Abstract
Purpose
The aim of this study was to evaluate whether indomethacin eye drops and intravitreal ranibizumab (IVR) injections would provide additional benefit over ranibizumab alone in the treatment of choroidal neovascularization (CNV).
Participants and methods
This was a randomized, prospective pilot study of eyes with new-onset CNV. Fifty-eight patients were randomized 1:1 into a ranibizumab monotherapy (RM) group and a ranibizumab plus indomethacin (RI) group. All patients received monthly 0.5 mg IVR injections for 3 months, followed by monthly injections administered as needed. RI group patients also self-administered one drop of 0.5% indomethacin three times a day for 12 months. All patients were followed up for 12 months.
Results
At 12 months, both groups showed significant improvement in best-corrected visual acuity (BCVA) and central retinal thickness (CRT). The mean BCVA change from baseline to 12 months was −0.12±0.04 LogMAR and −0.20±0.04 LogMAR in the RM and RI groups, respectively, with the degree of change being significantly different between the two groups (P=0.04). At 12 months, the mean CRT in the RM group (316±41.2 µm) was significantly higher than that in the RI group (287±31.5 µm; P=0.004). The mean required number of IVR injections was 7.38±0.78 and 6.34±0.67 in the RM and RI groups, respectively (P<0.001).
Conclusion
Compared to IVR monotherapy, combination therapy with indomethacin eye drops and IVR provides superior anatomical and visual outcomes in patients with naive CNV lesions. Moreover, topical indomethacin might reduce the frequency of IVR injections, which is very beneficial considering the chronic and expensive nature of IVR therapy.
Introduction
Age-related macular degeneration (AMD) is still the primary cause of visual impairment and blindness in patients older than 60 years in developed countries.Citation1 In the neovascular form of AMD, the choroidal neovascularization (CNV) beneath the macula leads to fibrous metaplasia, permanent loss of photoreceptors, and disciform scarring, which often results in the loss of central vision.Citation2
Large-scale clinical trials have shown that monthly or bimonthly intravitreal injection of vascular endothelial growth factor (VEGF) antagonists prevents vision loss and may even improve visual acuity in patients with neovascular AMD.Citation3,Citation4 However, it is important to note that VEGF is not the only causative factor in CNV. In particular, free radicals and oxidized lipoproteins in the aging retina are major local triggers of parainflammation, which is the chronic status responsible for the initiation and progression of age-related chorioretinal damage.Citation5,Citation6 Thus, inflammation itself plays an important role in the pathogenesis of CNV and administration of a topical nonsteroidal anti-inflammatory drug (NSAID) has been shown to supplement the effectiveness of anti-VEGF agents in reducing central retinal thickness (CRT)Citation7,Citation8 and the rate of re-injection in CNV.Citation9 A recent assessment of the vitreous penetration of NSAIDs and their effects on prostaglandin E2 (PGE2) revealed a significant reduction in vitreous PGE2 levels in response to indomethacin, bromfenac, and nepafenac.Citation10 Considering these results, in this study, we prospectively evaluated whether the addition of 0.5% indomethacin eye drops three times a day (TID) to the standard treatment of intravitreal ranibizumab (IVR) injections would provide better efficacy than that achieved with ranibizumab alone in patients with CNV.
Participants and methods
Study design
This was an open-label, pilot study in eyes affected by new neovascular AMD. This study was conducted in the Ophthalmic Center of “Spedali Civili di Brescia”, according to the ethical principles of the Declaration of Helsinki. The institutional review board of the “Spedali Civili di Brescia” hospital approved the study protocol (registered with ClinicalTrials.gov, identifier NCT03261635). All study participants provided written informed consent.
Participants
Sixty consecutive participants were enrolled from September 2016 to March 2017 at the Spedali Civili Ophthalmic Center (Spedali Civili di Brescia) in Brescia, Italy. Patients were randomized (online statistical computing web programming www.graphpad.com/quickcalcs) at a 1:1 ratio into the following two groups: monotherapy with IVR injections (ranibizumab monotherapy [RM] group; n=29) and IVR injections plus off-label topical 0.5% indomethacin eye drops (ranibizumab plus indomethacin [RI] group; n=29). One patient per group was excluded because they moved to another eye clinic after the initial ranibizumab-loading phase.
Inclusion and exclusion criteria
Inclusion criteria were as follows: 1) provision of written informed consent, 2) age >40 years, and 3) presence of treatment-naive neovascular AMD, which was defined as neovascularization, fluid, or hemorrhage underneath the fovea. The presence of new active CNV was confirmed by the evidence of leakage on fluorescein angiography and fluid on spectral domain optical coherence tomography (OCT) (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany). The diagnosis was made by one investigator (AR) and thereafter confirmed by the second investigator (EG).
Exclusion criteria were as follows: 1) any prior intravitreal or retinal laser treatment, 2) pathological myopia (>7 D), 3) any concomitant eye disease, and 4) corneal epithelial defects or any condition that would affect the cornea.
Study treatments
Participants in both groups received an initial loading phase of 3 monthly IVR. Retreatment criteria for further injections performed by a masked examiner were as follows: 1) any intraretinal or subretinal fluid on OCT, 2) new or persistent hemorrhage, and 3) decrease in visual acuity. In the case of the absence of fluid on OCT or visual acuity drop, a fluorescein leakage of >25% of the lesion circumference or the expansion of CNV was needed for retreatment in suspicious cases.
Patients in the RI group were given a bottle of eye drops of 0.5% indomethacin ophthalmic solution for self-administration, which was provided free of charge by Alfa Intes Srl (Casoria, Italy). Over the 12-month study period, indomethacin was administered at a dose of one drop in the study eye TID. To verify and increase their compliance, patients were asked to bring their used bottles of indomethacin during each visit.
Clinical evaluation
The following examinations were performed at each visit by a blinded examiner: 1) measurement of best-corrected visual acuity (BCVA) as measured by the Early Treatment Diabetic Retinopathy study letter score, 2) fully dilated slit-lamp ophthalmic examination, 3) OCT (Spectral Domain Spectralis OCT) measurement of CRT, and 4) evaluation of adverse ocular events. Fundus photography (fluorescein and indocyanine green angiography) was performed at baseline, 3 months, and 12 months and at any monthly visit between 4 and 11 months on the basis of retreatment criteria.
Endpoints and outcome measures
Outcome measures were as follows: 1) mean change in study eye visual acuity; 2) mean change in CRT; 3) mean number of IVR injections over the 6-month period; and 4) adverse ocular events at 12 months.
Statistical analyses
To determine if there was a significant difference in changes in BCVA and CRT, repeated measures of analysis of variance with Greenhouse–Geisser and Bonferroni corrections were performed. Independent samples t-tests were conducted to determine the statistical significance of differences in visual acuity, CRT, and number of injections between the RM and RI groups. All statistical analyses were performed using the SPSS software V.20 (IBM Corporation, Armonk, NY, USA). P-values <0.05 were considered significant, and all values are reported as mean ± standard deviation.
Results
Baseline demographic and clinical data of the participants are shown in . In general, the baseline demographics between the two groups were well matched, with no significant differences between the groups. All 58 patients completed the study.
Table 1 Baseline patient characteristics
Visual acuity
The mean BCVA score improved significantly in both treatment groups, with the largest mean change observed in the RI group from baseline ().
Figure 1 Change in visual acuity in both groups over 12 months of treatment.
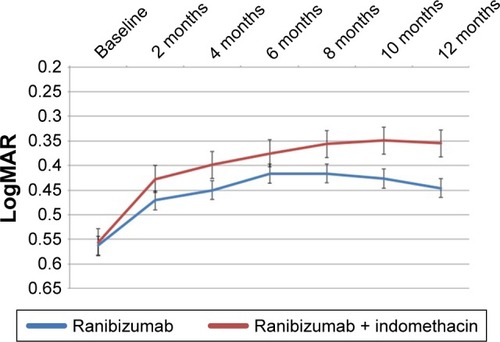
The mean change in BCVA from baseline to 12 months was −0.12±0.04 LogMAR in the RM group (P=0.04) and −0.20±0.04 LogMAR in the RI group (P=0.001), with the degree of change being significantly different between the two groups (P=0.04).
At 12 months, the mean BCVA in the RI group (0.35±0.14 LogMAR) was significantly higher than that in the RM group (0.45±0.18 LogMAR; P=0.04). shows the changes in visual acuity at key time points.
Table 2 Changes in visual acuity and central retinal thickness over 12 months of treatment
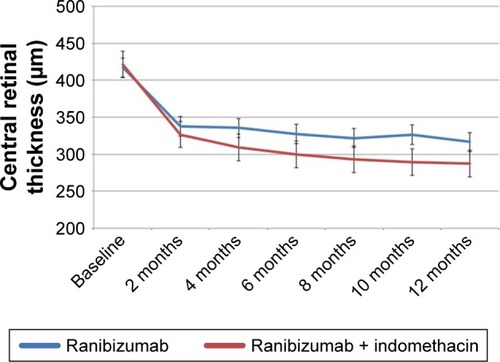
CRT
A constant and significant reduction in mean CRT was observed in the RM group (−101±12.6 µm; P<0.0001) and in the RI group (−134±14.8 µm; P<0.0001) from baseline to 12 months. shows the changes in CRT in both groups over the 12-month treatment period. At 12 months, the mean CRT in the RM group (316±41.2 µm) was significantly higher than that in the RI group (287±31.5 µm; P=0.004). Changes in CRT over the 12-month treatment period are shown in .
Figure 2 Change in central retinal thickness in both groups over 12 months of treatment.
Number of injections
The mean number of required IVR injections was 7.38±0.78 in the RM group and 6.34±0.67 in the RI group (P<0.001).
Safety and adverse events
No serious adverse events were observed during the study period. There was no significant difference in the number of adverse ocular events described by either group. Mild burning/stinging was reported more frequently in the RI group, while pain was similar between groups. Adverse ocular events are reported in . No issues regarding compliance with the indomethacin therapy were recorded for any patient.
Table 3 Ocular adverse events in the study eyes during the 12-month study period
Discussion
The results of this prospective pilot study suggest that the topical administration of indomethacin, when used in combination with ranibizumab, may produce further improvements in BCVA, retinal thickness, and the number of required IVR injections.
Although anti-VEGF agents constitute the first line of therapy against CNV in AMD, topical NSAIDs targeting specific inflammatory processes have recently been tested both experimentallyCitation11,Citation12 and clinically and have been found to ameliorate AMD more effectively and synergistically.Citation7–Citation9,Citation13 Moreover, these results are supported by the recent finding of significant vitreous penetration by NSAIDs, which reach therapeutic concentrations that allow a significant reduction in PGE2 and IL-8 vitreous production.Citation10,Citation14,Citation15
We believe that the combination of IVR and indomethacin has the potential to reduce the burden associated with monthly IVR injections. Indeed, the results of this pilot study suggest that the addition of topical 0.5% indomethacin may result in a significant reduction in the 12-month CRT in patients with neovascular AMD, with a small but significant BCVA improvement over IVR monotherapy.
The anatomical and functional improvements shown in this study are consistent with the results of previous similar studies with other topical NSAIDs, including ketorolac,Citation7,Citation8 bromfenac,Citation9,Citation13 and nepafenac.Citation16 Although a comparison between different studies is not feasible, the results coming from this study show a greater synergistic effect with topical 0.5% indomethacin. This might be explained by lower PGE2 vitreous levels after treatment with 0.5% indomethacin, compared to 0.09% bromfenac and 0.1% nepafenac in a prospective, investigator-masked, and randomized study.Citation10
Interestingly, we observed that the addition of indomethacin resulted in a significant reduction in the number of IVR injections, which could be a pivotal class effect with NSAIDs. Moreover, this finding is consistent with a previous report by Gomi et al,Citation9 wherein patients who were prescribed bromfenac in combination with IVR required one less IVR injection during the first 6 months of therapy. Reduced frequency of IVR injections, although slight, is greatly beneficial in light of chronic and expensive (the mean national Medicare drug payment per anti-VEGF injection in the USA is reported to be $1,078 per injection)Citation17 intravitreal therapy, which can be associated with serious adverse events such as endophthalmitis, retinal tears, and retinal detachment.
No serious adverse events were reported in our study. The results show that the treatments were well tolerated in all groups, with a safety profile comparable to that observed in previous studies. Furthermore, compliance with eye drop use was very high and the incidence of reported adverse events was mild to moderate.
Our study has a few limitations: first, we included a limited number of patients in a pilot study design. Second, the evaluation of adverse events was limited to eye and headache symptoms. Third, we only assessed indomethacin TID. Further studies with a larger sample size, various dosing regimens, and more in-depth follow-up are warranted to validate the results of this pilot study, especially considering the substantial visual decline during the late stages of neovascular AMD.Citation18
Conclusion
The results of this pilot study suggest that topical 0.5% indomethacin administered TID with IVR has an additive effect on CRT reduction in CNV. As previously reported, further larger studies are needed to confirm these data, especially evaluating the long-term efficacy of NSAIDs because AMD is a chronic disease with an inflammatory component. In particular, careful attention should be paid to the corneal complications associated with long-term use of topical NSAIDs.
Acknowledgments
This study was presented in the AAO 2016 Meeting; Chicago, IL, USA. October 15, 2016 – October 18, 2016.
Disclosure
The authors report no conflicts of interest in this work.
References
- KleinRKleinBEKKnudtsonMDPrevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosisOphthalmology2006113337338016513455
- D’AmicoDJDiseases of the retinaN Engl J Med19943312951068208273
- RosenfeldPJBrownDMHeierJSRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- HeierJSBrownDMChongVIntravitreal aflibercept (VEGF trap-eye) in wet age-related macular degenerationOphthalmology2012119122537254823084240
- NitaMGrzybowskiAAscasoFJHuervaVAge-related macular degeneration in the aspect of chronic low-grade inflammation (pathophysiological parainflammation)Mediators Inflamm2014201493067125214719
- LinTWalkerGBKurjiKParainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eyeCytokine201362336938123601964
- SemeraroFRussoADelcassiLTreatment of exudative age-related macular degeneration with ranibizumab combined with ketorolac eyedrops or photodynamic therapyRetina20153581547155425784358
- RussoACostagliolaCDelcassiLRomanoMRSemeraroFA randomised controlled trial of ranibizumab with and without ketorolac eyedrops for exudative age-related macular degenerationBr J Ophthalmol201397101273127623873901
- GomiFSawaMTsujikawaMNishidaKTopical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degenerationRetina20123291804181022718152
- RussoAMorescalchiFVezzoliSReduction of vitreous prostaglandin E2 levels after topical administration of indomethacin 0.5%, bromfenac 0.09%, and nepafenac 0.1Retina Phila Pa201636612271231
- KimSJTomaHSInhibition of choroidal neovascularization by intravitreal ketorolacArch Ophthalmol2010128559620457981
- SchoenbergerSDKimSJNonsteroidal anti-inflammatory drugs for retinal diseaseInt J Inflamm2013201318
- FlaxelCSchainMBHamonSCFrancisPJProspective randomized controlled trial of combination ranibizumab (Lucentis) and bromfenac (Xibrom) for neovascular age-related macular degeneration: a pilot studyRetina201232341742321862953
- SchoenbergerSDKimSJShengJCalcuttMWReduction of vitreous prostaglandin E2 levels after topical administration of ketorolac 0.45%JAMA Ophthalmol2014132215015424264034
- SchoenbergerSDKimSJShahRShengJCherneyEReduction of interleukin 8 and platelet-derived growth factor levels by topical ketorolac, 0.45%, in patients with diabetic retinopathyJAMA Ophthalmol20141321323724336915
- ChenEBenzMSFishRHUse of nepafenac (Nevanac) in combination with intravitreal anti-VEGF agents in the treatment of recalcitrant exudative macular degeneration requiring monthly injectionsClin Ophthalmol201041249125221151329
- ErieJCBarkmeierAJHodgeDOMahrMAHigh variation of intravitreal injection rates and medicare anti-vascular endothelial growth factor payments per injection in the United StatesOphthalmology201612361257126226976701
- RofaghaSBhisitkulRBBoyerDSSaddaSRZhangKSEVEN-UP Study GroupSeven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP)Ophthalmology2013120112292229923642856
