Ophthalmol2016101385139627555745
- FriedlandBRFlemingCPBlackieCAKorbDRA novel thermodynamic treatment for meibomian gland dysfunctionCurr Eye Res2011362798721281063
- GreinerJVLong-term (3 year) effects of a single thermal pulsation system treatment on meibomian gland function and dry eye symptomsEye Contact Lens20164229910726222095
- EpitropoulosATGoslinKBedaRBlackieCAMeibomian gland dysfunction patients with novel Sjögren’s syndrome biomarkers benefit significantly from a single vectored thermal pulsation procedure: a retrospective analysisClin Ophthalmol20171170170628458508
- FinisDKönigCHayajnehJBorrelliMSchraderSGeerlingGSix-month effects of a thermodynamic treatment for MGD and implications of meibomian gland atrophyCornea201433121265127025321941
- NgoWSituPKeirNKorbDBlackieCASimpsonTPsychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaireCornea20133291204121023846405
- NicholsJJMitchellGLNicholsKKChalmersRBegleyCThe performance of the contact lens dry eye questionnaire as a screening survey for contact lens-related dry eyeCornea200221546947512072721
- KorbDRBlackieCADebridement-scaling: a new procedure that increases meibomian gland function and reduces dry eye symptomsCornea201332121554155724145633
- WanTJinXLinLXuYZhaoYIncomplete blinking may attribute to the development of meibomian gland dysfunctionCurr Eye Res201641217918525835130
- KorbDRBlackieCAMeibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland locationCornea200827101142114719034129
- AritaRItohKInoueKAmanoSNoncontact infrared meibography to document age-related changes of the meibomian glands in a normal populationOphthalmology2008115591191518452765
- AritaRItohKMaedaSProposed diagnostic criteria for obstructive meibomian gland dysfunctionOphthalmology2009116112058.e12063.e119744718
- AritaRItohKInoueKKuchibaAYamaguchiTAmanoSContact lens wear is associated with decrease of meibomian glandsOphthalmology2009116337938419167077
- KorbDRGreinerJVHermanJComparison of fluorescein break-up time measurement reproducibility using standard fluorescein strips versus the Dry Eye Test (DET) methodCornea200120881181511685057
- LempMAReport of the National Eye Institute/Industry workshop on Clinical Trials in Dry EyesCLAO J19952142212328565190
- NicholsKKMorrisSGaddieIBEvansDEpinastine 0.05% ophthalmic solution in contact lens-wearing subjects with a history of allergic conjunctivitisEye Contact Lens2009351263119125045
- NicholsJJBickleKMZinkRCSchieweMDHaqueRMNicholsKKSafety and efficacy of topical azithromycin ophthalmic solution 1.0% in the treatment of contact lens-related dry eyeEye Contact Lens2012382737922157392
- NicholsJJMitchellGLNicholsKKAn assessment of self-reported disease classification in epidemiological studies of dry eyeInvest Ophthalmol Vis Sci200445103453345715452049
- SchiffmanRMChristiansonMDJacobsenGHirschJDReisBLReliability and validity of the ocular surface disease indexArch Ophthalmol2000118561562110815152
- MillerKLWaltJGMinkDRMinimal clinically important difference for the ocular surface disease indexArch Ophthalmol201012819410120065224
- SchallhornCSSchallhornJMHannanSSchallhornSCEffectiveness of an eyelid thermal pulsation procedure to treat recalcitrant dry eye symptoms after laser vision correctionJ Refract Surg2017331303628068444
- PetzoldGBediRBlackieCAManagement of dry-eye syndrome after laser in situ keratomileusis with a vectored thermal pulsation systemJCRS Online Case Rep201643437
- BerberianDThe contact lens ‘drop-out’ – a dissatisfied person?Ophthalmic Optician198317551
- FonnDTargeting contact lens induced dryness and discomfort: what properties will make lenses more comfortableOptom Vis Sci200784427928517435511
- PritchardNFonnDBrazeauDDiscontinuation of contact lens wear: a surveyInt Contact Lens Clin199926615716211384832
- RichdaleKSinnottLTSkadahlENicholsJJFrequency of and factors associated with contact lens dissatisfaction and discontinuationCornea200726216817417251807
- LintonRGCurnowDHRileyWJThe meibomian glands. An investigation into the secretion and some aspects of the physiologyBr J Ophthalmol196l4571872318170726
- McMonniesCWIncomplete blinking: exposure keratopathy, lid wiper epitheliopathy, dry eye, refractive surgery, and dry contact lensesCont Lens Anterior Eye2007301375117251052
- McMonniesCWBlink efficiency: a neglected area of ocular surface disease management?Invest Ophthalmol Vis Sci2011527448421700718