Abstract
Background
While loteprednol etabonate (LE) suspension 0.5% is approved for the treatment of postoperative ocular inflammation, there have been no reported studies of its use in glaucoma patients undergoing canaloplasty.
Methods
This was a retrospective medical chart review conducted at a single US center. Data were collected on patients with glaucoma who underwent canaloplasty with or without cataract surgery, and were prescribed LE suspension 0.5% postoperatively. Outcomes evaluated included postsurgical inflammation (anterior chamber [AC] cells and flare), intraocular pressure (IOP), number of IOP-lowering medications, and postsurgical complications.
Results
Data were collected on 204 patients (262 eyes) with a mean (SD) age of 71.6 (11.3) years. The most frequent LE dosing regimens at day 1, week 1, and month 1 postsurgery were QID (92.3%; 241/261), TID (52.6%; 133/253), and QD (65.5%; 78/119), respectively. Inflammation (AC flare and cells), mostly mild, was noted in 33.2% (86/259) of eyes on postoperative day 1 and 8.6% (21/244) of eyes at month 1. Mean IOP and mean number of IOP-lowering medications were significantly reduced from baseline (P<0.001) at all time points postoperatively. Complete (no IOP-lowering medication) or qualified (use of ≤2 IOP-lowering medications) surgical success was achieved in 78.8% and 90.6% of eyes, respectively, at month 6 and 63.4% and 92.7% of eyes at month 36. The most frequently observed postoperative complication was hyphema in 48.7% (126/259) eyes at day 1, which decreased to 0.4% (1/244) of eyes by month 1. IOP ≥30 mmHg was noted in 13 (5.3%) eyes at postoperative week 1 and rarely thereafter, and no patient discontinued therapy because of an IOP increase.
Conclusion
These real-world data suggest that canaloplasty with or without cataract surgery managed postoperatively with LE suspension 0.5% is effective and safe in the glaucoma patient.
Introduction
Intraocular pressure (IOP) lowering is a proven treatment strategy that slows progression of glaucomatous optic nerve injury and visual field loss in patients with glaucoma.Citation1–Citation7 Initial strategies for IOP lowering include use of medications and laser trabeculoplasty.Citation8,Citation9 However, if these approaches fail to adequately control IOP, incisional glaucoma surgery is usually required.Citation4,Citation8,Citation9 The traditional surgical technique for lowering IOP is trabeculectomy, which increases aqueous humor outflow through a partial removal of the trabecular meshwork/Schlemm’s canal and the creation of a subconjunctival filtering bleb.Citation10,Citation11 However, this procedure may be associated with complications, including bleb infections, choroidal detachment, and scarring of the conjunctival tissue leading to failure of the bleb.Citation8,Citation12–Citation15
Canaloplasty is a minimally invasive, nonpenetrating surgical technique that lowers IOP by restoring, rather than partially removing, the trabecular meshwork/Schlemm’s canal.Citation16–Citation21 As the procedure does not create a permanent fistula in the eye wall, the likelihood of postsurgical complications is reduced, and patient follow-up is simplified relative to trabeculectomy.Citation15,Citation22–Citation26 Canaloplasty enhances natural transtrabecular outflow over the long term by tensioning of the meshwork and opening of the trabecular plates, viscodilation of the Schlemm’s canal, and the creation of a scleral lake and Descemet’s window.Citation27,Citation28 Performing the procedure without a tensioning suture spares conjunctival manipulation for possible future procedures and is termed ab-interno canaloplasty.Citation29 In follow-up studies of glaucoma patients who underwent canaloplasty, IOP reduction was maintained as long as 4 years, with a significant decline in the need for medication and/or additional surgical treatment.Citation17,Citation18,Citation20,Citation26,Citation30–Citation32 Canaloplasty can be performed in conjunction with cataract surgery with reported improved outcomes over cataract surgery alone in the majority of studies.Citation16,Citation21,Citation24,Citation28,Citation30,Citation31,Citation33
Postoperative management following canaloplasty typically involves topical antibiotics for 1 week and topical corticosteroids tapered over 3–4 weeks.Citation29,Citation34 Although routinely used for reducing postsurgical inflammation and improving patient comfort following ocular surgery, corticosteroid use can be associated with a number of potential adverse effects of particular concern to the glaucoma patient, such as steroid-induced IOP increases, decreased wound healing, and cataract formation in phakic eyes.Citation35–Citation40
Loteprednol etabonate (LE) is a topical ophthalmic corticosteroid that contains a chloromethyl ester group at the carbon 20 position instead of the ketone group present in other corticosteroids. This unique structure facilitates rapid metabolism of LE molecules that are unbound to glucocorticoid receptors into inactive metabolites,Citation41–Citation44 allowing LE to exert the desired anti-inflammatory activity while reducing the likelihood of unwanted effects.Citation41,Citation43,Citation45,Citation46 LE ophthalmic suspension 0.5% (Lotemax® suspension; Bausch + Lomb, Bridgewater, NJ, USA) was approved in 1998 for the treatment of postoperative inflammation following ocular surgery, in addition to steroid-responsive inflammatory conditions such as seasonal allergic conjunctivitis and uveitis.
Multiple studies have demonstrated the anti-inflammatory efficacy and safety (including minimal effect on IOP) of LE suspension 0.5% used postsurgically in patients undergoing cataract surgery, photorefractive keratectomy, and laser-assisted in situ keratomileusis.Citation47–Citation52 Further studies have demonstrated that LE suspension has a low propensity to elevate IOP,Citation53 including in known corticosteroid respondersCitation54,Citation55 and in comparison with other corticosteroids.Citation56–Citation61
To date, two retrospective chart reviews have evaluated the use of LE suspension 0.5% in patients undergoing glaucoma surgery.Citation62,Citation63 Patients treated with LE 0.5% experienced a minimal effect on IOP and a significant reduction in the number of glaucoma medications needed following combined phacoemulsification and trabecular microbypass stent implantation.Citation62 Similarly, there was no difference in IOP in patients undergoing selective laser trabeculoplasty who received LE 0.5% at the time of surgery compared to those not receiving corticosteroid.Citation63 However, the effect of LE on inflammation was not reported in these studies, and, to date, there are no published data addressing outcomes with the use of LE in patients following canaloplasty. This retrospective study evaluated postsurgical success and safety outcomes, including IOP findings, in patients managed with LE 0.5% suspension postoperatively after canaloplasty with or without phacoemulsification and intraocular lens (IOL) implantation.
Methods
Study design
This retrospective chart review was conducted at a single center (Dean McGee Eye Institute, University of Oklahoma, Oklahoma City, OK, USA). Retrospective data were collected on the routine care and follow-up of patients who underwent canaloplasty with or without simultaneous phacoemulsification and IOL implantation between January 19, 2010, and March 5, 2013, for whom LE suspension 0.5% was prescribed as part of postoperative management. All patient data were de-identified and kept confidential, as specified by the International Conference on Harmonization Guidelines for Good Clinical Practice.Citation64 The Institutional Review Board at the University of Oklahoma approved the protocol and waived the need for informed consent, given that all patient data were de-identified for the purposes of the study.
Eligible patients were ≥18 years of age with a diagnosis of glaucoma who underwent canaloplasty (either with or without simultaneous phacoemulsification and IOL implantation) and were treated postoperatively with LE suspension 0.5%. Exclusion criteria included use of a topical corticosteroid other than LE during the preoperative evaluation period and/or within 1 day of the postoperative period. If an included eye had documented the use of another topical corticosteroid at any of the subsequent follow-up visits, data from that eye were included only up to the time of receipt of the non-LE corticosteroid.
Surgical procedures
The details of the canaloplasty procedure have been described previously.Citation16–Citation18,Citation20,Citation26,Citation27,Citation29,Citation30,Citation34,Citation65 For the cases included in this chart review, one surgeon (MK) performed all canaloplasty procedures according to the same standard of care. Surgical preparation included a peribulbar or retrobulbar block followed by conjunctival and subsequent superficial flap dissection. For those patients undergoing a combined procedure including phacoemulsification and IOL implantation, a temporal clear corneal incision and posterior chamber IOL implantation were performed before dissection of the deep flap creating a Descemet window.
Schlemm’s canal was unroofed through the surgical creation and removal of a deep scleral flap. A microcatheter (iTRACK-250; Ellex) was inserted and guided through this opening for the entire 360° of Schlemm’s canal. Before reversing and removing the catheter from Schlemm’s canal, those eyes undergoing the ab-externo approach had a stent suture tied under tension at the catheter’s distal tip to maintain inward distention of the trabecular meshwork. If there was failure to completely catheterize the eye, no suture was tied. Postoperative management, initiated 1 day postsurgery, included third- or fourth-generation fluoroquinolone drops TID or QID for 2 weeks and LE suspension 0.5% QID for 1 week and tapered over the next 3–4 weeks.
Data collected and reported outcomes
Data were collected for the presurgical visit (baseline) and all postsurgical visits starting on day 1 (the day after surgery) and extending to the longest available follow-up visit up to a maximum of 3 years. Data extracted from patient charts included the following: patient demographics; surgical characteristics; LE suspension dosing, frequency, and duration; ocular inflammation (anterior chamber [AC] cells and flare); IOP (by Goldmann applanation tonometry); concomitant medications; visual acuity (VA; Snellen); and adverse events (AEs). Inflammation (AC cells and AC flare data) was scored using a 6-point severity scale (0, 0.5, 1, 2, 3, 4, or unknown [inflammation present using terms such as rare or occasional, but no grade]). Hyphema was scored according to the presence of red blood cells in the AC using a 6-grade scale (none, micro [trace], grade I [≤33% filling of the AC], grade II [33%–50% filling of the AC], grade III [>50%–<100% filling of the AC], grade IV [100% filling of the AC], or unknown).
Outcomes evaluated included mean postsurgical inflammation (AC cells and flare), IOP, VA, number of concomitant IOP-lowering medications, postsurgical complications (injection and hyphema), and AEs.
Data analysis
Eyes of patients in which both eyes qualified for inclusion were treated independently for all outcomes. Where data for IOP and/or VA were available, a lack of recorded data for outcomes other than IOP and VA was imputed as “zero” or “none”. When neither IOP nor VA was recorded, a lack of recorded data for other outcomes was imputed as missing. Data for hyphema and inflammation were not collected past postoperative month 3.
When calculating the number of glaucoma medications at follow-up visits, combination medications were counted as two medications. The percentage of eyes achieving postoperative IOPs of ≤21, ≤18, and ≤15 mmHg without use of IOP-lowering medications at the postoperative time points of 6 months, 12 months, 18 months, 2 years, and 3 years were calculated. Canaloplasty surgery was deemed a complete success if eyes attained target IOP without the use of IOP-lowering medications. Qualified success was defined similarly, but with adjunctive use of ≤2 IOP-lowering medications.
Postoperative inflammation was reported as the percentage of eyes with any noted inflammation (AC cells and/or AC flare) and the percentage of eyes graded by severity of inflammation in terms of AC cells and AC flare. Hyphema findings were reported as the percentage of eyes with any observed hyphema and the percentage of eyes by severity grade. VA was converted from Snellen to logarithm of the minimum angle of resolution or logMAR.
The number of IOP-lowering medications and IOP (analyzed as change from baseline) at each time point postsurgery was compared to baseline using a paired t-test (Statistical Analysis Software Version 7; SAS Institute). Statistical significance was defined as P<0.05. All other outcomes were analyzed descriptively.
Results
Data were collected on 204 patients (262 eyes) with a mean (SD) age of 71.6 (11.3) years, mostly Caucasian (70.3%), and evenly divided between men and women (). Fifty-eight (28.4%) patients had surgery performed in both eyes on separate days. Primary open-angle glaucoma (POAG) with or without nuclear sclerotic cataract was the most frequent diagnosis at the time of surgery (91.8% of eyes) (). The majority of eyes (90.8%) underwent canaloplasty with suture tensioning, and 47.3% of eyes underwent canaloplasty combined with phacoemulsification and IOL implantation. The total number of eyes available for analysis by visit was 262 at day 1, 255 at week 1, 244 at month 1, 210 at month 3, 179 at month 6, 164 at month 12, 151 at month 18, 132 at month 24, and 47 at month 36. The primary reason eyes were not available for analysis at later visits was that patients could be sent back to their referral sources within 3–6 months following surgery. Other reasons included lack of a recorded outcome measure (eg, IOP) at a particular visit, discontinuation from the study because of the use of a steroid other than LE (further detail provided below), a missed visit, or being lost to follow-up.
Table 1 Baseline and surgical characteristics of study subjects and eyes
Among included eyes, the first instillation of LE occurred at the postoperative day 1 visit, though one eye did not receive LE until the postoperative week 1 visit. The majority of patients were prescribed LE for <1 month. The most frequent dosing regimen at day 1, week 1, and month 1 post-surgery was QID (92.3%; 241/261), TID (52.6%; 133/253), and QD (65.5%; 78/119) respectively (). A total of 27 eyes (10.3%) were switched to another corticosteroid at a time point after postoperative day 1 and were, therefore, excluded from analyses from that point onwards. In six eyes, this occurred within the first postoperative week, and in eight eyes, between 1 week and 1 month following surgery. Thereafter, between one and three eyes were discontinued for using a non-LE corticosteroid at each of the following postsurgery visits: month 3, month 6, month 12, month 18, year 2, and year 3. Patients were switched to another steroid primarily because after initially receiving a sample of LE, they were unable to pay for a follow-up LE prescription and/or LE was not covered by their health care insurance prescription plan.
Table 2 Dosing frequency for LE suspension 0.5% over the first 3 month of postcanaloplasty
There was no recorded use of antibiotics or nonsteroidal anti-inflammatory agents (NSAIDs) for included eyes prior to surgery (preoperative visit). Concomitant use of topical antibiotics at postoperative day 1, week 1, month 1, and month 3 following surgery was reported in 100% (262/262), 67.4% (172/255), 4.9% (12/244), and 1% (2/210) eyes, respectively. Besifloxacin ophthalmic solution 0.6% (Besivance®; Bausch + Lomb) was the most commonly utilized antibiotic and was administered TID in 60% eyes on postoperative day 1. Concomitant use of NSAIDs (bromfenac, ketorolac, or nepafenac) following surgery was reported in 27.8% (n=72/259), 20% (n=51/255), 2% (n=5/244), and 1% (n=2/210) of eyes at these time points, respectively.
Resolution of inflammation
On postoperative day 1, 86 (33.2%) eyes were noted to have some degree of inflammation (AC cells and/or flare) compared to 56 (22.0%) eyes at week 1, 21 (8.6%) eyes at month 1, and 2 (1.0%) eyes at month 3. Grading of AC cells and AC flare indicated that inflammation was mostly mild (grade of either 0.5 or 1) in severity ().
Table 3 Inflammation grading for AC cells and AC flare by postoperative visit
Surgical outcomes
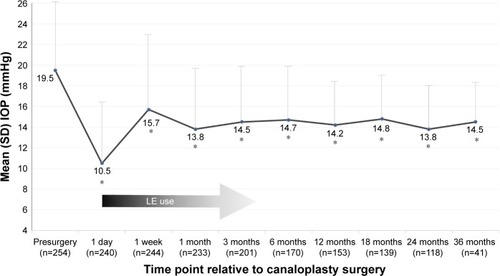
shows mean IOP in included eyes before and following surgery. Mean IOP was significantly reduced from baseline (P<0.001) at all postoperative time points. The mean preoperative (SD) IOP was 19.5 (6.6) mmHg, with 21 eyes noted to have an IOP ≥30 mmHg. At postoperative day 1, mean IOP decreased by 46.7% (P<0.001 vs presurgery) and remained significantly lower compared with baseline at each subsequent follow-up visit through year 3.
Figure 1 Mean intraocular pressure in eyes treated with LE suspension 0.5% postcanaloplasty.
Abbreviations: IOP, intraocular pressure; LE, loteprednol etabonate.
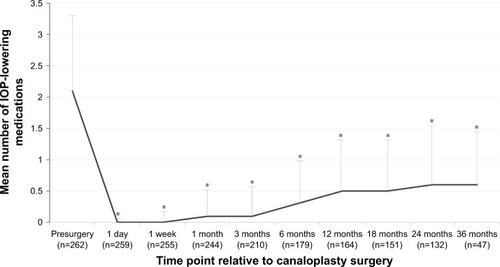
The mean (SD) number of IOP-lowering medications used per eye decreased significantly from 2.1 (1.22) presurgery to 0.0 (0.9) at day 1, 0.0 (0.22) at week 1, 0.1 (0.43) at month 1, 0.1 (0.51) at month 6, and 0.6 (0.83) at month 36 (P<0.001 at all postsurgical time points vs baseline) ().
Figure 2 Intraocular pressure-lowering medication use following canaloplasty.
Abbreviation: IOP, intraocular pressure.
Mean (SD) VA in logMar prior to surgery was 0.32 (0.37). A worsening in VA from baseline was observed at the day 1, week 1, and month 1 postoperative visits, with values of 0.83 (0.52), 0.56 (0.42), and 0.39 (0.34), respectively. However, for all subsequent visits, mean VA either appeared similar to baseline (month 3 and year 3) or improved over baseline (month 6, month 12, month 18, and year 2).
Surgical success rates at IOP values of ≤21, 18, and 15 mmHg are presented in . Among 170 eyes with data at 6 months follow-up, complete success (no use of IOP-lowering medication) was achieved in 78.8% of eyes with IOP values ≤21 mmHg. Qualified success (use of ≤2 IOP-lowering medications) at month 6 was noted in 90.6% of eyes with IOP values ≤21 mmHg. Surgical success at all subsequent time points through 36 months of follow-up generally paralleled these findings, though there appeared to be a slight reduction in eyes with complete success at longer follow-up intervals. At months 12, 24, and 36, complete success decreased from 67.3% to 66.1% to 63.4% of eyes with IOP values ≤21 mmHg, respectively. Additionally, the number of eyes with available data at each time point decreased.
Table 4 CompleteTable Footnotea and qualifiedTable Footnoteb surgical success rates (%) at postoperative month 6, month 12, year 2, and year 3
Safety outcomes
As expected, most postsurgical complications occurred in the early postoperative period. Hyphema was the most frequently observed early postoperative complication, occurring in 126/259 (48.7%), 39/255 (15.3%), and 1/244 (0.4%) of eyes at postoperative day 1, week 1, and month 3, respectively. The majority of observed cases were classified as microhyphema (54.5% of day 1 cases and 73.7% of week 1 cases), with a maximum severity recording of Grade II found in only three eyes at day 1. All cases of hyphema resolved spontaneously with no interventions required. Injection was noted in ≤4% of eyes at day 1, week 1, and months 1, 3, and 6.
The proportion of subjects with categorically high or low IOP was recorded at each follow-up visit. A high IOP (≥30 mmHg) was noted in 13/244 (5.3%) eyes at week 1, and rarely (≤1.8% eyes) thereafter. IOP elevations did not lead to discontinuation of LE use. Instances of hypotony (IOP <5 mmHg) occurred in 50/240 (20.8%), 7/244 (2.9%), 13/233 (5.6%), and 5/201 (2.5%) of eyes at postoperative day 1, week 1, month 1, and month 3, respectively, and in smaller percentages of eyes at subsequent visits. There was no intervention in cases of high or low IOP. Cataract formation was not noted in any of the phakic eyes during the follow-up period. There were no AE reports considered related to postoperative treatment with LE.
Discussion
These real-world findings in glaucoma patients who underwent canaloplasty with or without cataract surgery and were treated postoperatively with LE support the long-term safety and efficacy of this minimally invasive surgical procedure. It also corroborates a growing body of research on a variety of ocular surgery types supporting the clinical benefits and safety of LE formulations when used for control of inflammation during the postoperative period.Citation47,Citation48,Citation56,Citation66–Citation68 While the gold standard for surgical management of glaucoma has traditionally been trabeculectomy, canaloplasty is increasingly being recognized as a much less invasive and maximally effective technique for achieving long-term IOP control.Citation29
Canaloplasty lowers IOP by restoring the eye’s natural trabeculocanalicular outflow system, thereby obviating the need for a subconjunctival filtering bleb, simplifying postoperative care, and reducing the likelihood of serious surgical complications inherent to trabeculectomy.Citation15–Citation21,Citation30,Citation31 Canaloplasty patients have also been found to experience a higher degree of satisfaction and less quality-of-life impairment after surgery when compared to trabeculectomy patients.Citation25
In this observational study, mean IOP was reduced significantly from baseline and sustained over follow-up periods of up to 3 years along with significantly reduced use of adjunctive IOP-lowering medications. At 3 years of follow-up, about half of the eyes met the most stringent criteria for complete success, defined as an IOP ≤15 mmHg and no concurrent use of IOP-lowering medications. About two-thirds of eyes at 3 years of follow-up had IOP ≤21 mmHg without concurrent IOP-lowering medication use. Although VA decreased during the first postoperative month relative to baseline, all subsequent measures were either similar or significantly improved compared to baseline.
Previous studies evaluating the efficacy of canaloplasty with 2–3 years of follow-up closely corroborate these findings, reporting mean IOPs of 13.1–17.0 mmHg (34%–42.2% reductions from baseline), significant decreases in medication use, and complete (IOP ≤21 mmHg without medications) and qualified (IOP ≤21 mmHg with or without medications) success rates of 44.8%–77.5% and 81.6%–86.2%, respectively.Citation15–Citation18,Citation20,Citation21,Citation27 A recently reported canaloplasty study with 5 years of follow-upCitation69 noted the need for additional surgery for IOP control in 13 of 20 (65%) eyes at this time point, though the sample size was small. Ongoing collection of longer term data in larger studies will be needed to fully measure the long-term success of the procedure.
Despite the increasing literature on favorable outcomes with canaloplasty, there are no studies specific to pharmacologic management of these patients in the postoperative period. The typically recommended regimen of antibiotics coupled with a tapered dose of corticosteroids over 3–4 weeksCitation29,Citation34,Citation66 is based on general guidelines for ocular surgery and does not account for the unique challenges of canaloplasty patients, who (unlike the trabeculectomy patient) continue to rely on the eye’s natural outflow system to regulate IOP.Citation29,Citation34 Recent in vitro and in vivo studies suggest that eyes diagnosed with POAG have a smaller, less observable Schlemm’s canal, decreased trabecular meshwork thickness, and increased trabecular meshwork stiffness when compared to healthy eyes,Citation70–Citation73 and, therefore, are more susceptible to IOP spikes.Citation74,Citation75 The canaloplasty procedure may illicit early postsurgical IOP spikes because of the eye’s inflammatory response to surgery. This may impede aqueous humor passing into the collector channels in the immediate postoperative period.Citation17,Citation18,Citation29,Citation34 Similar findings of early postoperative IOP spikes have also been reported in glaucoma patients undergoing cataract surgery.Citation76–Citation78
While topical corticosteroids are important for the control of postoperative inflammation, their use in and of themselves can be associated with IOP elevations, especially in patients with a history of glaucoma.Citation35,Citation36,Citation38,Citation40 Corticosteroids can elevate IOP by causing a decrease in aqueous flow through the trabecular meshwork, the primary pathway for outflow. Possible mechanisms include increased extracellular matrix deposition, increased cross-linking of actin fibers between cells, and an inhibition of phagocytosis.Citation38,Citation40 Considering the aforementioned sensitivity of the canaloplasty patient to changes in the trabeculocanalicular outflow system following surgery, corticosteroid-induced IOP elevation has particular relevance in this setting.
In the author’s practice, LE suspension or now the newer LE gel formulation is a standard part of postcanaloplasty surgery management. LE was developed using a retrometabolic design with the intention of lessening risks of IOP elevation and other unwanted effects of topical corticosteroid therapy.Citation43 The chemical structure of LE is such that any drug that is not bound to the glucocorticoid receptor undergoes rapid conversion into inactive metabolites, allowing for localized, controlled suppression of ocular inflammation with minimized potential for causing unwanted side effects.Citation41,Citation42,Citation44,Citation45 Studies comparing LE suspension or gel to other corticosteroids, including dexamethasone,Citation51,Citation57,Citation59–Citation61 prednisolone,Citation54–Citation56,Citation58,Citation79,Citation80 and flurometholone,Citation50,Citation81,Citation82 have consistently found that LE has lower impact on IOP, while retaining the desired anti-inflammatory effects of a corticosteroid. A recent review by Sheppard et al of the available published data on the effect of marketed LE formulations on IOP found that LE consistently demonstrated a low propensity to elevate IOP, regardless of the formulation, dosage regimen, or treatment duration, including in known steroid responders.Citation53
In this real-world cohort of canaloplasty patients, postoperative management with LE suspension 0.5% appeared to be safe and effective. In accordance with numerous previous studies of LE suspension or LE gel following ocular surgery,Citation47,Citation48,Citation56,Citation66–Citation68 postoperative inflammation was reduced as evidenced by AC cell and flare data with minimal clinically significant IOP elevations. In the current study, no AEs considered related to treatment with LE suspension were reported. The immediate and sustained decreases in IOP beginning 1 day after surgery and maintained over a period of up to 3 years likely reflect both the efficacy of the canaloplasty procedure as well as the low propensity of LE for inducing IOP elevations in the short term following the procedure. Early postoperative IOP spikes have been found in up to 20.3% of canaloplasty cases in the literature,Citation31 though this complication is generally reported at lower rates even in the presence of postoperative topical corticosteroid use.Citation16–Citation20 However, differences in drug regimen and definition of an IOP spike confound comparisons across studies.
Other postoperative complications among patients in this review were mostly transient, required few interventions, and were typical for this type of surgical procedure.Citation16,Citation17,Citation83 Hyphema was the most commonly observed and occurred in 48.7% of eyes at postoperative day 1, with the majority of cases classified as microhyphema. Spontaneous resolution by month 3 was recorded for all eyes. Previous studies have reported similar findings of inconsequential hyphema in 6.1%–85.2% of canaloplasty cases,Citation16–Citation18,Citation21 and one studyCitation84 concluded that the presence of microhyphema on the first postoperative day following canaloplasty might have positive prognostic value with regard to IOP reduction, as a reflux of blood may signify a patent trabecular meshwork. Hypotony, reported in 0.6%–27.6% of cases in the literature,Citation16–Citation18,Citation21,Citation30 was documented in 20.8% of eyes at day 1 in this retrospective review, and all cases resolved without intervention.
The main limitations of this study are those typical of retrospective chart review studies, including nonrandomization, which might lead to selection bias, incompleteness of chart data for outcomes of interest, and the lack of a control group. Furthermore, data gathering was limited primarily to objective findings, precluding evaluations of pain relief or patient satisfaction. A small number of eyes received a non-LE steroid in the early postoperative period, and thus, these data were excluded from the analysis. However, it may be that these cases would have had less favorable outcomes if continued solely on LE. Given safety data were limited to information previously recorded during routine patient follow-up visits, minor complaints and less serious AEs may have been missed, though it is presumed that events of clinical significance would have been recorded.
Conclusion
Findings from this retrospective chart review point to the long-term safety and effectiveness of the canaloplasty procedure performed with or without cataract surgery and managed postoperatively with LE suspension 0.5%. Complications related to the surgical procedure were mostly transient and comparable to those noted in previous canaloplasty studies. No adverse reactions considered related to LE were noted. Significant IOP reduction was achieved rapidly and sustained over a period of years with minimal need for adjunct medication use. Postoperative use of LE was associated with good control of postsurgical inflammation without apparent exacerbation of IOP. These data add to the increasing body of research supporting the clinical benefits and safety of LE 0.5% use during the postoperative period for a variety of ocular surgery types.
Author contributions
MK substantially contributed to concept and design, data acquisition, and analysis and interpretation; he contributed to article drafting and revision and gave final approval of this version to be published; he is accountable for all aspects of the work and will ensure that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Acknowledgments
Writing assistance was provided by Rachel Hathcock, BSN, and Sandra Westra, PharmD, of Churchill Communications (Maplewood, NJ, USA) and was funded by Bausch + Lomb, Inc. The author retained full control over the manuscript content. This study was sponsored by Bausch + Lomb as an independent research grant.
Disclosure
The author reports no conflicts of interest in this work.
References
- HeijlALeskeMCBengtssonBReduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma TrialArch Ophthalmol2002120101268127912365904
- KassMAHeuerDKHigginbothamEJOcular Hypertension Treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol2002120670171312049574
- LeskeMCHeijlAHusseinMFactors for glaucoma progression and the effect of treatment: the early manifest glaucoma trialArch Ophthalmol20031211485612523884
- The Advanced Glaucoma Intervention Study (AGIS): 7The relationship between control of intraocular pressure and visual field deterioration. AGIS investigatorsAm J Ophthalmol2000130442944011024415
- LeePPWaltJWRosenblattLCSiegartelLRSternLSGlaucoma CareSGAssociation between intraocular pressure variation and glaucoma progression: data from a United States chart reviewAm J Ophthalmol2007144690190717919446
- MuschDCGillespieBWNiziolLMLichterPRVarmaRCigtsSGIntraocular pressure control and long-term visual field loss in the collaborative initial glaucoma treatment studyOphthalmology201111891766177321600658
- StewartWCKolkerAESharpeEDFactors associated with long-term progression or stability in primary open-angle glaucomaAm J Ophthalmol200013027427911020404
- FingeretMMancilGLBaileyILOptometric Clinical Practice Guideline. Care of the Patient with Open Angle GlaucomaSt. Louis, MOAmerican Optometric Association2010 Available from: http://www.aoa.org/documents/optometrists/CPG-9.pdfAccessed March 6, 2017
- PrumBERosenbergLFGeddeSJPrimary open-angle glaucoma preferred practice pattern® guidelinesOphthalmology20161231P41P11126581556
- CairnsJETrabeculectomy preliminary report of a new methodAm J Ophthalmol19686646736794891876
- RazeghinejadMRFudembergSJSpaethGLThe changing conceptual basis of trabeculectomy: a review of past and current surgical techniquesSurv Ophthalmol201257112522137574
- DeBryPWPerkinsTWHeatleyGKaufmanPBrumbackLCIncidence of late-onset bleb-related complications following trabeculectomy with mitomycinArch Ophthalmol2002120329730011879132
- PoulsenEAllinghamRCharacteristics and risk factors of infections after glaucoma filtering surgeryJ Glaucoma20009643844311131749
- JampelHMuschDGillespieBLichterPWrightMGuireKPerioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS)Am J Ophthalmol20051401162215939389
- MatlachJDhillonCHainJSchlunckGGrehnFKlinkTTrabeculectomy versus canaloplasty (TVC study) in the treatment of patients with open-angle glaucoma: a prospective randomized clinical trialActa Ophthalmol201593875376125847610
- BullHvon WolffKKörberNTetzMThree-year canaloplasty outcomes for the treatment of open-angle glaucoma: European study resultsGraefes Arch Clin Exp Ophthalmol2011249101537154521732110
- BrusiniPCanaloplasty in open-angle glaucoma surgery: a four-year follow-upScientificWorldJournal2014162014 469609
- BrusiniPCaramelloGBenedettiSTosoniCCanaloplasty in open-angle glaucoma: mid-term results from a multicenter studyJ Glaucoma201425403407
- GandolfiSAUngaroNGhirardiniSTardiniMGMoraPComparison of surgical outcomes between canaloplasty and Schlemm’s canal scaffold at 24 months’ follow-upJ Ophthalmol20162016341046926989497
- GrieshaberMCPienaarAOlivierJStegmannRCanaloplasty for primary open-angle glaucoma: long-term outcomeBr J Ophthalmol201094111478148220962352
- LewisRAvon WolffKTetzMCanaloplasty: three-year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucomaJ Cataract Refract Surg201137468269021420593
- RulliEBiagioliERivaIEfficacy and safety of trabeculectomy vs. nonpenetrating surgical procedures: a systematic review and meta-analysisJAMA Ophthalmol2013131121573158224158640
- LinZXuSHuangSZhangXZhongYComparison of canaloplasty and trabeculectomy for open angle glaucoma: a meta-analysisInt J Ophthalmol20169121814181928003985
- MatlachJFreibergFJLeippiSGrehnFKlinkTComparison of phacotrabeculectomy versus phacocanaloplasty in the treatment of patients with concomitant cataract and glaucomaBMC Ophthalmol201313123360243
- KlinkTSauerJKörberNJQuality of life following glaucoma surgery: canaloplasty versus trabeculectomyClin Ophthalmol2015971625565763
- SchoenbergEDChaudhryALChodRZurakowskiDAyyalaRSComparison of surgical outcomes between phacocanaloplasty and phacotrabeculectomy at 12 months’ follow-up: a longitudinal cohort studyJ Glaucoma201524754354924240873
- LewisRAvon WolffKTetzMCanaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: interim clinical study analysisJ Cataract Refract Surg20073371217122617586378
- ShingletonBTetzMKorberNCircumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: one-year resultsJ Cataract Refract Surg200834343344018299068
- KhaimiMACanaloplasty: a minimally invasive and maximally effective glaucoma treatmentJ Ophthalmol2015201548506526495135
- RekasMByszewskaAPetzKWierzbowskaJJünemannACanaloplasty versus non-penetrating deep sclerectomy – a prospective, randomised study of the safety and efficacy of combined cataract and glaucoma surgery; 12-month follow-upGraefes Arch Clin Exp Ophthalmol2015253459159925795059
- ArthurSNCantorLBWuDunnDEfficacy, safety, and survival rates of IOP lowering effect of phacoemulsification alone or combined with canaloplasty in glaucoma patientsJ Glaucoma201423531632023377581
- AyyalaRSChaudhryALOkogbaaCBZurakowskiDComparison of surgical outcomes between canaloplasty and trabeculectomy at 12 months’ follow-upOphthalmology2011118122427243321856008
- TetzMKoerberNShingletonBJPhacoemulsification and intraocular lens implantation before, during, or after canaloplasty in eyes with open-angle glaucoma: 3-year resultsJ Glaucoma201524318719423429620
- CaginiCPeruzziCFioreTSpadeaLLipperaMLipperaSCanaloplasty: current value in the management of glaucomaJ Ophthalmol20162016708047527239337
- McGheeCNDeanSDanesh-MeyerHLocally administered ocular corticosteroids: benefits and risksDrug Saf2002251335511820911
- ClarkAFWilsonKde KaterAWAllinghamRRMcCartneyMDDexamethasone-induced ocular hypertension in perfusion-cultured human eyesInvest Ophthalmol Vis Sci1995364784897843916
- RajpalRKDigbyDD’AversaGMahFHollanderDAConwayTIntraocular pressure elevations with loteprednol etabonate: a retrospective chart reviewJ Ocul Pharmacol Ther201127330530821574815
- PleyerUUrsellPGRamaPIntraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same?Ophthalmol Ther201322557225135807
- YablonskiMEBurdeRMKolkerAEBeckerBCataracts induced by topical dexamethasone in diabeticsArch Ophthalmol1978963474476629687
- KerseyJPBroadwayDCCorticosteroid-induced glaucoma: a review of the literatureEye200620440741615877093
- BhardwajYRPareekAJainVKishoreDChemical delivery systems and soft drugs: retrometabolic approaches of drug designSaudi Pharm J201422429030225161372
- HochhausGChenLDerendorfHPharmacokinetic characterization and tissue distribution of the new glucocorticoid soft drug loteprednol etabonate in rats and dogsJ Pharm Sci19928112121012151491342
- BodorNLoftssonTWuWMMetabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonatePharm Res1992910127512781448425
- DruzgalaPWuWMBodorNOcular absorption and distribution of loteprednol etabonate, a soft steroid, in rabbit eyesCurr Eye Res199110109339371959381
- WuWHuangFLeeYBuchwaldPBodorNPharmacokinetics of the sequential metabolites of loteprednol etabonate in ratsJ Pharm Pharmacol200860329129718284808
- HowesJNovackGDFailure to detect systemic levels, and effects of loteprednol etabonate and its metabolite, PJ-91, following chronic ocular administrationJ Ocul Pharmacol Ther19981421531589572541
- StewartRHorwitzBHowesJNovackGDHartKDouble-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol etabonate post-operative inflammation study group 1J Cataract Refract Surg19982411148014899818338
- A double-masked, placebo-controlled evaluation of 0.5% loteprednol etabonate in the treatment of postoperative inflammation. The loteprednol etabonate postoperative inflammation study group 2Ophthalmology19981059178017869754192
- ZhangQ-SWangXWangZ-HZhangS-ZWangLXuW-XInfluence of topical Lotemax on intraocular pressure following excimerChin J Exp Ophthalmol2013316551554
- GaoDHLiKJLuHClinical observations on the effect of Lotemax on changes in intra-ocular pressure after LASEK [sic: LASIK]Int J Ophthalmol2010109806807
- WuJSZhongXWZhangXXLiuQA randomized controlled study on the application of 0.5% loteprednol etabonate eye drops after LASIK surgeryChin J Exp Ophthalmol2012307641645
- MifflinMDLeishmanLLChristiansenSMSikderSHsuMMoshirfarMUse of loteprednol for routine prophylaxis after photo-refractive keratectomyClin Ophthalmol2012665365922570546
- SheppardJDComstockTLCavetMEImpact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressureAdv Ther201633453255226984315
- BartlettJDHorwitzBLaibovitzRHowesJFIntraocular pressure response to loteprednol etabonate in known steroid respondersJ Ocul Pharmacol1993921571658345288
- HollandEJDjalilianARSandersonJPAttenuation of ocular hypertension with the use of topical loteprednol etabonate 0.5% in steroid responders after corneal transplantationCornea200928101139114319770719
- LaneSSHollandEJLoteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgeryJ Cataract Refract Surg201339216817323232255
- ThanathaneeOSriphonPAnutarapongpanOA randomized controlled trial comparing dexamethasone with loteprednol etabonate on postoperative photorefractive keratectomyJ Ocular Pharmacol Ther2015313165168
- Loteprednol Etabonate US Uveitis Study GroupControlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitisAm J Ophthalmol199912753754410334346
- WhiteEMMacyJIBatemanKMComstockTLComparison of the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitisCurr Med Res Opin20082428729618062846
- ChenMGongLSunXA multicenter, randomized, parallel-group, clinical trial comparing the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of Chinese patients with blepharokeratoconjunc-tivitisCurr Med Res Opin20122838539422256909
- HollandEJBartlettJDPaternoMRUsnerDWComstockTLEffects of loteprednol/tobramycin versus dexamethasone/tobramycin on intraoc-ular pressure in healthy volunteersCornea2008271505518245967
- WangQHarasymowyczPShort-term intraocular pressure elevations after combined phacoemulsification and implantation of two trabecular micro-bypass stents: prednisolone versus loteprednolJ Ophthalmol2015201534145026266045
- RebenitschRLBrownENBinderNREffect of topical loteprednol on intraocular pressure after selective laser trabeculoplasty for open-angle glaucomaOphthalmol Ther20132211312025135811
- International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonized tripartite guideline: guideline for good clinical practiceJ Postgrad Med2001471455011590294
- KhaimiMACanaloplasty using iTrack 250 microcatheter with suture tensioning on schlemm’s canalMiddle East Afr J Ophthalmol200916312712920142977
- RajpalRKRoelLSiou-MermetRErbTEfficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgeryJ Cataract Refract Surg201339215816723218817
- ComstockTLPaternoMRSinghAErbTDavisESafety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgeryClin Ophthalmol2011517718621383946
- FongRLeitritzMSiou-MermetRErbTLoteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicenter trialClin Ophthalmol201261113112422888209
- VoykovBBlumenstockGLeitritzMDimopoulosSAlnahrawyOTreatment efficacy and safety of canaloplasty for open-angle glaucoma after 5 yearsClin Exp Ophthalmol201543876877125952140
- YanXLiMChenZZhuYSongYZhangHSchlemm’s canal and trabecular meshwork in eyes with primary open angle glaucoma: a comparative study using high-frequency ultrasound biomicroscopyPLoS One2016111e014582426726880
- HongJXuJWeiASpectral-domain optical coherence tomographic assessment of Schlemm’s canal in Chinese subjects with primary open-angle glaucomaOphthalmology2013120470971523352198
- HannCRVercnockeAJBentleyMDJorgensenSMFautschMPAnatomic changes in Schlemm’s canal and collector channels in normal and primary open-angle glaucoma eyes using low and high perfusion pressuresInvest Ophthalmol Vis Sci20145595834584125139736
- HuangJCamrasLJYuanFMechanical analysis of rat trabecular meshworkSoft Matter201511142857286525710888
- StamerWThe cell and molecular biology of glaucoma: mechanisms in the conventional outflow pathwayInvest Ophthalmol Vis Sci20125352470247222562843
- BrubakerRTargeting outflow facility in glaucoma managementSurv Ophthalmol200348Suppl 1S17S2012852430
- Levkovitch-VerbinHHabot-WilnerZBurlaNIntraocular pressure elevation within the first 24 hours after cataract surgery in patients with glaucoma or exfoliation syndromeOphthalmology2008115110410817561259
- GuptaAVernonSAIs the 1-day postoperative IOP check needed post uncomplicated phacoemulsification in patients with glaucoma and ocular hypertension?Eye201529101299130725697456
- ChenPPLinSCJunkAKRadhakrishnanSSinghKChenTCThe effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of OphthalmologyOphthalmology201512271294130725943711
- NovackGDHowesJCrockettRSSherwoodMBChange in intraocular pressure during long-term use of loteprednol etabonateJ Glaucoma1998742662699713785
- PriceMOFengMTScanameoAPriceFWLoteprednol etabonate 0.5% gel vs. prednisolone acetate 1% solution after descemet membrane endothelial keratoplasty: prospective randomized trialCornea201534885385826020827
- LiWTLiHWeiJClinical study on the application of 5 g/L loteprenol etabonate ophthalmic suspension after LASIKInt J Ophthalmol201010917921793
- CoffeyMJDecoryHHLaneSSDevelopment of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic dropClin Ophthalmol2013729921223430378
- BrandãoLMGrieshaberMCUpdate on minimally invasive glaucoma surgery (MIGS) and new implantsJ Ophthalmol2013201370591524369494
- GrieshaberMCSchoetzauAFlammerJOrgülSPostoperative microhyphema as a positive prognostic indicator in canaloplastyActa Ophthalmol201391215115622151545
