Abstract
Macular edema is one of the leading causes of vision loss among patients with retinal vein occlusion, diabetic retinopathy, and posterior chamber inflammatory disease. However, the treatment of macular edema is considerably limited by the difficulty in delivering effective doses of therapeutic agents into the vitreous cavity. In recent years, the development of a sustained-release dexamethasone intravitreal implant (Ozurdex®) has enabled more controlled drug release at a stable rate over a long period of time, with a potentially lower rate of adverse events. Clinical studies indicate that this dexamethasone implant is a promising new treatment option for patients with persistent macular edema resulting from retinal vein occlusion, diabetic retinopathy, and uveitis or Irvine-Gass syndrome.
Introduction
Macular edema is thought to be due to abnormal retinal capillary permeability, manifesting by extravascular swelling in the macula.Citation1 Macular edema is associated with a variety of underlying diseases, but is most commonly seen in patients with retinal venous occlusion, diabetic retinopathy, and posterior-segment inflammatory disease.Citation1–Citation3 Current evidence suggests that the pathological processes leading to macular edema involve numerous inflammatory cells, cytokines, growth factors, and intercellular adhesion molecules, which are associated with increased vascular permeability, breakdown of the blood–retinal barrier, remodeling of the extracellular matrix, and upregulation of proangiogenic factors.Citation4–Citation8
Macular edema is one of the leading causes of vision loss among patients with retinal vein occlusion, diabetic retinopathy, and posterior-segment inflammatory disease. However, the treatment of macular edema is considerably limited by the difficulty in delivering effective doses of therapeutic agents into the vitreous cavity. While various therapeutic agents, such as antivascular endothelial growth factor, along with an array of technologies to circumvent the blood–retinal barrier are under development,Citation9–Citation12 a sustained-release dexamethasone implant has recently become available for the treatment of macular edema secondary to a variety of underlying diseases.
Treatment of macular edema
Traditionally, the main treatment options for macular edema are laser photocoagulation and anti-inflammatory therapy depending on etiology. Laser photocoagulation prevents further vision loss in patients with macular edema caused by diabetes and retinal vein occlusion.Citation13–Citation15 However, the prevention of visual decline is not always uniform, and some patients can be refractory to laser treatment. In addition, this mode of therapy is associated with moderate visual loss, a diminished visual field, and reduced color vision and contrast sensitivity.Citation16–Citation18
Corticosteroids are potent anti-inflammatory agents that can counteract many of the pathological processes thought to play a role in the development of macular edema. Corticosteroids prevent leukocyte migration, reduce fibrin deposition, stabilize endothelial cell tight junctions, and inhibit synthesis of vascular endothelial growth factor, prostaglandins, and proinflammatory cytokines.Citation19 However, the route of corticosteroid administration dramatically affects the risk to benefit ratio of corticosteroid therapy (). Oral corticosteroids are complicated with many adverse events, including osteoporosis, a Cushingoid state, adrenal suppression, and exacerbation of diabetes.Citation20–Citation23 Topical, peribulbar, and subconjunctival corticosteroid administrations deliver suboptimal vitreous drug levels () with a very short half-life (approximately 3.5 hours) and are associated with relatively high systemic corticosteroid concentrations, which can potentially be accompanied by significant adverse events.Citation24–Citation28 On the other hand, direct intravitreal corticosteroid administration bypasses the blood–retinal barrier, leading to high local drug concentrations with no or little systemic adverse events.
Table 1 Traditional routes of dexamethasone administration provide suboptimal vitreous levels and high systemic concentrations
Given the short half-life of dexamethasone in the vitreous cavity, the crystalline form of a lipophilic corticosteroid, triamcinolone acetonide (Kenalog®-40, Bristol-Myers Squibb, Princeton, NJ), with a vitreous residence time of several months, gained widespread use for treatment of macular edema secondary to retinal vein occlusion, diabetic retinopathy, and uveitis, in spite of the lack of controlled, randomized studies demonstrating that its efficacy exceeds the risks.Citation29–Citation33 Recent randomized trials demonstrated that intravitreal triamcinolone acetonide was more effective than observation in patients with macular edema secondary to central retinal vein occlusion, and as effective as laser therapy in patients with macular edema secondary to branch retinal vein occlusion in improving visual acuity.Citation34,Citation35 In diabetic patients with macular edema, laser therapy was more effective than intravitreal triamcinolone acetonide in improving visual acuity at the study primary endpoint of two years, although triamcinolone acetonide was initially more effective than laser at four months.Citation36 However, in those studies, intravitreal triamcinolone acetonide was associated with a high rate of elevated intraocular pressure and cataract formation, two ocular adverse events that have been linked most commonly to corticosteroid therapy.Citation37–Citation39 The incidence of a ≥10 mmHg increase in intraocular pressure from baseline ranged from 24% to 50% in the intravitreal triamcinolone acetonide 4 mg group compared with 2% to 13% in the observation or laser therapy group.Citation34–Citation36 The incidence of cataract surgery ranged from 23% to 51% in the intravitreal triamcinolone acetonide 4 mg group compared with 0% to 13% in the observation or laser therapy group.
In recent years, the development of a sustained-release intravitreal dexamethasone implant (Ozurdex®, Allergan Inc, Irvine, CA) enabled more controlled delivery of drug, with a potentially lower rate of adverse events. The dexamethasone implant is now emerging as a potential treatment for macular edema arising from retinal vein occlusion, diabetic retinopathy, and uveitis.
Sustained-release dexamethasone implant
Among the corticosteroids, dexamethasone is one of the most potent, with an anti-inflammatory activity that is six-fold greater than that of triamcinolone and 30-fold greater than cortisol.Citation40 In the dexamethasone implant, the active drug is dispersed through a biodegradable copolymer of lactic acid and glycolic acid (PLGA), forming a matrix structure (Novadur™, Allergan Inc).Citation41 These polymers have been used in a number of products, including absorbable sutures.Citation42,Citation43 For several years, PLGA has been used to prepare nanoparticles and microparticles for intraocular drug delivery. These drug delivery systems have been tested in animal models and humans.Citation44–Citation47
In the early clinical studies, the dexamethasone implant was surgically implanted into the vitreous cavity via a pars plana incision.Citation48–Citation50 Subsequently, a single-use, sutureless dexamethasone posterior-segment drug delivery system (DDS) applicator was developed, allowing injection of the dexamethasone implant in the office rather than in a surgical setting ().Citation51 The dexamethasone implant was observed for six months after implantation in monkey eyes.Citation52 The dexamethasone implant releases the drug by diffusion in a biphasic fashion, with higher doses for up to six weeks followed by lower doses for up to six months ().Citation52 Experience has shown that PLGA is biocompatible and, inside the eye, is metabolized into carbon dioxide and water. Thus, sequential implants can be placed in an office setting without the need for surgical removal. The dexamethasone implant is indicated for the treatment of macular edema following retinal vein occlusion.Citation41 The safety and efficacy of the dexamethasone implant have also been investigated in uveitic, diabetic, and vitrectomized diabetic patients with macular edema.
Figure 1 Dexamethasone Posterior Segment Drug Delivery System® Applicator and approximate vitreous location of dexamethasone implant after insertion. The implant is approximately 6 mm long and is inserted into the vitreous cavity through the 22-gauge needle of the applicator.
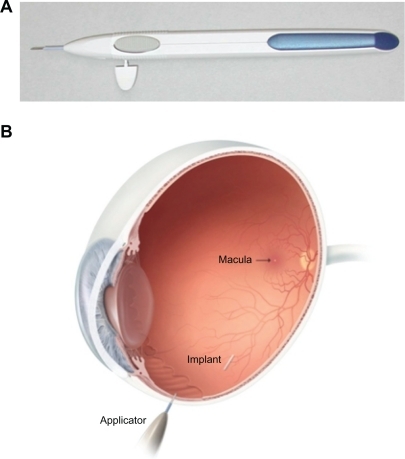
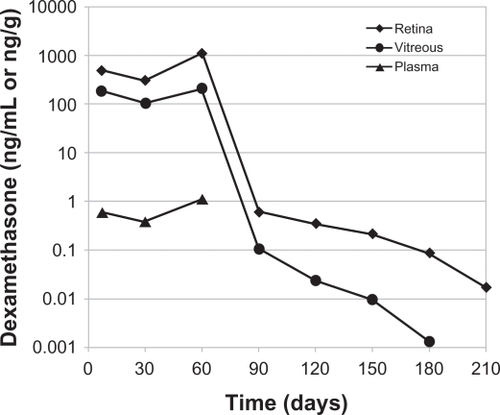
Figure 2 Temporal kinetics of dexamethasone concentrations in the vitreous cavity, retina, and plasma following placement of the 0.7 mg dexamethasone implant in monkeys. The dexamethasone concentration was below the minimum detection limit in plasma after day 60.
Phase II studies
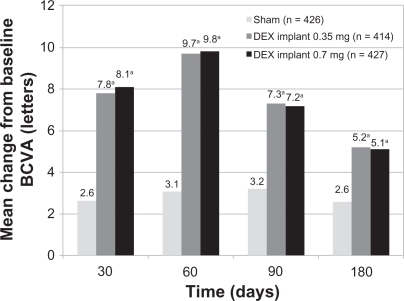
A Phase II trial evaluated the safety and efficacy of the dexamethasone implant in patients with persistent macular edema.Citation49 This study enrolled patients ≥12 years of age with a best-corrected visual acuity (BCVA) of 20/40 to 20/200 because of clinically detectable macular edema persisting for ≥90 days after laser or medical therapy. Macular edema was secondary to central retinal vein occlusion, branch retinal vein occlusion, diabetic retinopathy, uveitis, or Irvine-Gass syndrome. A total of 315 patients were randomized in a 1:1:1 ratio to observation or to treatment with a surgically placed dexamethasone implant at two doses, ie, 0.35 mg or 0.7 mg. The primary outcome measure was the proportion of patients who achieved at least a 10-letter improvement in BCVA at day 90.Citation49
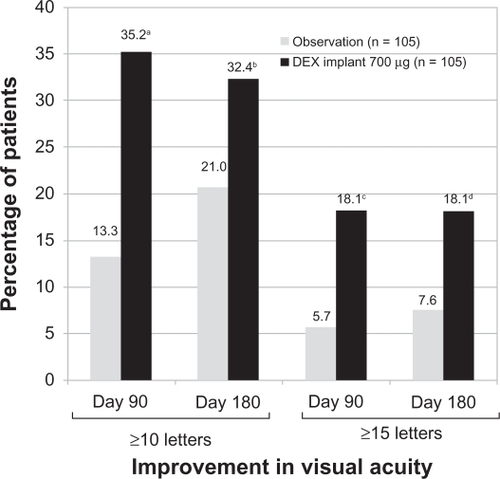
The proportion of patients with a ≥10-letter or ≥15-letter improvement in BCVA at day 90 was significantly higher in the 0.7 mg dexamethasone implant group compared with the observation group. At day 180, a significantly greater proportion of patients treated with the 0.7 mg dexamethasone implant achieved a ≥10-letter or ≥15-letter improvement in BCVA (). There were no statistically significant differences in improvement of BCVA between the 0.35 mg dexamethasone implant group and the observation group at day 90 or 180.Citation49 Treatment with dexamethasone implant at either dose (0.35 mg or 0.7 mg) significantly decreased central retinal thickness and fluorescein angiographic leakage at day 90 compared with the observation group.Citation49
Figure 3 Percentage of patients with a ≥10-letter or a ≥15-letter improvement in best corrected visual acuity following treatment with the 0.7 mg dexamethasone implant at days 90 and 180.
Notes: aP < 0.001, bP = 0.06, cP = 0.006, and dP = 0.02 compared with observation.
A P < 0.025 was considered a statistically significant difference.
In the Phase II study, the dexamethasone implant was well tolerated and had a favorable safety profile. The incidence of a ≥10 mmHg increase in intraocular pressure from baseline was 3% in the observation group, 12% in the 0.35 mg dexamethasone implant group, and 17% in the 0.7 mg dexamethasone implant group. Most of these patients (>65%) had only a single occurrence of an intraocular pressure increase of this magnitude or greater. No significant between-group differences were found in the number of reports of cataract. However, treatment-related cataract formation may take longer than 180 days to become apparent.Citation49
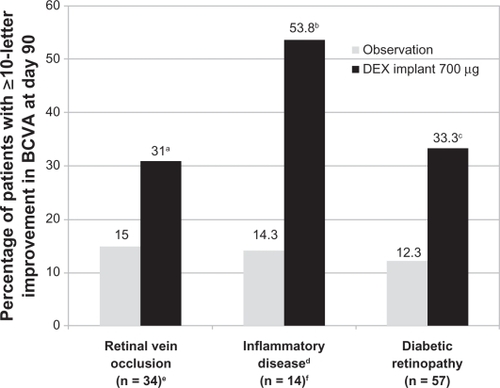
In the Phase II study, subset analyses were performed to evaluate whether the treatment effect was similar across the underlying causes of persistent macular edema. These analyses showed that the improvement in BCVA at day 90 was more favorable in the 0.7 mg dexamethasone implant group than in the observation group, regardless of the underlying cause of macular edema ().Citation48–Citation50 In a subset of patients with retinal vein occlusion, an improvement in BCVA of ≥10 letters at day 90 was observed in 31% of patients treated with the 0.7 mg dexamethasone implant compared with 15% of patients in the observation group.Citation49 In a subset of patients with uveitis or Irvine-Gass syndrome, an improvement in BCVA of ≥10 letters at day 90 was observed in 53.8% of patients treated with the 0.7 mg dexamethasone implant compared with 14.3% of patients in the observation group.Citation50 In a subset of patients with diabetic macular edema, an improvement in BCVA of ≥10 letters at day 90 was observed in 33.3% of patients treated with the 0.7 mg dexamethasone implant compared with 12.3% of patients in the observation group.Citation48 Among diabetic patients, this significant difference was maintained when patients were stratified according to their pattern of diabetic macular edema, ie, focal, diffuse, cystoid, and both cystoid and diffuse.Citation53 Overall, the pattern of adverse events seen in these subpopulations was similar to that seen in the overall population of patients included in the Phase II study.Citation48–Citation50
Figure 4 Efficacy of the dexamethasone implant in improving BCVA stratified by the underlying cause of macular edema in patients who participated in the Phase II trial.
Abbreviations: DEX, dexamethasone; BVCA, best corrected visual acuity.
Phase III studies
Two identical, multicenter, masked, randomized, clinical trials evaluated the safety and efficacy of the dexamethasone implant as compared with sham in eyes with vision loss due to clinically detectable macular edema associated with either central or branch retinal vein occlusion.Citation54 A total of 1267 patients was randomized in a 1:1:1 ratio to either a sham procedure or treatment with the dexamethasone implant at the dose of 0.35 mg or 0.7 mg. The dexamethasone implant was inserted into the vitreous cavity through the pars plana by a single-use DDS applicator. The primary efficacy outcome for the pooled data from the two Phase III studies was the time to reach a 15-letter improvement from baseline BCVA.Citation54
Eyes receiving dexamethasone implants achieved a 15-letter improvement in BCVA significantly faster than did eyes receiving sham treatment ().Citation54 The proportion of eyes achieving at least a 15-letter improvement from baseline BCVA was significantly greater in both dexamethasone implant groups than in the sham group from day 30 through day 90, with the greatest response (29%) at day 60 (P < 0.001).Citation54 The mean increase from baseline visual acuity was also significantly greater in both dexamethasone implant groups than in the sham group from day 30 through day 180 (P ≤ 0.006, ), with the greatest between-group difference (approximately 10 letters) at day 60.Citation54 The mean decrease in central retinal thickness was significantly greater in both dexamethasone implant groups compared with the sham group at day 90 (P < 0.001) but not at day 180.Citation54
Figure 5 Time to achieve 15 letters of improvement from baseline BVCA in patients with macular edema secondary to central retinal vein occlusion or branch retinal vein occlusion who participated in the Phase III trials.
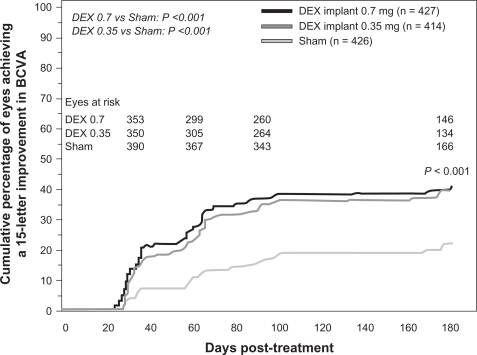
Figure 6 Mean change from baseline BVCA in patients with macular edema secondary to central retinal vein occlusion or branch retinal vein occlusion who participated in the Phase III trials.
Abbreviations: DEX, dexamethasone; BVCA, best corrected visual acuity.
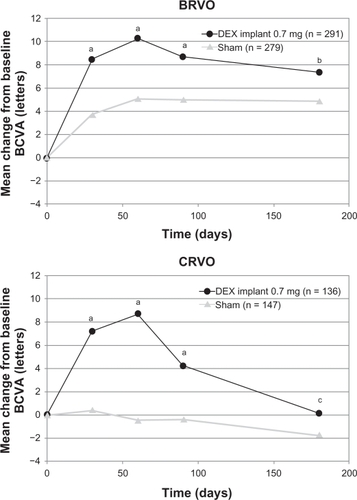
In a prospectively defined subgroup analysis, the key efficacy outcomes (time to 15-letter improvement, proportion of eyes achieving at least a 15-letter improvement, and mean change from baseline BCVA) were evaluated for the branch and central retinal vein occlusion populations separately. In general, the response to the dexamethasone implant in both subgroups was qualitatively similar to the response seen in the overall population, but the response in the sham group was greater in the branch retinal vein occlusion subgroup than in the central retinal vein occlusion subgroup in all efficacy analyses. The difference between the sham groups was particularly marked in the analysis of mean change from baseline BCVA (). These findings support previous observations that central retinal vein occlusion is a more visually disabling disorder than branch retinal vein occlusion. Eyes with central retinal vein occlusion did not respond as well to therapy as eyes with branch retinal vein occlusion, and they were not improved without therapy.Citation54
Figure 7 Mean change from baseline BCVA stratified by the underlying cause of macular edema of patients who participated in the Phase III trials.
Abbreviations: DEX, dexamethasone; BVCA, best corrected visual acuity; BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion.
In the Phase III study, a post hoc subgroup analysis based on the duration of macular edema at baseline found that the response to the dexamethasone implant (proportion of eyes improving by ≥15 letters and mean change from baseline BCVA) was often greater among eyes with a shorter duration of macular edema at baseline (≤90 days) as compared with a longer duration (>90 days) of macular edema.Citation54 A similar effect of macular edema duration was seen in SCORE (Standard Care versus cOrticosteroid for REtinal vein occlusion study).Citation34,Citation54
In the Phase III study, the dexamethasone implant was well tolerated and associated with generally transient, moderate, and readily manageable adverse events. Those adverse events that occurred significantly more frequently in either dexamethasone implant group than in the sham group were eye pain (P = 0.023), anterior chamber cells (P ≤ 0.031), and ocular hypertension (P ≤ 0.002).Citation54 The percentage of eyes receiving intraocular pressure-lowering medication increased in the dexamethasone implant groups from approximately 6% at baseline to 24% at day 180, while there was no change in the sham group. Overall, the proportion of patients experiencing an intraocular pressure elevation of ≥10 mmHg from baseline peaked at day 60, and was less than 1% in the sham group and approximately 15% in both dexamethasone implant groups.Citation54 There was no statistically significant difference between the treatment groups in the incidence of cataract.Citation54 As with the Phase II studies, 180 days may not be long enough for detection of treatment-related cataract formation.
Recent studies for other indications
Recent clinical studies have evaluated the safety and efficacy of the dexamethasone implant as monotherapy for uveitic and diabetic macular edema. The efficacy of the dexamethasone implant in vitrectomized eyes is of particular interest because preclinical studies show that after implantation, vitreous and retinal dexamethasone concentrations in vitrectomized eyes are similar to those in nonvitrectomized eyes.Citation55 The dexamethasone implant was also investigated as a combination therapy with laser photocoagulation in diabetic macular edema patients and with ranibizumab (Lucentis®, Genentech Inc, San Francisco, CA) in patients with choroidal neovascularization secondary to exudative age-related macular degeneration. The findings of these studies indicate that the dexamethasone implant significantly improves intraocular inflammation and visual acuity in uveitic macular edema patients, increases visual acuity in a difficult-to-treat vitrectomized population with chronic diabetic macular edema, and reduces the need for repeated ranibizumab injections in patients with choroidal neovascularization secondary to exudative age-related macular degeneration.Citation55
Conclusion
Phase II and III studies indicate that the 0.7 mg dexamethasone implant is consistently more efficacious than the 0.35 mg dexamethasone implant. The response to treatment with the 0.7 mg dexamethasone implant lasts for up to 180 days. Further studies are warranted to determine the response to repeated treatments, as well as the optimum retreatment interval in patients who require a longer duration of treatment. Nevertheless, clinical studies demonstrate that the dexamethasone implant is a promising new treatment option for patients with persistent macular edema resulting from retinal vein occlusion, diabetic retinopathy, and uveitis or Irvine-Gass syndrome.Citation48–Citation50,Citation54 Further studies are warranted to support the clinical value of the dexamethasone implant in these patient populations.
Given the differences in patient populations, efficacy endpoints, and reporting of adverse events, it is not possible to compare directly the findings of the dexamethasone implant trials with those of the intravitreal triamcinolone acetonide trials. Head-to-head clinical studies are warranted to compare the safety and efficacy of the dexamethasone implant with those of intravitreal triamcinolone acetonide in macular edema patients. Mathematical models predict that placement of the dexamethasone implant in a more posterior vitreous location than the current anterior vitreous location maximizes macular drug exposure while minimizing anterior chamber drug exposure. Such a change in anatomical localization of the dexamethasone implant may further decrease the incidence of intraocular pressure elevation.Citation56
Disclosure
Editorial assistance in the preparation of this manuscript was provided by Hadi Moini, PhD, of Pacific Communications, a wholly-owned subsidiary of Allergan, Inc.
References
- FerrisFLIIIPatzAMacular edema. A complication of diabetic retinopathySurv Ophthalmol198428Suppl4524616379946
- DurraniOMTehraniNNMarrJEMoradiPStavrouPMurrayPIDegree, duration, and causes of visual loss in uveitisBr J Ophthalmol20048891159116215317708
- OrthDHPatzARetinal branch vein occlusionSurv Ophthalmol1978226357376354066
- AntonettiDABarberAJKhinSLiethETarbellJMGardnerTWVascular permeability in experimental diabetes is associated with reduced endothelial occludin content: Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research GroupDiabetes19984712195319599836530
- CampochiaroPAHafizGShahSMRanibizumab for macular edema due to retinal vein occlusions: Implication of VEGF as a critical stimulatorMol Ther200816479179918362932
- FunatsuHYamashitaHNomaHMimuraTYamashitaTHoriSIncreased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edemaAm J Ophthalmol20021331707711755841
- RossettiLAutelitanoACystoid macular edema following cataract surgeryCurr Opin Ophthalmol2000111657210724830
- PatelJITombran-TinkJHykinPGGregorZJCreeIAVitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profilesExp Eye Res200682579880616324700
- KiernanDFMielerWFThe use of intraocular corticosteroidsExpert Opin Pharmacother200910152511252519761356
- MansoorSKuppermannBDKenneyMCIntraocular sustained-release delivery systems for triamcinolone acetonidePharm Res200926477078419184374
- CiullaTARosenfeldPJAnti-vascular endothelial growth factor therapy for neovascular ocular diseases other than age-related macular degenerationCurr Opin Ophthalmol200920316617419381089
- SimoRHernandezCAdvances in the medical treatment of diabetic retinopathyDiabetes Care20093281556156219638526
- ArnarssonAStefanssonELaser treatment and the mechanism of edema reduction in branch retinal vein occlusionInvest Ophthalmol Vis Sci200041387787910711707
- BattagliaPMSavianoSRavalicoGGrid laser treatment in macular branch retinal vein occlusionGraefes Arch Clin Exp Ophthalmol1999237121024102710654172
- LardenoyeCWvan SchooneveldMJFritsTWRothovaAGrid laser photocoagulation for macular oedema in uveitis or the Irvine-Gass syndromeBr J Ophthalmol1998829101310169893590
- MorganCMSchatzHAtrophic creep of the retinal pigment epithelium after focal macular photocoagulationOphthalmology1989961961032919053
- RoiderJLaser treatment of retinal diseases by subthreshold laser effectsSemin Ophthalmol1999141192610790572
- SchatzHMadeiraDMcDonaldHRJohnsonRNProgressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edemaArch Ophthalmol199110911154915511755735
- LeopoldIHNonsteroidal and steroidal anti-inflammatory agentsSearsMLTarkkanenASurgical Pharmacology of the EyeNew YorkRaven Press1985
- PaganoGBrunoACavallo-PerinPCescoLImbimboBGlucose intolerance after short-term administration of corticosteroids in healthy subjects. Prednisone, deflazacort, and betamethasoneArch Intern Med19891495109811012655543
- RobinsonBHMattinglyDCopeCLAdrenal function after prolonged corticosteroid therapyBr Med J1962152921579158414492888
- SaagKGGlucocorticoid-induced osteoporosisEndocrinol Metab Clin North Am2003321135157vii12699296
- StanburyRMGrahamEMSystemic corticosteroid therapy-side effects and their managementBr J Ophthalmol19988267047089797677
- WeijtensOvan der SluijsFASchoemakerRCPeribulbar corticosteroid injection: Vitreal and serum concentrations after dexamethasone disodium phosphate injectionAm J Ophthalmol199712333583639063245
- WeijtensOSchoemakerRCCohenAFDexamethasone concentration in vitreous and serum after oral administrationAm J Ophthalmol199812556736799625551
- WeijtensOFeronEJSchoemakerRCHigh concentration of dexamethasone in aqueous and vitreous after subconjunctival injectionAm J Ophthalmol1999128219219710458175
- WeijtensOSchoemakerRCRomijnFPCohenAFLentjesEGvan MeursJCIntraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphateOphthalmology2002109101887189112359610
- KwakHWD’AmicoDJEvaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injectionArch Ophthalmol199211022592661736876
- BeerPMBakriSJSinghRJLiuWPetersGBIIIMillerMIntraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injectionOphthalmology2003110468168612689886
- KamppeterBACejAJonasJBIntraocular concentration of triamcinolone acetonide after intravitreal injection in the rabbit eyeOphthalmology200811581372137518355920
- AntcliffRJSpaltonDJStanfordMRGrahamEMFfytcheTJMarshallJIntravitreal triamcinolone for uveitic cystoid macular edema: An optical coherence tomography studyOphthalmology2001108476577211297495
- IpMSKumarKSIntravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusionArch Ophthalmol200212091217121912215101
- MartidisADukerJSGreenbergPBIntravitreal triamcinolone for refractory diabetic macular edemaOphthalmology2002109592092711986098
- IpMSScottIUvan VeldhuisenPCA randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5Arch Ophthalmol200912791101110419752419
- ScottIUIpMSvan VeldhuisenPCA randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6Arch Ophthalmol200912791115112819752420
- Diabetic Retinopathy Clinical Research NetworkA randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edemaOphthalmology200811591447144918662829
- ArmalyMFStatistical attributes of the steroid hypertensive response in the clinically normal eye. I. The demonstration of three levels of responseInvest Ophthalmol1965418719714283012
- BeckerBIntraocular pressure response to topical corticosteroidsInvest Ophthalmol1965419820514283013
- ButcherJMAustinMMcGalliardJBourkeRDBilateral cataracts and glaucoma induced by long term use of steroid eye dropsBMJ19943096946438044069
- GoldfienAAdrenocorticosteroids and adrenocortical antagonistsKatzungBGBasic and Clinical Pharmacology6th edLondonPrentice Hall International1995592607
- Ozurdex® [Package insert]Irvine, CAAllergan Inc2009
- KobayashiHShirakiKIkadaYToxicity test of biodegradable polymers by implantation in rabbit corneaJ Biomed Mater Res19922611146314761332973
- VisscherGERobisonRLMauldingHVFongJWPearsonJEArgentieriGJBiodegradation of and tissue reaction to 50:50 poly (DL-lactide-co-glycolide) microcapsulesJ Biomed Mater Res19851933493654077887
- BarciaEHerrero-VanrellRDiezAVarez-SantiagoCLopezICalongeMDownregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasoneExp Eye Res200989223824519341729
- CardilloJASouza-FilhoAAOliveiraAGIntravitreal Bioerudivel sustained-release triamcinolone microspheres system (RETAAC). Preliminary report of its potential usefulness for the treatment of diabetic macular edemaArch Soc Esp Oftalmol2006811267568117199160
- Herrero-VanrellRRefojoMFBiodegradable microspheres for vitreoretinal drug deliveryAdv Drug Deliv Rev200152151611672871
- KompellaUBBandiNAyalasomayajulaSPSubconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expressionInvest Ophthalmol Vis Sci20034431192120112601049
- HallerJAKuppermannBDBlumenkranzMSRandomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edemaArch Ophthalmol2010128328929620212197
- KuppermannBDBlumenkranzMSHallerJARandomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edemaArch Ophthalmol2007125330931717353400
- WilliamsGAHallerJAKuppermannBDDexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndromeAm J Ophthalmol200914761048105419268890
- HallerJADugelPWeinbergDVChouCWhitcupSMEvaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edemaRetina2009291465118827732
- Chang-LinJEAttarMAcheampongAAPharmacokinetics and pharmacodynamics of the sustained-release dexamethasone intravitreal implantInvest Ophthalmol Vis Sci2010811 [Epub ahead of print]
- KuppermannBDChouCWeinbergDVWhitcupSMHallerJABlumenkranzMSIntravitreous dexamethasone effects on different patterns of diabetic macular edemaArch Ophthalmol2010128564264320212194
- HallerJABandelloFBelfortRJrRandomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusionOphthalmology201011761134114620417567
- Data on fileIrvine, CAAllergan Inc2010
- LeeSSD’ArgenioDZMoatsRPharmacokinetic modeling to improve the design of intravitreal corticosteroid implants for treating macular edemaInvest Ophthalmol Vis Sci2007 Abstr 0288.