Abstract
Objective
There is little evidence of real-life outcomes of dietary supplementation with high-dose docosahexaenoic acid (DHA) and carotenoids in patients with diabetic retinopathy (DR). We assessed the effect of supplementation with DHA triglyceride (1,050 mg/d) + xanthophyll carotenoid multivitamin on macular function in nonproliferative DR.
Methods
Asymptomatic patients with nonproliferative DR were included in a prospective controlled study and assigned (1:1) to the DHA supplementation group or the control group. Macular sensitivity and macular integrity area were the main outcome measures. Functional vision measures (macular function [MAIA™ CenterVue], best-corrected visual acuity), structural retinal measures (central subfield macular thickness), and biochemical parameters (plasma total antioxidant capacity, DHA content of the erythrocyte membrane, and plasma IL-6) were evaluated at baseline and after 45 and 90 days of DHA supplementation.
Results
The study included 24 patients (48 eyes) (12 patients, 24 eyes in each group). Baseline clinical characteristics of patients in both groups were similar. Macular sensitivity increased from a mean (SD) of 25.9 (2.4) dB at baseline to 27.3 (2.3) dB at 90 days (P=0.030) in the DHA group only (between-group differences P<0.19). The macular integrity index decreased from 71.2 (33.2) at baseline to 63.5 (36.4) at 45 days and to 51.6 (35.9) at 90 days (P=0.002) in the DHA group only (between-group differences P<0.05). Best-corrected visual acuity and central subfield macular thickness did not vary significantly in any of the comparisons and in none of the groups. DHA content of erythrocyte membrane and total antioxidant capacity levels increased significantly only in the DHA group. Plasma IL-6 levels decreased significantly only in the DHA group.
Conclusion
In an early stage of DR, supplementation with high-dose DHA plus xanthophyll carotenoid multivitamin during 90 days was associated with a progressive and significant improvement of macular function measured by microperimetry. Biochemical changes supported the effect of DHA.
Introduction
Diabetic retinopathy (DR) is a microangiopathic complication of diabetes and the leading cause of vision loss in working-age adults.Citation1 Approximately 1 in 3 people living with diabetes have some degree of DR, and 1 in 10 will develop a vision-threatening form of the disease. Diabetes mellitus is becoming a global epidemic, with 415 million people having diabetes, and the number is expected to increase to 642 million by 2040.Citation2 Approximately 75% of the global diabetes burden occurs in low- and middle-income countries, and 46.5% of adults with the disease are underdiagnosed.Citation2 These alarming statistics urgently require effective strategies for prevention, early diagnosis, and appropriate treatment. Good glycemic control and treatment of modifiable risk factors have been extensively recognized as essential factors for the successful ophthalmic care of patients with diabetes.Citation3–Citation5
The pathogenesis of DR is multifactorial, and a number of interconnecting events including capillary endothelial vascular dysfunction and retinal pericyte loss as a result of hyperglycemia; local inflammatory activity with upregulation of proinflammatory mediators, interleukins, and growth factors; cellular hypoxia; oxidative stress; breakdown of the blood–retinal barrier; and retinal neurodegeneration have been reported.Citation6–Citation11 Full understanding and the sequence of these underlying mechanisms remains unclear. The pleotropic mechanisms of action of long-chain polyunsaturated fatty acids (ω-3 PUFAs), particularly docosahexaenoic acid (DHA),Citation12–Citation22 as well as the xanthophylls, lutein and zeaxanthin,Citation23–Citation25 are involved in the molecular pathways implicated in DR ()Citation15,Citation17,Citation23–Citation34 and support the rationale of dietary supplementation with DHA and xanthophyll carotenoids in DR.
Figure 1 Effects of DHA/ω-3 fatty acids and lutein/zeaxanthin on pathways leading to DR.
However, there is little evidence of real-life outcomes of dietary supplementation with DHA in patients with DR. In a randomized controlled study of patients with diabetic macular edema (DME) treated with intravitreal ranibizumab, the addition of a dietary supplement rich in DHA reduced macular thickness after 2 years of follow-up as compared with ranibizumab alone.Citation35 This anatomical improvement was accompanied by a trend for an amelioration of visual acuity.Citation35 In patients with DR and well-controlled diabetes, increasing PUFA intake was associated with a reduced likelihood of the presence and severity of DR.Citation36 Interestingly, in a study of lipidomic analyses on red blood cell membranes from controls and type 2 diabetes patients, a significant decrease in levels of DHA and arachidonic acid in erythrocytes of diabetic patients with and without retinopathy was observed.Citation37 This observations provide a good rationale to supplement the diet with DHA, although DHA is mainly used for its anti-inflammatory, antioxidant, and antiangiogenic effects.Citation14–Citation17,Citation22
The possibility that DHA supplementation could prevent the progression of DR acting on an early stage of the disease is an appealing hypothesis. To this end, a prospective controlled study was designed to assess the effectiveness of dietary supplementation with a high rich DHA oral nutraceutical formulation in patients with non-proliferative DR (NPDR). Microperimetry, a relatively new and extremely sensitive method for studying retinal and optic nerve diseases, was used to assess macular function. Evaluation of subtle changes in macular function with an objective method, such as microperimetry, may support the advantage of early DHA supplementation in diabetic subjects with incipient DR. To our knowledge, studies on macular function using microperimetry in asymptomatic patients with type 2 diabetes and NPDR have not been previously reported.
Methods
This study was approved by the Ethics Committee of Hospital Universitario Virgen de la Arrixaca (Murcia, Spain) and adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent. The study was registered in the European Clinical Trials Database (EudraCT) (EudraCT trial number 2017-000856-25 for the Sponsor’s Protocol code number DHA/RDNP).
Study design and participants
A prospective controlled study was conducted at the outpatient clinics of the Service of Ophthalmology of an 860-bed acute care teaching hospital in Murcia, Spain. Patients of both sexes aged >18 years were invited to participate in the study during an ophthalmologic appointment at the study center. All patients had type 2 diabetes mellitus and were asymptomatic at the time of enrollment. They had been diagnosed with NPDR elsewhere and were referred to our service for a full vision evaluation between June 2014 and June 2016. The diagnosis of NPDR in all referred asymptomatic type 2 diabetes patients was confirmed, with fundus examination showing the presence of microaneurysms and some isolated hard exudate. Clinically significant macular edema was absent. Patients unable to participate in the study according to the criteria of the ophthalmologist and those who refused to sign the written consent were excluded from the study as were patients using vitamin/mineral or fatty acids supplements and those with hypersensitivity to these compounds.
Study intervention
Each participant contributed 2 study eyes to the protocol. The diagnosis of NPDR according to criteria of the Early Treatment Diabetic Retinopathy Study (ETDRS)Citation38 was confirmed before enrollment. The diagnosis was based on clinical examination and retinography and was made by 2 specialized ophthalmologists. Study patients were consecutively assigned with a 1:1 sequential allocation to the DHA supplementation (experimental) group or to the control group. The inclusion criteria were identical for all study patients. Subjects in the control group received no treatment at all and were masked regarding the existence of the experimental group. Patients in the DHA group received a high rich DHA (1,050 mg/d) nutraceutical formulation (Brudyretina 1.5 g; Brudy Lab, S.L., Barcelona, Spain). This is a concentrated DHA triglyceride having a high antioxidant activity patented to prevent cellular oxidative damage.Citation39,Citation40 shows the composition of the nutraceutical formulation, which includes a high dose of DHA (1 g), eicosapentaenoic acid, a mixture of B vitamins, vitamins C and E, lutein, zeaxanthin, and minerals. All fatty acids were present in the form of triglycerides (>95%) or ethyl esters (<5%). Patients were instructed to take 3 capsules of Brudyretina 1.5 g once daily. The treatment evaluator (M.E.R.G-H.) was masked of which subjects were receiving DHA supplementation.
Table 1 Composition of Brudyretina 1.5 g (Brudy Lab S.L.), per capsule
Study procedures and outcome
The duration of the study was 90 days. All patients were evaluated at baseline and at 45 and 90 days thereafter. At each visit, best-corrected visual acuity (BCVA) and measurement of central subfield macular thickness (CSMT) by spectral-domain optical coherence tomography (Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA, USA) were assessed. BCVA was assessed using the ETDRS optotype at 2 m distance from the observer. Other investigations included retinography (VISUCAM®PRO NM, Zeiss) and assessment of macular function by microperimetry (MAIA 1™ CenterVue, Topcon España, S.A., Sant Just Desvern, Barcelona, Spain). Macular function included assessment of retinal sensitivity (10° diameter area) and macular integrity index. The MAIA sensitivity scale is 0–36 dB. The macular integrity index is a numerical value that describes the likelihood that a patient’s responses are normal, suspect, or abnormal when compared to age-adjusted normative data. Higher numbers suggest a greater likelihood of abnormal findings, while lower values suggest a greater likelihood of normal findings.
At the beginning of the study and at 90 days, the following were measured: erythrocyte membrane DHA content, plasma total antioxidant capacity (TAC), and plasma levels of IL-6. The National Eye Institute Visual Function Questionnaire-25 (NEI-VFQ-25)Citation41 was also evaluated at baseline and at 45 and 90 days. Total score of the NEI-VFQ-25 ranges between 0 and 100 (a high score represents better functioning).
At each visit, the nutraceutical formulation was delivered to the patient for 45-day treatment. Compliance with DHA supplementation was assessed at the study visits by return of supplementation tablet counts and analytical data, especially erythrocyte membrane DHA content. Ophthalmologists paid special care to insist on the importance of compliance with the dietary supplement and the benefit that the patient may receive from the supplement.
TAC in plasma samples was measured using the OxiSelect Total Capacity Assay kit (STA-360, Cell Biolabs Inc., San Diego, CA, USA) following the manufacturer’s instructions. High values in the TAC assay reflect high antioxidant capacity (ie, greater protection). Uric acid equivalent was used to calculate copper-reducing equivalent values (µM copper-reducing equivalent). The composition of fatty acids on the erythrocyte membrane (ω-3 DHA) was determined using the method described by Lepage and Roy,Citation42 analyzed by gas chromatography–mass spectrometry and identified by comparing the elution pattern and relative retention times of fatty acid (FA) methyl esters with a reference FA methyl esters mixture (GLC-744 Nu-Chek Prep. Inc., Elysian, MN, USA). The results were expressed in relative amounts (% of total FA). Plasma levels of IL-6 were measured by a solid-phase sandwich enzyme-linked immunosorbent assay with a commercial kit (Human IL-6 ELISA Kit, Cat. No 950.030 purchased from Diaclone SAS, Besancon Cedex, France). This assay recognizes both natural and recombinant human IL-6. The instructions provided by the manufacturer were followed for the qualitative and quantitative determination of IL-6. The samples or standards were added in wells of the microtiter strip plate coated with anti-IL-6 monoclonal. Then, a biotin-conjugated monoclonal anti-IL-6 antibody was added and bonded to IL-6 captured by the first antibody. Later enzyme-linked secondary antibody (Streptavidin-HRP) was added, and bonded to detecting antibody. Ultimately, a chromogen substrate was added and enzymatically converted to detectable form by absorbance and measured at a wavelength of 450 nm with a microplate reader Synergy H1 Hybrid Multimode (BioTek Instruments, Winooski, VT, USA).
The primary outcome of the study was the changes in macular sensitivity and macular integrity index during the study period. Secondary outcome variables were BCVA, CSMT, plasma TAC, erythrocyte membrane DHA content, plasma IL-6 levels, and the NEI-VFQ-25 score.
Statistical analysis
The sample size calculation was based macular sensitivity as the main variable of the study. Considering a standard deviation of 2.3 dB as reported by Roisman et alCitation43 with a precision of 1.7 dB, an α risk of 5% and a power of 80%, the sample size per group was 23 eyes. Assuming 5% losses at follow-up, 24 eyes per group were needed (total 48 eyes).
Analysis of visual function tests, including microperimetry, BCVA, and CSMT, was based on 48 eyes (24 eyes in each study group), whereas analysis of laboratory tests was based on 24 patients (12 patients in each study group). Categorical data are expressed as frequencies and percentages and continuous data as mean and standard deviation (±SD). Mixed linear model analysis was used to assess differences in the study variables between DHA supplementation and control groups over the 90-day study period (covariate: baseline data of variables; random factor: patients). Statistical significance was set at P<0.05. Statistical analyses were performed with the Statistical Package for the Social Sciences, version 11.0 software (SPSS Inc., Chicago, IL, USA).
Results
A total of 62 eyes from 31 patients diagnosed with NPDR were referred for a full vision evaluation during the study period, but 7 patients (14 eyes) were excluded because of the presence of neovessels, high risk for proliferative retinopathy, or doubts regarding adherence to the study protocol. No drop outs were recorded, and results were based on analysis of the per-protocol data set. The study population included 24 patients (48 eyes) with type 2 diabetes. There were 17 men and 7 women, with a mean age of 60.6 years (range 36–79 years). As shown in , the clinical characteristics of patients were similar in the 2 study groups.
Table 2 Demographics and clinical characteristics of the study population
The mean ± SD values of all study variables at baseline and at 45 and 90 days are shown in . In relation to the primary outcome of the study (), macular sensitivity increased from a mean of 25.9±2.4 dB at baseline to 27.3±2.3 dB at 90 days (P=0.030) only in the DHA supplementation group (between-group differences P<0.19). Also, in the DHA supplementation group, the macular integrity index decreased from 71.2±33.2 at baseline to 51.6±35.9 at 90 days (P=0.002); differences between values at 45 days (63.5±36.4) and 90 days (51.6±35.9) were also statistically significant (P=0.03) (between-group differences P<0.05).
Figure 2 Changes of macular sensitivity (A) and macular integrity index (B) in the 2 study groups at 90 days as compared with baseline (n=24 eyes in each study group).
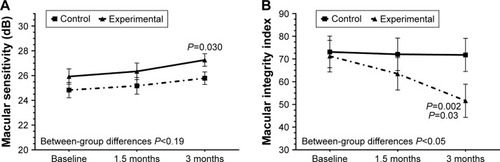
Table 3 Changes of macular function variables, visual acuity, CSMT, biochemical markers, and vision-related quality of life from baseline to 90 days after treatment with DHA supplementation as compared with controls
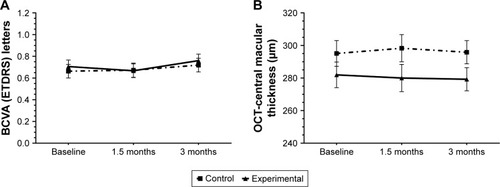
Statistically significant changes in BCVA () and CSMT () throughout the study period were not found in any of the comparisons and in none of the study groups. In relation to ω-3 DHA on the erythrocyte membrane, a significant increase at 90 days as compared with baseline (5.6%±0.8% vs 3.9%±0.6% total fatty acids, P<0.001) was only observed in the DHA supplementation group. In this case, between-group differences were also statistically significant (P<0.05).
Figure 3 Changes of BCVA (ETDRs letters) (A) and CSMT (B) in the 2 study groups at 90 days as compared with baseline.
Abbreviations: OCT, optical coherence tomography; BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; CSMT, central subfield macular thickness.
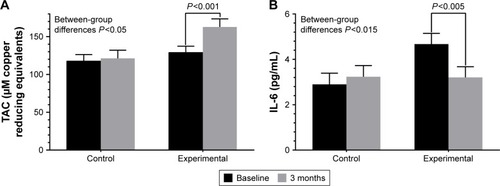
Plasma TAC values increased significantly from baseline as compared with 90 days only in the DHA supplementation group (between-group differences P<0.05) (), whereas serum levels of IL-6 decreased significantly from baseline as compared to 90 days only in the DHA supplementation group (between-group differences P<0.015) ().
Figure 4 Changes of plasma TAC (A) and plasma levels of IL-6 (B) the study groups at 90 days as compared with baseline (n=12 patients in each study group).
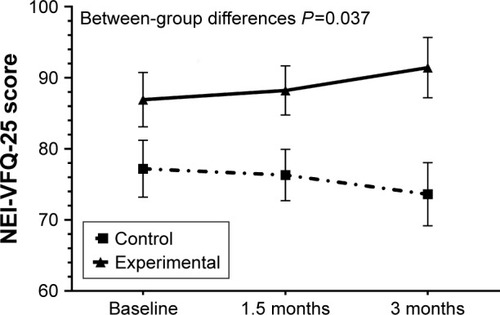
Vision-related quality of life showed a trend toward improvement in the DHA supplementation group at 45 and 90 days as compared with baseline, and a trend toward worsening in the control group (between-group differences P=0.037) ().
Figure 5 Changes in vision-related quality of life in the 2 study groups at 45 and 90 days as compared with baseline, with a clear trend toward improvement in the DHA supplementation group and toward worsening in the control group.
Abbreviations: NEI-VFQ-25, National Eye Institute Visual Function Questionnaire, near activities subscale score; DHA, docosahexaenoic acid.
The nutraceutical formulation was well tolerated and no adverse events were registered. In relation to compliance with the nutraceutical supplement, all patients in the DHA supplementation group reported having taking the three capsules each day of the study.
Discussion
Results of the present prospective controlled study performed in routine daily practice shows that oral supplementation based on a high-dose DHA formulation had a beneficial effect on macular function in asymptomatic patients with NPDR. After 3 months of oral supplementation with DHA, significant differences in macular sensitivity and macular integrity index as compared with baseline were observed only in the supplementation group. This finding is clinically relevant and may indicate that antioxidant and anti-inflammatory properties of DHA could play a contributing role on maintenance of macular function at early stages of DR. Also, maintenance and improvement of the quality of vision rather than simply visual acuity should be important in the prevention of visual loss in patients with diabetes.
The favorable results of macular function obtained in the DHA supplementation group are further enhanced by biochemical findings, including a significant decrease of plasma IL-6 levels and significant increases of plasma antioxidant protection and level of DHA in the erythrocyte membrane. In all cases, significant between-group differences were found. Although evidence of dietary supplementation with PUFAs to reduce the risk of age-related macular degeneration and the progression of the disease is based on numerous studies published in the literature,Citation44,Citation45 data on the use of DHA supplementation in DR is scarce,Citation46 particularly in asymptomatic patients with incipient retinopathy.
In a cross-sectional study of 51 patients with type 2 diabetes and NPDR, oxidative deregulation as compared with healthy volunteers was found, with increased levels of lipid peroxidation products, nitrites and nitrates, erythrocyte catalase activity, and glutathione peroxidase activity and decreased levels of TAC.Citation47 Other studies have shown that lipid peroxidation increases with the increase in severity and duration of diabetes.Citation48 In studies of experimental DR in rats, DHA and lutein were capable of normalizing all the diabetes-induced biochemical, histological, and functional modifications,Citation49,Citation50 which allowed their proposal as potential adjuvant therapies to help prevent vision loss in diabetic patients.Citation51
Newer technologies, such as microperimetry, are aimed at earlier detection of subtle deficits and enhancing diagnostic accuracy. In the last 15 years, microperimetry has been successfully used in the diagnosis and follow-up of different macular disorders, including age-related macular degeneration, myopic maculopathy, macular dystrophies, and DME. Different studies have shown a correlation between macular sensitivity determined by microperimetry and visual acuity in patients with clinically significant macular edema, as well as reduced macular sensitivity in relation to increasing macular thickness.Citation52 Microperimetry has also been useful for detailed study of the macular region for the assessment of morphological and functional outcome after intravitreal ranibizumab in patients with clinically significant DME.Citation53 However, as far as we are aware, studies assessing macular function by microperimetry in early stages of DR have not been previously published. In this respect, our study demonstrates the usefulness of microperimetry in this disease setting.
The present results should be interpreted taking into account some limitations of the study, especially the few patients included in the study groups and the fact that duration of the study was limited to 90 days only. However, different studies have shown that 90 days is a sufficient period of time for incorporation of ω-3 PUFAs in the erythrocyte membrane.Citation54,Citation55 Also, in previous studies using the same nutraceutical formulation for 90 days in ocular surface disorders, reduced expression of inflammatory biomarkers (IL-6, IL-10, IL-1β, and TNFα) in tear samples as well as improvements of dry eye symptoms as compared with the nonsupplemented groups were found.Citation56–Citation58 The study was not designed as a masked study because patients in the control group were not treated with placebo. Therefore, this situation could have influenced the results of variables that are more dependent on the subject’s subjectivity, such as the results of the NEI-VFQ-25 questionnaire. Also, the small sample size of only 12 patients in each study group may account for baseline differences in the baseline scores of the NEI-VFQ-25 questionnaire.
Compliance with the nutraceutical formulation was checked at the study visits by asking the patient to bring the empty box, but control over dietary intake of participants was lacking. No measure of dietary DHA was contemplated in the study protocol as it would be very unlikely that the usual Mediterranean diet consumed in our country would have a high DHA content of 1,050 mg/d similar to that administered with the nutraceutical supplementation. However, the significant increase in DHA content of the erythrocyte membrane is a reliable measure of good compliance. It may be argued that the fixed DHA daily dose of 1,050 mg/d does not fill all depending of body weight. In a study of 64 youth (7–14 years) with a diagnosis of mood disorder, a linear relationships between body weight and body mass index percentile with ω-3 PUFA accumulation has been reported.Citation59 However, the daily dose of DHA of 200 mg was markedly lower to that used in our patients, and DHA levels were determined in blood samples rather than in the erythrocyte membrane.
On the other hand, the rationale for use of DHA triglyceride form is based on a higher bioavailability of the compound.Citation60,Citation61 In addition, IL-6 was chosen because this cytokine has been found elevated in the vitreous fluid of diabetic patients with DR and has been shown to be a main contributor to the pathogenesis of DR.Citation62,Citation63
In a randomized, controlled clinical trial of patients with diabetes with no retinopathy or mild to moderate NPDR assigned to twice daily consumption of placebo (n=28) or a multi-component formula (n=39) containing vitamins, zinc oxide, eicosapentaenoic acid, DHA, α-lipoic acid, coenzyme Q10, mixed tocotrienols/tocopherols, zeaxanthin, lutein, benfotiamine, N-acetyl cysteine, and selected botanical extracts, visual function and macular pigment optical density improved significantly in the supplemented group after 6 months of treatment.Citation64 The authors suggest that, theoretically, multiple and overlapping mechanisms implicated in DR may have been targeted by the various components of the test formula, although synergistic or inhibitory constituent effects were not analyzed. In our study, the synergistic influence of carotenoids on microperimetry was not evaluated, which is an interesting area of research for further studies.
Conclusion
In asymptomatic patients with NPDR, dietary supplementation with a nutraceutical formulation based on high dose of DHA (1 g) plus antioxidant vitamins, minerals, and xanthophylls for 3 months was associated with a significant improvement of macular function as compared with controls. Changes of biochemical parameters were consistent with the antioxidant and anti-inflammatory effects of DHA and lutein/zeaxanthin. Further studies are needed to confirm these promising preliminary results. Replication of the present findings in a randomized trial could offer a new approach to prevention of progression of early-stage DR in subjects with diabetes.
Acknowledgments
The authors thank Jaume Borrás, MD, for his coordination and monitoring of the study and to Marta Pulido, MD, PhD, for editing the manuscript and for her editorial assistance. This study was presented at the XXI Congress of the Spanish Vitreo-Retinal Society (SERV), Madrid, Spain, March 3, 2017.
Disclosure
The authors report no conflicts of interest in this work.
References
- CheungNMitchellPWongTYDiabetic retinopathyLancet2010376973512413620580421
- Executive SummaryIFD Diabetes Atlas8th editionBrussels, BelgiumInternational Diabetes Federation Available from: http://www.diabetesatlas.org/Accessed July 15, 2017
- ZhangXZhaoJZhaoTLiuHEffects of intensive glycemic control in ocular complications in patients with type 2 diabetes: a meta-analysis of randomized clinical trialsEndocrine2015491788925355306
- ChewEYThere is level 1 evidence for intensive glycemic control for reducing the progression of diabetic retinopathy in persons with type 2 diabetesEndocrine20154911325722012
- DoDVWangXVedulaSSBlood pressure control for diabetic retinopathyCochrane Database Syst Rev20151CD00612725637717
- OguraSKurataKHattoriYSustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdownJCI Insight201723e9090528194443
- RuiaSSaxenaSPrasadSSharmaSRAkdumanLKhannaVKCorrelation of biomarkers thiobarbituric acid reactive substance, nitric oxide and central subfield and cube average thickness in diabetic retinopathy: a cross-sectional studyInt J Retina Vitreous20162827847626
- TarrJMKaulKChopraMKohnerEMChibberRPathophysiology of diabetic retinopathyISRN Ophthalmol2013201334356024563789
- CaiJBoultonMThe pathogenesis of diabetic retinopathy: old concepts and new questionsEye (Lond)200216324226012032713
- HernándezCSimó-ServatABogdanovPSimóRDiabetic retinopathy: new therapeutic perspectives based on pathogenic mechanismsJ Endocrinol Invest201740992593528357783
- SimóRHernándezCEuropean Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR)Neurodegeneration is an early event in diabetic retinopathy: therapeutic implicationsBr J Ophthalmol201296101285129022887976
- ShindouHKosoHSasakiJDocosahexaenoic acid preserves visual function by maintaining correct disc morphology in retinal photoreceptor cellsJ Biol Chem201729229120541206428578316
- SanGiovanniJPChewEYThe role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retinaProg Retin Eye Res20052418713815555528
- ChenWEsselmanWJJumpDBBusikJVAnti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cellsInvest Ophthalmol Vis Sci200546114342434716249517
- RotsteinNPPolitiLEGermanOLGirottiRProtective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptorsInvest Ophthalmol Vis Sci20034452252225912714668
- GermanOLInsuaMFGentiliCRotsteinNPPolitiLEDocosahexaenoic acid prevents apoptosis of retina photoreceptors by activating the ERK/MAPK pathwayJ Neurochem20069851507152016923163
- MukherjeePKMarcheselliVLSerhanCNBazanNGNeuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stressProc Natl Acad Sci U S A2004101228491849615152078
- DecsiTMindaHHermannRPolyunsaturated fatty acids in plasma and erythrocyte membrane lipids of diabetic childrenProstaglandins Leukot Essent Fatty Acids200267420321012401433
- CalderPCOmega-3 fatty acids and inflammatory processesNutrients20102335537422254027
- GalliCCalderPCEffects of fat and fatty acid intake on inflammatory and immune responses: a critical reviewAnn Nutr Metab2009551–312313919752539
- SzymczakMMurrayMPetrovicNModulation of angiogenesis by omega-3 polyunsaturated fatty acids is mediated by cyclooxygenasesBlood200811173514352118216296
- MatesanzNParkGMcAllisterHDocosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cellsInvest Ophthalmol Vis Sci201051126815682520702831
- ThomsonLRToyodaYLangnerAElevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quailInvest Ophthalmol Vis Sci200243113538354912407166
- LiSYFungFKFuZJWongDChanHHLoACAnti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: in vivo and in vitro studiesInvest Ophthalmol Vis Sci201253105976598422871829
- MaresJLutein and zeaxanthin isomers in eye health and diseaseAnnu Rev Nutr20163657160227431371
- MadonnaRBalistreriCRGengYJDe CaterinaRDiabetic microangiopathy: pathogenetic insights and novel therapeutic approachesVascul Pharmacol2017901728137665
- ChucairAJRotsteinNPSangiovanniJPDuringAChewEYPolitiLELutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acidInvest Ophthalmol Vis Sci200748115168517717962470
- ConnorKMSanGiovanniJPLofqvistCIncreased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesisNat Med200713786887317589522
- LafuenteMOrtíLArgenteMThree-year in a randomized single-blind controlled trial of intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edemaRetina Epub2018222
- BazanNGNeuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stressBrain Pathol200515215916615912889
- ZurierRBFatty acids, inflammation and immune responseProstaglandins Leukot Essent Fatty Acids199348157628424124
- FuZLofqvistCAShaoZDietary ω-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectinAm J Clin Nutr2015101487988825833984
- HuntSIncreased dietary intake of omega-3-PUFA reduces pathological retinal angiogenesisOphthalmologe20071048727729 German17674004
- OpreanuMLydicTAReidGEMcSorleyKMEsselmanWJBusikJVInhibition of cytokine signaling in human retinal endothelial cells through downregulation of sphingomyelinases by docosahexaenoic acidInvest Ophthalmol Vis Sci20105163253326320071681
- LafuenteMOrtínLArgenteMCombined intravitreal ranibizumab and oral supplementation with docosahxaenoic acid and antioxidants for diabetic macular edema. Two-year randomized single-blind controlled trial resultsRetina20173771277128627787443
- SasakiMKawasakiRRogersSThe associations of dietary intake of polyunsaturated fatty acids with diabetic retinopathy in well-controlled diabetesInvest Ophthalmol Vis Sci201556127473747926595607
- KoehrerPSaabSBerdeauxOErythrocyte phospholipid and polyunsaturated fatty acid composition in diabetic retinopathyPLoS One201499e10691225188352
- Early Treatment Diabetic Retinopathy Study Research GroupGrading diabetic retinopathy from stereoscopic color fundus photographs – an extension of the modified Airlie House classification: ETDRS report number 10Ophthalmology199198Suppl 57868062062513
- Results shown in the European Patent EP 1 962 825 B1 (held by BRUDY TECHNOLOGY SL) related to the use of DHA for treating a pathology associated with cellular oxidative damage European patent granted, date April 2, 2014
- BogdanovPGassoFDomingoJCDocosahexaenoic acid improves endogenous antioxidant defense in ARPE-19 cellsInvest Ophthalmol Vis Sci2008495932
- MangioneCMLeePPGutierrezPRDevelopment of the 25-item National Eye Institute Visual Function Questionnaire (VFQ-25)Arch Ophthalmol20011191050105811448327
- LepageGRoyCCDirect transesterification of all classes of lipids in a one-step reactionJ Lipid Res19862711141203958609
- RoismanLRibeiroJCFechineFVDoes microperimetry have a prognostic value in central serous chorioretinopathy?Retina201434471371823975001
- SinHPLiuDTLamDSLifestyle modification, nutritional and vitamins supplements for age-related macular degenerationActa Ophthalmol201391161122268800
- EvansJRLawrensonJGAntioxidant vitamin and mineral supplements for slowing the progression of age-related macular degenerationCochrane Database Syst Rev201211CD00025423152201
- WilliamsMHoggREChakravarthyUAntioxidants and diabetic retinopathyCurr Diab Rep201313448148723649947
- Rodríguez-CarrizalezADCastellanos-GonzálezJAMartínez-RomeroECOxidants, antioxidants and mitochondrial function in non-proliferative diabetic retinopathyJ Diabetes20146216717523875878
- GuptaMMChariSLipid peroxidation and antioxidant status in patients with diabetic retinopathyIndian J Physiol Pharmacol200549218719216170987
- ArnalEMirandaMJohnsen-SorianoSBeneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic ratsCurr Eye Res2009341192893819958109
- MirandaMMuriachMJohnsenSOxidative stress in a model for experimental diabetic retinopathy: treatment with antioxidantsArch Soc Esp Oftalmol2004796289294 Spanish15221675
- KowluruRAKennedyATherapeutic potential of anti-oxidants and diabetic retinopathyExpert Opin Investig Drugs200110916651676
- MidenaEVujosevicSMicroperimetry in diabetic retinopathySaudi J Ophthalmol201125213113523960914
- MalagolaRSpinucciGCofoneCPattavinaLProspective microperimetry and OCT evaluation of efficacy of repeated intravitreal bevacizumab injections for persistent clinically significant diabetic macular edemaInt Ophthalmol201333326126723242589
- ArterburnLMHallEBOkenHDistribution, interconversion, and dose response of n-3 fatty acids in humansAm J Clin Nutr200683Suppl 61467S1476S16841856
- BrowningLMWalkerCGManderAPIncorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fishAm J Clin Nutr201296474875822932281
- Pinazo-DuránMDGalbis-EstradaCPons-VázquezSCantú-DibildoxJMarco-RamírezCBenítez-del-CastilloJEffects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disordersClin Interv Aging2013813914823430672
- Galbis-EstradaCPinazo-DuránMDCantú-DibildoxJMarco-RamírezCDíaz-LlópisMBenítez-del-CastilloJPatients undergoing long-term treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acidsClin Interv Aging2013871171923818768
- RibellesAGalbis-EstradaCParrasMAVivar-LlopisBMarco-RamírezCDiaz-LlopisMOcular surface and tear film changes in older women working with computersBiomed Res Int2015201546703926557673
- ChristianLMYoungASMitchellAMBody weight affects ω-3 polyunsaturated fatty acid (PUFA) accumulation in youth following supplementation in post-hoc analyses of a randomized controlled trialPLoS One2017124e017308728379964
- DyerbergJMadsenPMøllerJMAardestrupISchmidtEBBioavail-ability of marine n-3 fatty acid formulationsProstaglandins Leukot Essent Fatty Acids201083313714120638827
- NeubronnerJSchuchardtJPKresselGMerkelMvon SchackyCHahnAEnhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl estersEur J Clin Nutr201165224725421063431
- ChenHZhangXLiaoNWenFIncreased levels of IL-6, sIL-6R, and sgp130 in the aqueous humor and serum of patients with diabetic retinopathyMol Vis2016221005101427563232
- Simó-ServatOSimóRHernándezCCirculating biomarkers of diabetic retinopathy: an overview based on physiopathologyJ Diabetes Res20162016526379827376090
- ChousAPRicherSPGersonJDKowluruRAThe Diabetes Visual Function Supplement Study (DiVFuSS)Br J Ophthalmol2016100222723426089210
