Abstract
We describe three cases of submacular hemorrhage that occurred two to four days after anti-VEGF intravitreal injection for occult choroidal neovascularisation in age-related macular degeneration and their management with 25 gauge pars plana vitrectomy with injection of subretinal recombinant tissue plasminogen activator (rTPA) followed by fluid-air exchange and postoperative prone position. Vitrectomy, subretinal rTPA injection and fluid-gas exchange apply as a safe and effective treatment in these cases. Functional results seem to be positive especially if surgical treatment is promptly performed.
Introduction
We describe three cases of submacular hemorrhage (SMH) that occurred two to four days after anti-vascular endothelial growth factor (anti-VEGF) intravitreal injection for occult choroidal neovascularisation (CNV) in age related macular degenerationCitation1 (AMD) and their management.
Methods
Almost 2500 anti-VEGF intravitreal injections for neovascular AMD were performed in our Department between January 2007 and December 2009. Three patients developed an acute SMH after the injection involving most of the macular region. Fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA) and optical coherence tomography (OCT) were carried out. After diagnosis, patients underwent 25 gauge pars plana vitrectomy with posterior hyaloid removal. An injection of 0.2 mL of subretinal recombinant tissue plasminogen activator (rTPA) (125 μg/mL) through a 41-gauge flexible translocation microcannula (DORC-dual bore BSS injection needle 0.1 mm tip) followed.Citation2 The rTPA was injected inferiorly to the SMH in order to create a bullous retinal detachment encompassing the entire blood clot. Finally a fluid-air exchange was performed and patients maintained a supine position for 45 minutes followed by a postoperative prone position.
Case 1
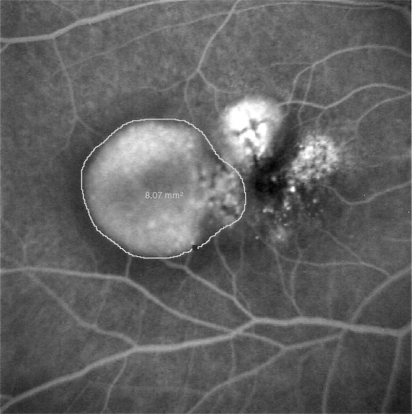
In January 2008, a 67-year old woman with 20/20 visual acuity and metamorphopsia in her right eye (RE) had been treated for seven months with an injection of ranibizumab each month for an occult CNV. The FFA performed at that time demonstrated, in the RE, a paramacular pigment epithelial detachment of 8.07 mm2 and a serous neuroepithelial detachment involving the fovea (). Four days after the 8th intravitreal anti-VEGF injection a SRH developed and the visual acuity dropped to light perception. The patient underwent surgical treatment the day after the appearance of the hemorrhage. No intraoperative or postoperative complications occurred. A complete displacement of submacular hemorrhage from the fovea was obtained. Visual acuity one month after treatment rose to 20/63 with metamorphopsia. The patient had four further intravitreal injections of bevacizumab between May 2008 and August 2008. Significant cataract developed which required extraction in August 2008. One year following cataract surgery the patient’s visual acuity was stable at 20/63.
Case 2
An 84-year old woman, affected by cardiac arrhythmia treated with amiodarone, presented in January 2008 complaining of metamorphopsia in her RE. An occult CNV due to AMD was identified and she underwent three intravitreal bevacizumab injections in the RE. In April 2008 her visual acuity was 20/125 in the RE and 20/63 in the left eye (LE), and the FFA and ICGA demonstrated an occult choroidal neovascularisation of 18.55 mm2 in the LE. Two days after the first intravitreal injection of bevacizumab, she developed a thick SMH and her visual acuity decreased to hand motion. Two days after the hemorrhage occurred, she underwent surgical treatment. No intra or postoperative complications were observed. Four days after surgery the hemorrhage was displaced from the fovea. The visual acuity in the LE one month after treatment was 20/160 with metamorphopsia. Two months after surgery she underwent another bevacizumab injection in the LE. Her visual acuity remained 20/160 for the following year.
Case 3
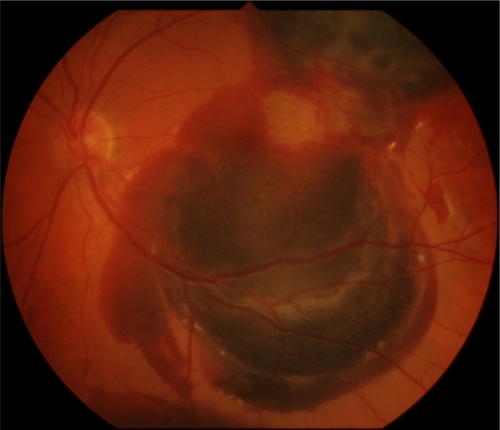
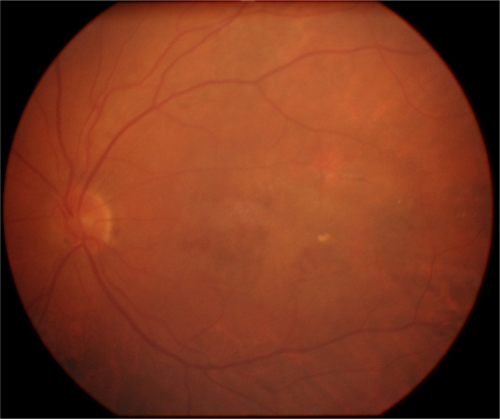
In June 2007 a 72-year old woman, who had already undergone in her RE four ranibizumab injections for a wet AMD, started complaining of metamorphopsia in her LE with a visual acuity of 20/20. FFA and ICGA demonstrated a lesion area of 4.15 mm2 due to occult CNV. A ranibizumab intravitreal injection was carried out in her LE and two days after a SMH occurred (). The visual acuity in the LE dropped to counting fingers. Two days later she underwent surgical treatment. No intraoperative complications were noticed. Four days after treatment an almost complete displacement of the hemorrhage was achieved. Visual acuity was 20/160 one month after surgery and she underwent another ranibizumab injection. One year after surgery () her visual acuity was unmodified and no other treatment was performed.
Discussion
Submacular hemorrhage is not a rare complication during the natural history of occult neovascularisation in AMD. It has been reported that it occurs in 17.0% of AMD cases with the presence of retinal pigment epithelial detachment (PED).Citation3 This complication has been also described after photodynamic therapy especially in cases of AMD with PED.Citation4–Citation9 In our cases only in one of the three patients was a PED present before the treatment (case 1). In that case the area involving the PED was 8.07 mm 2 ().
Recently a few studies have described the occurrence of large submacular hemorrhages after the intravitreal injection both of bevacizumab and ranibizumab. Karagiannis has hypothesized that this event might also be due to changing from bevacizumab to ranibizumab.Citation10 In the cases that we report, only one kind of anti-VEGF was injected in each patient before the occurrence of the hemorrhage.
The mechanism of submacular hemorrhage in these cases remains unknown, but it has been hypothesized that the contraction of the neovascular membrane could lead to new vessels rupturing, especially in large lesions.Citation11,Citation12 In addition, a reduction of tight junctions in retinal pigment epithelial and endothelial cells related to the decrease in VEGF availability could promote the vessel rupture,Citation13 even though recently Peng et al have demonstrated that permeability and selectivity of the junctions are not affected by VEGF, bevacizumab or ranibizumab.Citation14
Vascular endothelial growth factor regulates crucial processes, such as embryo- and organogenesis as well as immune system, endocrinology, hematopoiesis, (lymphoid) vessel architecture and reparative processes in adults.Citation15,Citation16 It might be therefore expected that its inhibition could cause multiple adverse effects. Although the intravitreal administration of smaller doses can drastically reduce systemic exposure, and current and past clinical trials do not provide sufficient statistical power when evaluating whether systemic events significantly differ between the treatment and control groups,Citation17–Citation21 possible local side effects on retinal perfusion and survival of neuronal tissue must be taken into consideration.Citation22
Goverdhan and LochheadCitation1 has described four cases of submacular hemorrhage after intravitreal bevacizumab all occurring in large neovascular lesions and the same finding was described by Baeteman et al reporting six cases of submacular hemorrhage after ranibizumab injection.Citation23 In these series the hemorrhages developed at a median of 14 to 25 days after the anti-VEGF injection, while in our cases the median was 2.6 days.
Moreover it is interesting to note that the patient described in our third case developed a large subretinal hemorrhage after the injection but the initial lesion was relatively small and no PED was noticed.
The occurrence of submacular hemorrhage has been also related to anticoagulant therapy and to increased blood pressure.Citation24–Citation26 In our series no patients had history of systemic hypertension or anticoagulant treatment.
The toxicity of subretinal blood to the neurosensory retina has been demonstrated in clinical studiesCitation27 and in animal models.Citation28 Possible mechanisms of blood-induced retinotoxicity include mechanical effects such as fibrotic shearing of photoreceptors, hypoxia, and metabolic disruption imposed by the clot as a diffusion barrier. Direct neurotoxicity seems to be induced by the migration of blood components, such as iron, to the photoreceptor layer.Citation29
The management of submacular hemorrhage has evolved greatly during the past 15 years. A variety of therapeutic approaches have been developed, all with the common goal of clearing the submacular blood to minimize permanent damage to the photoreceptors and retinal pigment epithelium. Many options have been proposed including intravitreal gas injection;Citation30,Citation31 pars plana vitrectomy and submacular surgery, both with and without the assistance of rTPA;Citation32–Citation38 intravitreal injection of gas and pneumatic displacement of the submacular hemorrhage with associated intravitreal injection of rTPA;Citation39–Citation41 pars plana vitrectomy; subretinal injection of r-TPA and fluid gas exchange;Citation42 pars plana vitrectomy and subretinal rTPA injection, followed by evacuation of the liquefied blood through a 500 μm retinotomy with the aid of perfluorocarbon compression of the overlying retina;Citation43 intravitreal anti-VEGF;Citation44 combined rTPA, expansile gas and bevacizumab;Citation45 and co-application of rTPA and bevacizumab.Citation46 Many procedures include the use of rTPA. Subretinal rTPA has recently been demonstrated to achieve best anatomical results than intravitreal rTPA.Citation47
Patients with subretinal hemorrhages secondary to AMD are psychologically distressed as a result of acute loss of vision.Citation48 In our series subretinal r-TPA injection followed by gas tamponade allowed the displacement of the hemorrhage in all the three cases enabling the follow-up and the further treatment of the AMD. No intraoperative or postoperative complications were noted.
In conclusion, large subretinal hemorrhage is a possible complication of intravitreal anti-VEGF treatment in AMD and it may occur days or weeks after the injection. Further studies are required for the complete comprehension of the mechanism of the pathogenesis.
The surgical approach with vitrectomy, subretinal rTPA injection and fluid-gas exchange is a safe and effective treatment. Functional results seem to be positive, especially if surgical treatment is promptly performed.
Disclosure
The authors report no conflicts of interest in the work.
References
- GoverdhanSVLochheadJSubmacular hemorrhages after intravitreal bevacizumab for large occult choroidal neovascularisation in age-related macular degenerationBr J Ophthalmol20089221021217965104
- OlivierSChowDRPackoKHMacCumberMWAwhCCSubretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in Age-Related macular degenerationOphthalmology200411112011208 Erratum in: Ophthalmology. 2004;111:1640.15177972
- PolinerLSOlkRJBurgessDGordonMENatural history of retinal pigment epithelial detachments in age-related macular degenerationOphthalmology1986935435512425324
- TheodossiadisGPPanagiotidisDGeorgalasIGMoschosMTheodossiadisPGRetinal hemorrhage after photodynamic therapy in patients with subfoveal choroidal neovascularization caused by age-related macular degenerationGraefes Arch Clin Exp Ophthalmol2003241131812545287
- ArnoldJJBlinderKJBresslerNMAcute severe visual acuity decrease after photodynamic therapy with verteporfin: case reports from randomized clinical trials TAP and VIP report no. 3Am J Ophthalmol200413768369615059708
- GeliskenFInhoffenWKarim-ZodaKSubfoveal hemorrhage after verteporfin photodynamic therapy in treatment of choroidal neovascularizationGraefes Arch Clin Exp Ophthalmol200524319820315258778
- DoDVBresslerNMBresslerSBLarge submacular hemorrhages after verteporfin therapyAm J Ophthalmol200413755856015013883
- ChaudhryNALavaqueAJTomDELiggettPELarge submacular hemorrhage following PDT with verteporfin in patients with occult CNVM secondary to age-related macular degenerationOphthalmic Surg Lasers Imaging200738646817278540
- MatsushitaSNaitoTTakebayashiMSatoHShiotaHThe prognosis of cases with massive subretinal hemorrhage after photodynamic therapyJ Med Invest20085523123518797136
- KaragiannisDALadasIDParikakisEChanging from bevacizumab to ranibizumab in age-related macular degeneration. Is it safe?Clin Interv Aging2009445746120054410
- HondaSHirabayashiHTsukaharaYNegiAAcute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurityGraefes Arch Clin Exp Ophthalmol20082461061106318320201
- GibranSKSachdevAStapplerTNewsomeRWongDHiscottPHistological findings of a choroidal neovascular membrane removed at the time of macular translocation in a patient previously treated with intravitreal bevacizumab treatment (Avastin)Br J Ophthalmol20079160260417166893
- GhassemifarRLaiCMRakoczyPEVEGF differentially regulates transcription and translation of ZO-1alpha+ and ZO-1alpha− and mediates trans-epithelial resistance in cultured endothelial and epithelial cellsCell Tissue Res200532311712516163490
- PengSAdelmanRARizzoloLJMinimal effects of VEGF and anti-VEGF drugs on the permeability or selectivity of RPE tight junctionsInvest Ophthalmol Vis Sci2010513216322520042644
- LohelaMBryMTammelaTAlitaloKVEGFs and receptors involved in angiogenesis versus lymphangiogenesisCurr Opin Cell Biol20092115416519230644
- ZiemssenFHeiduschkaPPetersSGrisantiSSchraermeyerUChances and risks of anti-VEGF therapyKlin Monbl Augenheilkd200822577077818759208
- Schmidt-ErfurthUClinical safety of ranibizumab in age-related macular degenerationExpert Opin Drug Saf2010914916520001757
- RosenfeldPJBrownDMHeierJSRanibizumab for neovascular age-related macular degenerationN Engl J Med20063551419143117021318
- BrownDMMichelsMKaiserPKHeierJSSyJPIanchulevTANCHOR Study GroupRanibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR studyOphthalmology2009116576519118696
- Schmidt-ErfurthUClinical safety of ranibizumab in age-related macular degenerationExpert Opin Drug Saf20109114916520001757
- BoyerDSHeierJSBrownDMFrancomSFIanchulevTRubioRGA Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degenerationOphthalmology20091161731173919643495
- ZiemssenFBartz-SchmidtKUGrisantiS(Side) effects of VEGF inhibitionOphthalmologe200610348449216763865
- BaetemanCHoffartLGallandFRidingsBConrathJSubretinal hemorrhage after intravitreal injection of anti-VEGF for age-related macular degeneration: a retrospective studyJ Fr Ophtalmol20093230931319769866
- TilanusMAVaandragerWCuypersMHVerbeekAMHoyngCBRelationship between anticoagulant medication and massive intraocular hemorrhage in age-related macular degenerationGraefes Arch Clin Exp Ophthalmol200023848248510943671
- KiernanDFHariprasadSMRusuIMMehtaSVMielerWFJagerRDEpidemiology of the association between anticoagulants and intraocular hemorrhage in patients with neovascular age-related macular degenerationRetina2010301573157821060269
- IguchiYItoYKikuchiMSeasonal variations of acute massive submacular hemorrhage associated with age-related macular degenerationBr J Ophthalmol2006901256125816837537
- GrossniklausHEWilsonDJBresslerSBClinicopathologic studies of eyes that were obtained postmortem from four patients who were enrolled in the submacular surgery trials: SST Report No. 16Am J Ophthalmol20061419310416386982
- GlattHMachemerRExperimental subretinal hemorrhage in rabbitsAm J Ophthalmol1982947627737180915
- BhisitkulRBWinnBJLeeOTNeuroprotective effect of intravitreal triamcinolone acetonide against photoreceptor apoptosis in a rabbit model of subretinal hemorrhageInvest Ophthalmol Vis Sci20084994071407718421081
- DaneshvarHKertesPJLeonardBCPeymanGAManagement of submacular hemorrhage with intravitreal sulfurhexafluoride: a pilot studyCan J Ophthalmol19993438538810649579
- OhjiMSaitoYHayashiALewisJMTanoYPneumatic displacement of subretinal hemorrhage without tissue plasminogen activatorArch Ophthalmol1998116132613329790631
- PeymanGANelsonNCJrAlturkiWTissue plasminogen activating factor assisted removal of subretinal hemorrhageOphthalmic Surg1991225755821961614
- LimJIDrews-BotschCSternbergPCaponeAAabergTMSubmacular hemorrhage removalOphthalmology1995102139313999097779
- ScheiderAGundischOKampikASurgical extraction of subfoveal choroidal new vessels and submacular hemorrhage in age-related macular degeneration: results of a prospective studyGraefes Arch Clin Exp Ophthalmol199923710159951635
- LewisHIntraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen activator and surgical drainageAm J Ophthalmol19941185595687977569
- YangPMKuoHKKaoMLChenYJTsaiHHPneumatic displacement of a dense submacular hemorrhage with or without tissue plasminogen activatorChang Gung Med J2005281285285916515019
- ThompsonJTSjaardaRNVitrectomy for the treatment of submacular hemorrhages from macular degeneration: a comparison of submacular hemorrhage/membrane removal and submacular tissue plasminogen activator-assisted pneumatic displacementTrans Am Ophthalmol Soc200510398107 discussion 107.17057793
- KameiMTanoYTissue plasminogen activator-assisted vitrectomy: surgical drainage of submacular hemorrhageDev Ophthalmol200944828819494655
- HassanASJohnsonMWSchneidermanTEManagement of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacementOphthalmology19991061900190610519583
- RatanasukonMKittantongAResults of intravitreal tissue plasminogen activator and expansile gas injection for submacular hemorrhage in ThaisEye (Lond)2005191328133215565185
- ChenCYHooperCChiuDChamberlainMKariaNHeriotWJManagement of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gasRetina20072732132817460587
- HaupertCLMcCuenBWJaffeGJPars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degenerationAm J Ophthalmol200113120821511228297
- KameiMTanoYMaenoTIkunoYMitsudaHYuasaTSurgical removal of submacular hemorrhage using tissue plasminogen activator and perfluorocarbon liquidAm J Ophthalmol19961212672758597269
- StifterEMichelsSPragerFIntravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhageAm J Ophthalmol200714488689217916314
- MeyerCHSchollHPEterNHelbHMHolzFGCombined treatment of acute subretinal hemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot studyActa Ophthalmol20088649049418221499
- TreumerFKlattCRoiderJHillenkampJSubretinal co-application of recombinant tissue plasminogen activator and bevacizumab for neovascular age-related macular degeneration with submacular hemorrhageBr J Ophthalmol201094485319946027
- HillenkampJSurguchVFrammeCGabelVPSachsHGManagement of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activatorGraefes Arch Clin Exp Ophthalmol201024851119669780
- MozaffariehMSacuSBeneschTWedrichASubretinal hemorrhages secondary to age-related macular degeneration: psychological and vision-related functional perspectivesOphthalmologica200822219920418497530