Abstract
Purpose:
The purpose of this study is to analyze the dose response of the mammalian target of rapamycin (mTOR) inhibitor, rapamycin, on tumor burden and hypoxia, and study the treatment effect on vasculature in LHBETATAG retinal tumors.
Methods:
This study was approved by the Institutional Animal Care and Use Committee and follows Association for Research in Vision and Ophthalmology guidelines. Eighteen-week-old LHBETATAG retinal tumor eyes (n = 30) were evaluated. Mice were divided into five groups and received periocular injections once weekly for two consecutive weeks of: a) 80% DMSO (dimethyl sulfoxide, vehicle control), b) 0.00333 mg/kg, c) 0.167 mg/kg, d) 3.33 mg/kg, and e) 6.67 mg/kg of rapamycin. Tumor sections were analyzed for hypoxia, tumor burden, and vasculature with immunohistochemistry techniques.
Results:
Reduction in tumor burden and hypoxia was significantly different between rapamycin doses and control (P < 0.002). Eyes treated with rapamycin at 0.167, 3.33, and 6.67 mg/kg showed a significant decrease in tumor burden in comparison with the vehicle control group (P = 0.019, P = 0.001, P = 0.009, respectively) and the 0.00333 mg/kg dose response (P = 0.023, P = 0.001, P = 0.010, respectively). Eyes treated with rapamycin at 3.33 mg/kg showed a significant reduction in the amount of hypoxia in comparison with the lower concentration groups (0.00333 and 0.167 mg/kg) of rapamycin (P = 0.024 and P = 0.052, respectively). The number of mature vessels was significantly lower in the 3.33 mg/kg treated versus vehicle control (P = 0.015; equal variances assumed, t-test for equality of means). The number of neovessels was not significantly different between both groups (P = 0.092).
Conclusion:
Inhibition of mTOR was shown to reduce tumor burden, hypoxia, and vasculature in the LHBETATAG retinoblastoma tumor model. Rapamycin may have a role in combination with chemotherapy or other adjuvant therapies to enhance retinoblastoma tumor control.
Introduction
Retinoblastoma is the most common primary intraocular malignancy in children.Citation1,Citation2 Associated risks of retinoblastoma include metastatic disease, choroidal invasion, and neovascularization.Citation3–Citation5 More than 95% long-term survival rates in the United States and other developed countries have led to a research focus on local tumor control and globe conservation with preservation of sight. However, present treatments (eg, chemotherapy) result in noteworthy complications including, but not limited to, neutropenia, anemia, thrombocytopenia, infections, and risk for second malignancies (eg, acute myeloid leukemia).Citation6 Enucleation is generally performed in about 20% of the cases of intraocular retinoblastoma due to advanced disease.Citation3,Citation7
The serine–threonine kinase, mammalian target of rapamycin (mTOR), assumes a key regulatory role in cell growth and angiogenesis through effects on cellular metabolism and protein translation. Upstream, mTOR is activated by PI3K/Akt signaling, which has been shown to be dysregulated in cancer cells.Citation8,Citation9 As a result, enhanced mTOR activity leads to altered cellular signaling, mediated through the downstream targets such as p70S6K. This pathway has recently been shown to be O2-sensitive. Hypoxia-induced proliferation of adventitial fibroblast was demonstrated to require the activation of mTOR.Citation10 Other studies have shown the effects of mTOR inhibition resulting in a reduction of numerous downstream targets including glucose transporter (GLUT)-1, vascular endothelial growth factor (VEGF), and hypoxia inducible factor (HIF)-1α.Citation11,Citation12 Notably, cell proliferation and retinoblastoma (Rb) protein suppression concomitantly inhibited the mTOR pathway.Citation13
mTOR as an upstream regulator of HIF, a transcription factor that promotes protein production and glycolytic enzymes and transporters involved in glucose uptake under hypoxic conditions, plays a key role in the metabolic shift from oxidative phosphorylation to anaerobic glycolysis.Citation11,Citation12,Citation14 Hypoxic retinoblastoma cells survive under low O2 tension conditions, which are most prevalent during advanced tumor development.Citation15 These cells have been shown to be resistant to chemotherapy and radiation, which specifically target the rapidly dividing cells.Citation16 Hypoxic cells, therefore, may not respond to conventional treatments.Citation15,Citation17 These cells rely on anaerobic glycolysis for adenosine triphosphate (ATP) production and survival, which is a significantly less efficient method than oxidative phosphorylation in generating energy from glucose. We have previously shown that hypoxic cells can be targeted by inhibiting aspects of cellular metabolism. Using the glycolytic inhibitor 2-deoxy-D-glucose (2-DG), tumor burden was significantly reduced while effectively decreasing the amount of intratumoral hypoxia in LHBETATAG retinal tumors.Citation15,Citation16,Citation18–Citation20
Novel therapeutic strategies are lacking to effectively control retinoblastoma without the use of systemic chemotherapy, radiation, or enucleation.Citation21–Citation23 With mTOR potentially being an O2-sensitive pathway that cells may utilize to adapt to harsh tumor microenvironments, treatment with the mTOR inhibitor, rapamycin, may provide a viable mode to modulate the killing of the chemo-resistant hypoxic cell population in LHBETATAG retinal tumors. The purposes of this study are to: 1) analyze the dose response of rapamycin on tumor burden and hypoxia in LHBETATAG retinal tumors, and 2) study the treatment effect on vasculature in these retinal tumors.
Materials and methods
LHBETATAG mouse model for retinoblastoma
The study protocol was approved by the University of Miami Institutional Animal Care and Use Review Board Committee. The LHBETATAG transgenic mouse model used in this study has been characterized previously.Citation24 This animal model develops bilateral multifocal retinal tumors that are stable and grow at a predictable rate (ie, tumor at 4 weeks is undetectable, at 8 weeks is small, at 12 weeks is medium, and at 16 weeks is large).Citation25
Subconjuctival injections of rapamycin
Eighteen-week-old LHBETATAG retinal tumor, right eyes, (n = 30) were treated and evaluated. Mice were divided into five groups and received periocular injections for two consecutive weeks of: a) 80% dimethyl sulfoxide (DMSO, vehicle control), b) 0.00333 mg/kg, c) 0.167 mg/kg, d) 3.33 mg/kg, and e) 6.67 mg/kg. Since rapamycin is lipophilic and, thus, has a poor water solubility, all the dosages of rapamycin were diluted in 80% DMSO. A total volume of 20 μL was administered in each injection. Treatment of rapamycin was given once a week, starting at 16 weeks of age. Eyes were enucleated at 1 week following the last treatment. To assess hypoxia, mice received 60 mg/kg of pimonidazole via intraperitoneal injection. Mice were euthanized with CO2 fumes and eyes were enucleated. Tumor sections were analyzed for hypoxia, tumor burden, and vasculature.
Tumor burden measurements
Eyes were sectioned serially and processed for standard hematoxylin-eosin (H&E) staining. Microscopic images of H&E-stained sections (50 8-μm sections per eye) were obtained with a digital camera at a magnification of 40×. The section of the eye containing the largest cross-sectional tumor area was chosen for analysis. Tumor boundaries were traced using imaging software (Image Pro Express Software; Media Cybernetics, Silver Spring, MD). Tumor areas for all eyes were averaged, yielding an average area for each group. Tumor burden was averaged, yielding an average area for each group. Tumor burden was expressed as the tumor/globe ratio by dividing the tumor area by the area of the globe to normalize the data as previously described.Citation26
Measuring hypoxic regions
To assess tumor hypoxia after treatment, LHBETATAG mice were injected intraperitoneally with a 0.16 mL suspension of pimonidazole (a drug used to detect hypoxia that penetrates all tissues, including the brain). This suspension consisted of 10 mg of pimonidazole hydrochloride (Chemicon, Temecula, CA) in 1 mL saline. Pimonidazole is known to bind to thiol-containing proteins in cells under low oxygen (O2) tension.Citation27 These adducts can be detected with specific antibodies and stained using immunohistochemical techniques. Animals were euthanized 2 hours after pimonidazole injection, and eyes were harvested and sectioned for histopathologic examination. Eyes were fixed with cold methanol for 10 minutes and immunostained with a directly labeled antibody recognizing pimonidazole adducts (Hypoxyprobe 1-Mab-1-FITC, clone 4.3.11.3; Chemicon) or the same concentration of a directly labeled isotype control antibody (mouse IgG1-FITC; Caltag, Burlingame, CA). Background signal intensities were minimal. All samples were normalized to intensities from isotype controls.
Immunohistochemistry
Tumor samples were frozen in OCT (optimal cutting temperature) compound immediately after enucleation, and serially sectioned (8 μm). Slides were fixed with methanol for 10 minutes (−20°C) and immunohistochemical analyses were performed. Mature vessels were detected with alpha-smooth muscle actin (α-sma) Cy3 conjugate (1:3,000; Sigma Chemical Co, St Louis, MO) which specifically binds to pericytes.Citation28 Neovessels were detected with anti-endoglin (CD105 Wi, 1:500; Abcam, Cambridge, MA), which has been shown to have specificity for endothelial cells undergoing angiogenesis.Citation29 Alexa Fluor 568 goat anti-mouse and 488 donkey anti-mouse were used as secondary antibodies for anti-collagen type IV and endoglin, respectively (1:500; Invitrogen, Carlsbad, CA). Omission of the primary antibody (secondary only) was used as a negative control for nonspecific binding. Cell nuclei were stained for 5 minutes with 4′,6′-diamidino-2-phenylindole (DAPI, 1:5,000; Invitrogen, Carlsbad, CA).
Image analysis
Serial cross-sections of eyes containing tumors were examined for the presence of the described markers with a BX51 Olympus upright fluorescence microscope (Olympus American Inc., Melville, NY). All images were obtained at 200× magnification using different filters for the DAPI, Alexa Fluor 488, and 568 signals.
Statistical methods
Analysis of variance followed by post hoc least-significant different tests was used to evaluate differences between treatment groups with respect to tumor burden and hypoxia. Differences in the number of new vessels and mature vessels between the vehicle control and the rapamycin treated group were evaluated by two sample t-test. Values were considered significant with P-values ≤0.05.
Results
Tumor growth is directly associated with advancing age in the LHBETATAG transgenic mouse model.Citation30 We have previously shown that hypoxia is significantly detected in large-size LHBETATAG retinal tumors, and minimal hypoxia is observed in small LHBETATAG retinal tumors.Citation15
To assess the impact of periocular administration of rapamycin on tumor burden and hypoxia, LHBETATAG mice were treated with varying dosages of this mTOR inhibitor. There was no apparent toxicity observed due to the drug at the doses used in the current study. There were highly significant differences between treatment doses for tumor burden and hypoxia (P < 0.001). Tumor burden was found to be significantly different between rapamycin doses (P < 0.002) ( and ). Eyes treated with rapamycin at 0.167, 3.33, and 6.67 mg/kg showed a significant decrease in tumor burden in comparison with the vehicle control group (P = 0.019, P = 0.001, P = 0.009, respectively) and the 0.00333 mg/kg dose response (P = 0.023, P = 0.001, P = 0.010, respectively). There was no difference between the lowest dose group (ie, 0.00333 mg/kg) and the vehicle control for tumor burden (P = 0.992).
Figure 1 Percentage of tumor burden following different doses of treatment with rapamycin alone. Tumor burden is significantly different between rapamycin doses (P < 0.002). Eyes treated with rapamycin at 0.167, 3.33, and 6.67 mg/kg showed a significant decrease in tumor burden in comparison with the vehicle control group (P = 0.019, P = 0.001, P = 0.009, respectively) and the 0.00333 mg/kg dose response (P = 0.023, P = 0.001, P = 0.010, respectively).
Abbreviation: DMSO, dimethyl sulfoxide.
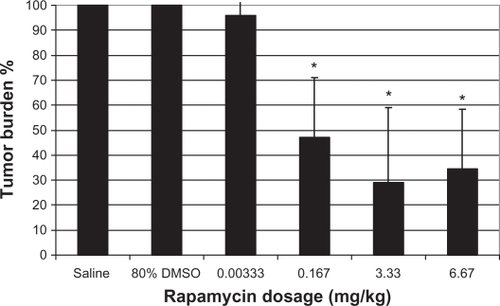
Figure 2 Hypoxia and tumor burden reduction following different doses of rapamycin treatment. There were highly significant interactions between the treatment dose for tumor burden and hypoxia (P < 0.001). DAPI stain (blue) for all the cell nuclei and pimonidazole stain (green) for hypoxic regions.

The percentage of hypoxia is significantly different between the rapamycin doses (P < 0.002) ( and ). Eyes treated with rapamycin at 3.33 mg/kg showed a significant decrease in the percentage of hypoxia in comparison with the lower concentration groups (0.00333 and 0.167 mg/kg) of rapamycin (P = 0.024 and P = 0.052, respectively). There was no difference between the highest doses of rapamycin (3.33 and 6.67 mg/kg) for the percentage of intratumoral hypoxia (P = 0.997). There is a significant correlation between tumor burden and the percentage of hypoxia (P < 0.001; Spearmen non-parametric correlation, r = 0.725).
Figure 3 Reduction in the percentage of tumor hypoxia following different doses of treatment with rapamycin. The percentage of hypoxia is significantly different between the rapamycin doses (P < 0.002). Eyes treated with rapamycin at 3.33 mg/kg showed a significant decrease in the percentage of hypoxia in comparison with the groups treated with 0.00333 and 0.167 mg/kg of rapamycin (P = 0.024 and P = 0.052, respectively).
Abbreviation: DMSO, dimethyl sulfoxide.
To assess the impact of periocular administration of rapamycin on blood vessels, the amount of neovessels and mature vessels were analyzed for the rapamycin dose with the highest impact on the reduction of tumor burden and hypoxia (ie, 3.33 mg/kg). Blood vessels were broken down by vessel caliber (ie, small and large).Citation31 The percentage of mature vessels was significantly lower in the rapamycin treated versus the vehicle control group (P = 0.015; equal variances assumed, t-test for equality of means) ( and ). The percentage of neovessels was not significantly different between the rapamycin treated and the vehicle control group (P = 0.092). This change was mainly due to the small-caliber blood vessels with a reduction of 41.1% for mature vessels versus 70.5% for neovessels compared with the vehicle control (P < 0.001). There were no significant changes for large-caliber vessels for neither neo- nor mature vessels compared with the vehicle control (101% and 54.7%, respectively).
Figure 4 Effect of rapamycin on the vasculature. Neovessels and mature vessels were analyzed for the rapamycin dose with the highest impact on reducing tumor burden and hypoxia (ie, 3.33 mg/kg). The percentage of mature vessels was significantly lower in the rapamycin treated versus the untreated group (P = 0.015), whereas the percentage of neovessels was not significantly different between the rapamycin treated and the control group (P = 0.092).
Abbreviation: DMSO, dimethyl sulfoxide.

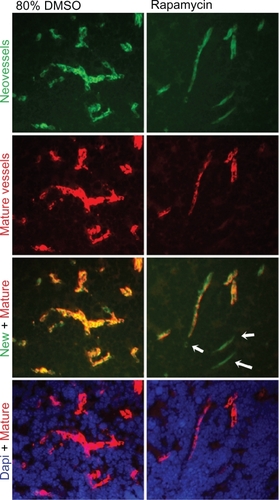
Figure 5 Effect of rapamycin on mature blood vessels. Neovessels and mature vessels were analyzed for the rapamycin dose with the highest impact on reducing tumor burden and hypoxia (ie, 3.33 mg/kg). Although the amount of both neo- and mature vessels decreased following treatment with rapamycin, only the amount of small-caliber, mature vessels decreased significantly (P = 0.015). DAPI stain (blue) for all the cell nuclei, endoglin stain (green) for neovessels, and α-sma stain (red) for mature blood vessels. Pictures were obtained at 200× high power field.
Abbreviations: DAPI, 4′,6′-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide.
Discussion
This study is the first to demonstrate that inhibition of mTOR significantly reduces hypoxia and tumor burden in LHBETATAG retinal tumors. Tumor cells thrive in a heterogeneous microenvironment that contains regions with low O2 tensions requiring neoplastic cells to adapt to hypoxic conditions as the tumor develops.Citation15,Citation17,Citation32–Citation34 In order to survive under hypoxia, metabolically active tumor cells alter their protein synthesis favoring enhanced anaerobic glucose metabolism. Additional stress adaptations include mTOR activation, which stimulates HIF-1 through downstream effectors,Citation35–Citation37 leading to altered cellular metabolism and angiogenesis.Citation38 As a result, hypoxic cells utilize anaerobic glycolysis for ATP production and rely less on oxidative phosphorylation. With this shift to anaerobic glycolysis, cells selectively alter gene expression to increase glucose transporters and glycolytic enzymes such as hexokinase, which catalyzes the phosphorylation of glucose in the first step of glycolysis. Maher et al recently showed that HIF-1 increases the expression of hexokinase.Citation35 Conversely, hypoxic cells with altered siRNAs or mutations that are unable to activate HIF-1, have a corresponding decrease in hexokinase levels.Citation35
The current study supports the hypothesis that hypoxic cells are targeted by blocking mTOR signaling with rapamycin, leading to a corresponding reduction in tumor burden. The reduction in hypoxia observed from different doses of rapamycin treatment suggests that mTOR upregulation is involved in the survival mechanism used by retinoblastoma cells under hypoxic conditions. In the absence of mTOR, the regular process of HIF-1 proteosome-executing degradation continues,Citation39 thus preventing hypoxic cells from having a corresponding increase in the levels of the enzymes and glucose transporters required to survive under anaerobic or low O2 partial pressure conditions, making hypoxic cells less adept to rely on anaerobic metabolism.Citation35 The current findings further support the essential role the tumor microenvironment, notably hypoxia, plays in advanced tumor progression.
We have previously shown that 2-DG and 2-fluorodeoxy-D-glucose (2-FDG) successfully kill the chemoresistant, hypoxic cell population and decrease tumor burden in the LHBETATAG transgenic mouse model of retinoblastoma (work in progress for 2-FDG).Citation15,Citation17,Citation32 These glycolytic inhibitors can be used in combination with chemotherapy and anti-angiogenic agents to have a synergistic effect on tumor burden.Citation32 Since mTOR and HIF-1 upregulate the production of glycolytic enzymes, higher amounts of the competitive inhibitor, 2-DG, are essential to inhibit glycolysis. In fact, increased sensitivity of hypoxic cells to 2-DG is correlated to the expression of HIF in different tumor cell lines.Citation40 It follows that mTOR inhibition should decrease this resistance to glycolytic inhibition found in tumor cells under hypoxia. Thus, blocking mTOR with rapamycin should not only interfere with the downstream upregulation of anaerobic metabolism but also cause hypoxic cells to become more susceptible to 2-DG. Results from the current study further demonstrate that mTOR may play an essential role in either the cellular shift to anaerobic glycolysis or the anaerobic uptake of glucose. The dose effect response curve obtained for tumor burden and hypoxia following rapamycin treatment provides baseline data to consider combination therapies of mTOR and chemotherapy for vasculature targeting to treat retinoblastoma.
Angiogenesis has been highly correlated with tumor proliferation and metastasis in a number of tumors.Citation41–Citation45 Protein synthesis for cell growth, proliferation, and angiogenesis is regulated by mTOR, which controls different signals from growth factor receptors to secure the cell with sufficient nutrients and energy for cell growth. Cancer cells have been shown to have a dysregulation in the angiogenic pathways mediated by mTOR.Citation46 Using the LHBETATAG mouse model of retinoblastoma, we have shown that anti-angiogenic therapy primarily targets areas with high angiogenic activity, while having little to no effect in established mature blood vessels.Citation30 We have also shown that the heterogeneity and spatial distribution of neovessels and mature vessels in ocular tumors may impact the efficacy of anti-angiogenic therapies, and may dictate the treatment modalities used.Citation31,Citation47 Other studies have shown that inhibition of mTOR can potentially inhibit angiogenesis by reducing VEGF-receptors (VEGFRs).Citation48,Citation49 VEGFR regulates angiogenic signaling in both endothelial cells and vascular pericytes, mediating tumor proliferation.Citation49,Citation50 In the current study, the number of mature blood vessels significantly decreased following treatment with rapamycin, whereas the number of neovessels remained stable following treatment. These results suggest that rapamycin affected pericytes, having little or no effect on endothelial cells. This drug may have had an indirect effect on vascular pericytes by mediating the angiogenic signaling from VEGFR in the tumor microenvironment. In addition, the use of DMSO as the vehicle control may cause nonapparent toxicities on the tumors, affecting the overall effects of angiogenesis.Citation51–Citation53 Since anti-angiogenic therapy (ie, anecortave acetate and combretastatin) has no effect in the established, pericyte protected mature blood vessels that are present in advanced LHBETATAG retinal tumors,Citation30 rapamycin may be an alternative treatment modulator to enhance the effects of anti-angiogenic agents and, thus, their modulation as adjuvant therapies in the treatment of retinoblastoma.Citation17,Citation26,Citation30
In conclusion, we have shown that the mTOR inhibitor rapamycin led to greater tumor control and decreased the amount of hypoxic regions in the LHBETATAG mouse model for retinoblastoma. The use of rapamycin as an mTOR inhibitor in a preclinical trial has several benefits. Rapamycin is commercially available, has been extensively studied in human clinical trials, and has a unique preparation (topical delivery).Citation54–Citation58 We believe that mTOR is a potential target in retinoblastoma and its modulation may allow a synergistic impact on tumor burden control in combination with standard treatment modalities (eg, chemotherapy) and other adjuvant therapies (eg, glycolytic inhibitors and anti-angiogenic agents) to treat retinoblastoma tumors. Future studies should include testing the compatibility of different treatment dose and schedule combinations for optimal retinoblastoma tumor burden control.
Disclosure
The authors report no conflicts of interest in this work.
References
- PendergrassTWDavisSIncidence of retinoblastoma in the United StatesArch Ophthalmol198098120412107396771
- TamboliAPodgorMJHormJWThe incidence of retinoblastoma in the United States: 1974 through 1985Arch Ophthalmol19901081281322288550
- AbramsonDHBeaversonKLChangSTDunkelIJMcCormickBOutcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastomaArch Ophthalmol20041221316132315364710
- KhelfaouiFValidirePAuperinAHistopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institutionCancer199677120612138635145
- ShieldsCLShieldsJABaezKACaterJde PotterPVChoroidal invasion of retinoblastoma: metastatic potential and clinical risk factorsBr J Ophthalmol1993775445488218048
- BenzMSScottIUMurrayTGKramerDToledanoSComplications of systemic chemotherapy as treatment of retinoblastomaArch Ophthalmol200011857757810766148
- RouicLLAertsILevy-GabrielCConservative treatments of intraocular retinoblastomaOphthalmologyIn press2008
- InokiKLiYXuTGuanKLRheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signalingGenes Dev2003171829183412869586
- JohannessenCMReczekEEJamesMFBremsHLegiusECichowskiKThe NF1 tumor suppressor critically regulates TSC2 and mTORProc Natl Acad Sci U S A20051028573857815937108
- GerasimovskayaEVTuckerDAStenmarkKRActivation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferationJ Appl Physiol20059872273115501927
- ZengZSarbassov dosDSamudioIJRapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AMLBlood20071093509351217179228
- WanXHarkavyBShenNGroharPHelmanLJRapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanismOncogene2007261932194017001314
- ChenWLuoYLiuLCryptotanshinone inhibits cancer cell proliferation by suppressing mammalian target of rapamycin-mediated cyclin d1 expression and rb phosphorylationCancer Prev Res (Phila)201031015102520628002
- ToschiALeeEThompsonSPhospholipase D-mTOR requirement for the Warburg effect in human cancer cellsCancer Lett2010299727920805015
- BoutridHJockovichMEMurrayTGTargeting hypoxia, a novel treatment for advanced retinoblastomaInvest Ophthalmol Vis Sci2008492799280518326690
- MaschekGSavarajNPriebeW2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivoCancer Res200464313414729604
- BoutridHPinaYCebullaCMIncreased hypoxia following vessel targeting in a murine model of retinoblastomaInvest Ophthalmol Vis Sci2009505537554319578014
- MaherJCKrishanALampidisTJGreater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditionsCancer Chemother Pharmacol20045311612214605866
- LampidisTJKurtogluMMaherJCEfficacy of 2-halogen substituted D-glucose analogs in blocking glycolysis and killing “hypoxic tumor cells”Cancer Chemother Pharmacol20065872573416555088
- BoutridHPinaYCebullaCVessel targeting increases hypoxia in a murine model of retinoblastomaInvest Ophthalmol Vis Sci2009505537554319578014
- BermanELDonaldsonCEGiblinMMartinFJOutcomes in retinoblastoma, 1974–2005: the Children’s Hospital, WestmeadClin Experiment Ophthalmol20073551217300564
- RouicLLAertsILevy-GabrielCConservative treatments of intraocular retinoblastomaOphthalmology2008
- ScheflerACCicciarelliNFeuerWToledanoSMurrayTGMacular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablationOphthalmology200711416216917070578
- WindleJJAlbertDMO’BrienJMRetinoblastoma in transgenic miceNature19903436656691689463
- JockovichMEPinaYAlegretACebullaCFeuerWMurrayTGHeterogeneous tumor vasculature in retinoblastoma: implications for vessel targeting therapyRetina200828S81S8618317352
- JockovichMEMurrayTGEscalona-BenzEHernandezEFeuerWAnecortave acetate as single and adjuvant therapy in the treatment of retinal tumors of LH(BETA)T(AG) miceInvest Ophthalmol Vis Sci2006471264126816565356
- VariaMACalkins-AdamsDPRinkerLHPimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinomaGynecol Oncol1998712702779826471
- NehlsVDrenckhahnDHeterogeneity of microvascular pericytes for smooth muscle type alpha-actinJ Cell Biol19911131471542007619
- TanakaFOtakeYYanagiharaKEvaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibodyClin Cancer Res200173410341511705856
- JockovichMEBajenaruMLPinaYRetinoblastoma tumor vessel maturation impacts efficacy of vessel targeting in the LH(BETA)T(AG) mouse modelInvest Ophthalmol Vis Sci2007482476248217525173
- PinaYBoutridHScheflerABlood vessel maturation in retinoblastoma tumors: spatial distribution of neovessels and mature vessels and its impact on ocular treatmentInvest Ophthalmol Vis Sci2009501020102418952925
- PinaYHoustonSKMurrayTGFocal, periocular delivery of 2-deoxy-D-glucose as adjuvant to chemotherapy for treatment of advanced retinoblastomaInvest Ophthalmol Vis Sci2010516149615620702830
- MatsumotoSYasuiHMitchellJBKrishnaMCImaging cycling tumor hypoxiaCancer Res201070100191002321159626
- BoxCRogersSJMendiolaMEcclesSATumour-microenvironmental interactions: paths to progression and targets for treatmentSemin Cancer Biol20102012813820599506
- MaherJCWangpaichitrMSavarajNKurtogluMLampidisTJHypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-D-glucoseMol Cancer Ther2007673274117308069
- HaradaHItasakaSKizaka-KondohSThe Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumorsJ Biol Chem20092845332534219098000
- RouschopKMWoutersBGRegulation of autophagy through multiple independent hypoxic signaling pathwaysCurr Mol Med2009941742419519399
- KurmashevaRTHarwoodFCHoughtonPJDifferential regulation of vascular endothelial growth factor by Akt and mammalian target of rapamycin inhibitors in cell lines derived from childhood solid tumorsMol Cancer Ther200761620162817483438
- YuBMiaoZHJiangYc-Jun protects hypoxia-inducible factor-1alpha from degradation via its oxygen-dependent degradation domain in a nontranscriptional mannerCancer Res2009697704771219738058
- WangpaichitrMSavarajNMaherJKurtogluMLampidisTJIntrinsically lower AKT, mammalian target of rapamycin, and hypoxia-inducible factor activity correlates with increased sensitivity to 2-deoxy-D-glucose under hypoxia in lung cancer cell linesMol Cancer Ther200871506151318566221
- RosenblattMIAzarDTAnti-angiogenic therapy: Prospects for treatment of ocular tumorsSemin Ophthalmol20062115116016912013
- StittAWGardinerTAAnti-angiogenic therapy for uveal melanoma – more haste, less speedBr J Ophthalmol20028636836911914199
- YangHGrossniklausHECombined immunologic and anti-angiogenic therapy reduces hepatic micrometastases in a murine ocular melanoma modelCurr Eye Res20063155756216769615
- ClarkAFMellonJLiXYInhibition of intraocular tumor growth by topical application of the angiostatic steroid anecortave acetateInvest Ophthalmol Vis Sci1999402158216210440274
- BergersGBenjaminLETumorigenesis and the angiogenic switchNat Rev Cancer2003340141012778130
- FaivreSKroemerGRaymondECurrent development of mTOR inhibitors as anticancer agentsNat Rev Drug Discov2006567168816883305
- PinaYCebullaCMMurrayTGBlood vessel maturation in human uveal melanoma: spatial distribution of neovessels and mature vasculatureOphthalmic Res20094116016919321938
- LangSAMoserCFichnter-FeiglSTargeting heat-shock protein 90 improves efficacy of rapamycin in a model of hepatocellular carcinoma in miceHepatology20094952353219085954
- AgarwalaSSCaseSEverolimus (RAD001) in the treatment of advanced renal cell carcinoma: a reviewOncologist20101523624520215359
- WilliamsRFMyersALSimsTLNgCYNathwaniACDavidoffAMTargeting multiple angiogenic pathways for the treatment of neuroblastomaJ Pediatr Surg2010451103110920620303
- MotzerRJHutsonTETomczakPOverall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinomaJ Clin Oncol2009273584359019487381
- EscudierBEisenTStadlerWMSorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trialJ Clin Oncol2009273312331819451442
- KimYMYangSXuWLiSYangXContinuous in vitro exposure to low-dose genistein induces genomic instability in breast epithelial cellsCancer Genet Cytogenet2008186788418940470
- DesarIMTimmer-BonteJNBurgerDMvan der GraafWTvan HerpenCMA phase I dose-escalation study to evaluate safety and tolerability of sorafenib combined with sirolimus in patients with advanced solid cancerBr J Cancer20101031637164321045832
- VerweijJDisisMLCannistraSAPhase I studies of drug combinationsJ Clin Oncol2010284545454620855831
- WangYEverolimus in renal cell carcinomaDrugs Today (Barc)20104655756620830316
- CalabroFSternbergCNNovel targeted therapy for advanced renal carcinoma: trials in progressCurr Opin Urol20102038238720625300
- HainsworthJDInfanteJRSpigelDRBevacizumab and everolimus in the treatment of patients with metastatic melanoma: a phase 2 trial of the Sarah Cannon Oncology Research ConsortiumCancer20101164122412920564157