Abstract
Purpose
To evaluate the efficacy of cryopreserved amniotic membrane (CAM) in reducing signs and symptoms of dry eye disease (DED) in a large patient population.
Methods
A retrospective chart review at 10 clinical sites was done of patients with refractory DED who received CAM and completed at least 3 months of follow-up. Data collected were demographics; medical history including previous and current ocular treatment, diagnosis, clinical presentations, comorbidity, duration and frequency of treatment with CAM; and concomitant medications. The primary outcome was the change in dry eye workshop (DEWS) score after treatment.
Results
A total of 97 eyes of 84 patients exhibited severe dry eye despite maximal medical treatments including topical artificial tears, cyclosporine-A, serum, antibiotics, and steroids. Patients manifested with superficial punctate keratitis (86%), filamentary keratitis (13%), exposure keratitis (19%), neurotrophic keratitis (2%), and corneal epithelial defect (7%). After CAM treatment for 5.4±2.8 days, 74 (88%) patients demonstrated an improved ocular surface along with a notable reduction of the severity as the overall DEWS score was significantly reduced from 3.25±0.5 at baseline to 1.44±0.6 at 1 week, 1.45±0.6 at 1 month, and 1.47±0.6 at 3 months (p<0.001). Ten eyes (10%) required repeated treatment to complete healing. Apart from discomfort during CAM placement, there were no adverse events.
Conclusion
Placement of CAM is promising to enhance the recovery of ocular surface health and reduce signs and symptoms in patients with moderate-to-severe DED.
Introduction
Dry eye disease (DED) is one of the most common ocular surface disorders in the USA and worldwide. It affects nearly 30% of the population, and its symptoms, such as ocular discomfort and visual fluctuations, represent the most frequent complaints in ophthalmic practice.Citation1–Citation4 DED is comprised of tear film insufficiency and ocular surface involvement. Despite different underlying pathogenic processes, inflammation is a common denominator in DED, which in turn induces further damage to the corneal epithelium and its underlying structures.Citation5 Various treatment modalities, such as steroids and cyclosporine, have been used to suppress inflammation. However, results are variable and refractory in some cases. In these cases, DED not only negatively impacts the quality of life,Citation2 but also increases the burden on health economics.
Recent progress has been made in understanding the pathogenesis of DED, and different treatment modalities have been introduced. Cryopreserved amniotic membrane (CAM) was used to treat DED with ocular surface involvement, and its short-term efficacy was attributed to its known potent anti-inflammatory effect.Citation6 More recently, John et alCitation7 further evaluated the potential effect of CAM in restoring corneal nerves in DED using in vivo confocal microscopy due to the known correlation between corneal nerve density and the severity of DED.Citation8–Citation10 They showed CAM treatment significantly increased corneal nerve density which was correlated with improved corneal sensitivity and reduced dry eye symptoms.Citation7 This treatment effect was seen for 3 months and may be attributed to CAM’s rich composition of neurotrophic factors, particularly nerve growth factor (NGF).Citation11–Citation13 However, these studies were conducted on a relatively small patient population, and further studies are needed to substantiate the findings. Hence, in this study, we retrospectively reviewed the effect of CAM in a larger patient population with moderate-to-severe dry eye.
Methods
Study design and participants
This is a retrospective study to evaluate the efficacy of self-retained CAM (PROKERA® Slim, Bio-Tissue, Miami, FL, USA) in reducing signs and symptoms of DED associated with ocular surface involvement. The study was exempted under 45 CFR §46.101(b)(4) by the Western Institutional Review Board (Puyallup, WA, USA), and patient consent was not required. The study was conducted at ten clinical sites across the USA in accordance with the Health Insurance Portability and Accountability Act and Declaration of Helsinki. The medical records of patients with DED associated with ocular surface disorders who were treated with CAM as a temporary bandage (PROKERA® Slim) before June 1, 2016, and completed 1 week, 1 month and 3 months of follow-up were reviewed. Inclusion criteria also included subjects aged 21 years and older who had moderate-to-severe DED, grades 2–4, as defined by the Report of the International Dry Eye WorkShop (DEWS).Citation1 Exclusion criteria included symblepharon, recent ocular surgery or injury within 3 months, contact lens wearers, and those who had undergone previous brain surgery or trigeminal nerve damage.
Data collected were demographics, medical history including previous and current ocular treatment, diagnosis, clinical presentations, comorbidity, duration and frequency of treatment with CAM, and concomitant medications. The DEWS score and the severity of DED including discomfort, visual symptoms, corneal staining, and corneal signs were graded from 1 (mild) to 4 (severe) as previously described.Citation1,Citation14 Posttreatment results were evaluated at 1 week, 1 month, and 3 months of follow-up and compared to the baseline. All data were recorded in such a manner that subjects could not be identified, directly or through identifiers linked to their records.
Statistical analysis
Descriptive statistics for continuous variables are reported as the mean ± SD and were analyzed using SPSS software, version 24.0 (SPSS Inc., Chicago, IL, USA). Differences between parameters before and after treatment were analyzed with the analysis of variance test and Student t-test. A p-value <0.05 was considered statistically significant.
Results
A total of 97 eyes of 84 patients [12 (14%) male, 69 (82%) female, and 3 (4%) unknown] were included in this study. They exhibited severe dry eye (DEWS 3.25±0.5) despite maximal medical treatments such as artificial tears (82%), steroids (44%), cyclosporine-A (40%), antibiotics (30%), serum drops (8%), and nonsteroidal anti-inflammatory drugs (5%). Punctal plugs were also noted in 29 cases (35%). The majority of patients presented with ocular discomfort (83%) and blurry vision (60%). Other symptoms included ocular pain (35%), redness (29%), and light sensitivity (14%). Most of the cases manifested with superficial punctate keratitis (86%) followed by exposure keratitis (19%), filamentary keratitis (13%), epithelial defect (7%), and neurotrophic keratitis (2%). Comorbidities included blepharitis (39%), cataract (36%), glaucoma (20%), lagophthalmos (7%), and conjunctivitis (5%).
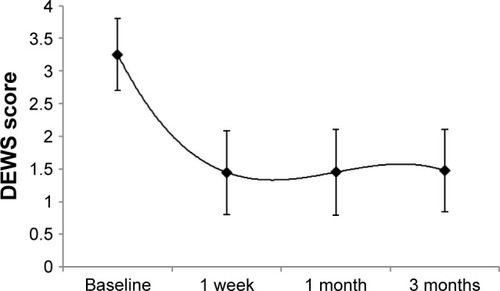
Placement and removal of CAM were uneventful in all cases. Tape-tarsorrhaphy was used in 26 cases (31%) to alleviate discomfort at the time of insertion. The average duration of CAM placement was 5.4±2.8 days, range 2–11 days. CAM was removed from 4 eyes (4%) after 2 days due to CAM intolerance, and another CAM fell out of one eye after 2 days. Upon removal, the AM was intact (28%), partially dissolved (20%), totally dissolved (42%), or not stated (10%). After removal, 74 patients (88%) demonstrated an improved ocular surface () along with notable reduction of the severity of dry eye symptoms. The overall DEWS score was significantly reduced from 3.25±0.5 at the baseline to 1.44±0.6 at 1 week (p<0.001), 1.45±0.6 at 1 month (p<0.001), and 1.47±0.6 at 3 months (p<0.001) (). Specifically, ocular discomfort scores improved from 3.0±0.8 at baseline to 1.3±0.7 at 3 months (p<0.001); visual symptoms scores improved from 2.6±0.9 to 1.0±1.0 (p<0.001); corneal staining scores improved from 2.6±0.7 to 1.0±1.0 (p<0.001); and the overall corneal signs scores improved from 3.5±0.7 at baseline to 2.0±1.0 at 3 months (p<0.001). Although there was significant improvement in visual symptom, the change in distant visual acuity was not statistically significant.
Figure 1 Case representatives: Self-retained CAM for severe DED.
Abbreviations: CAM, cryopreserved amniotic membrane; DED, dry eye disease; DEWS, dry eye workshop.
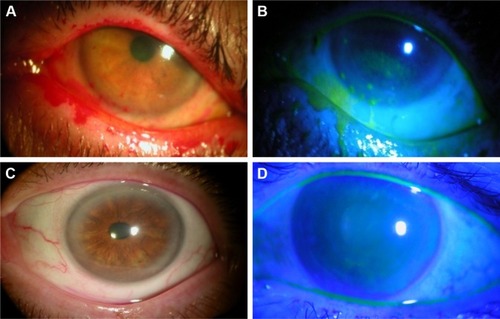
Figure 2 Changes of DEWS score.
Abbreviation: DEWS, dry eye workshop.
Ten patients (10%), who had exposure keratitis or epithelial defects, did not heal at the time of CAM removal and required repeat treatment to complete the healing. For these patients, the overall DEWS score was significantly reduced from 3.60±0.7 at the baseline to 2.5±0.7 at 1 week, 1.90±0.9 at 1 month, and 1.90±0.9 at 3 months. The corneal staining scores improved from 3.3±1.0 at baseline to 1.9±1.2 at 3 months.
The ocular surface remained stable during the follow-up period while the patients continued to use conventional treatment including artificial tears (96%), cyclosporine-A (57%), steroids (32%), antibiotics (23%), serum drops (11%), and nonsteroidal anti-inflammatory drugs (8%). There was no significant change in the number of topical medications after CAM placement. Apart from CAM intolerance, there were no adverse events.
Discussion
This retrospective study demonstrates that self-retained CAM can accelerate the recovery of corneal surface health in patients with moderate and severe DED. Single placement of CAM for 5.4±2.8 days resulted in a significant improvement of DED signs and symptoms with an overall significant reduction in DEWS scoring from 3.25±0.5 (baseline) to 1.44±0.6 at 1 week, 1.45±0.6 at 1 month, and 1.47±0.6 at 3 months. This improvement was associated with restoration of corneal surface health as evidenced by resolution of corneal punctuate staining and improvement of visual symptoms. These findings are consistent with previous studies.Citation6,Citation7
The therapeutic effect of CAM in the treatment of DED can be attributed to multiple mechanisms of action. First, CAM acts as a therapeutic bandage that keeps the eye moist by retaining tears and protects the ocular surface from the surrounding environment. The second mechanism is by controlling ocular surface inflammationCitation6 since it is well established that inflammation triggered by both innate and adaptive immune responses is critical to the pathogenesis and chronicity of DED.Citation15,Citation16 Taking the anti-inflammatory action as an example, CAM has been demonstrated to induce apoptosis of neutrophils,Citation17,Citation18 monocytes, and macrophages;Citation19 reduce infiltration of neutrophils,Citation17,Citation18 macrophages,Citation20,Citation21 and lymphocytes;Citation22 and promote polarization of M2 macrophages.Citation23 Such anti-inflammatory action exerted by CAM is retained in the water-soluble extractCitation24,Citation25 and replicated by HC-HA/PTX3 purified from AM.Citation11,Citation12 A third mechanism is CAM’s ability to regenerate corneal nerves as previously reported,Citation7,Citation26 and this may explain the lasting effect. This notion is supported by the fact that NGF is abundantly present in CAM and is known to play an important role in nerve regeneration and epithelial healing.Citation8,Citation13,Citation27 Other conventional topical anti-inflammatory therapies such as cyclosporine,Citation28 corticosteroids,Citation29 or non-steroidal anti-inflammatory drugsCitation30 have been found to compromise corneal nerves and may explain why some cases do not respond. Collectively, these actions mentioned above of CAM seem to be beneficial in treating DED.
In this study, the ocular surface did not heal in 10% of cases following single placement of CAM and required repeat treatment. These cases had exposure keratopathy, neurotrophic conditions, or persistent epithelial defect. These results are comparable with what has been reported previously by Suri et al,Citation31 who have published a recurrence rate of 14.3%. This recurrence may be explained by the nature of the underlying disease or associated comorbidities. In fact, 39% of the cases had associated blepharitis in this study, and the improvement of lid hygiene helped improve the dry eye symptoms. Therefore, it is essential to look for other comorbidities and treat them accordingly.
DED presents as a public health problemCitation32,Citation33 with substantial economic implications including increased prescription medication use and expenditures for DED. It has been shown that CAM can alleviate DED accompanied with a significant reduction in usage of concomitant topical medications.Citation6 However, the patients in the current study continued to use conventional treatment as per their usual routine even though their symptoms were refractory before CAM placement. Hence, it may be advisable to reduce the number and frequency of concomitant medications to minimize potential toxicity, economic burden, and impact on quality of life.
Conclusion
In conclusion, CAM is a promising treatment to enhance the recovery of ocular surface health and reduce signs and symptoms in patients with moderate-to-severe DED. Further studies are needed to determine longer-term effects (>3 months) and whether repetitive use of CAM generates a more lasting effect.
Acknowledgments
The abstract of this paper was partially presented at the American Society of Cataract and Refractive Surgery (ASCRS) annual meeting, Los Angeles, CA, May 2017, as a conference talk with interim findings. The abstract of this presentation is available at http://www.ascrs.org/node/29198. The ASCRS abstract was also discussed at the 34th Annual Cornea, Contact Lens, Contemporary Vision Care Symposium, Houston, TX, Dec 2017 available at https://ce.opt.uh.edu/live/2017/cornea-contact-lens-contemporary-vision-care-symposium/course-notes/. This work was also presented at the American Academy of Ophthalmology (AAO) annual meeting, New Orleans, LA, November 2017 as a poster presentation. The poster’s abstract is available at https://aao.scientificposters.com/epsAbstractAAO.cfm?id=1. This study was supported in part by a research grant from TissueTech, Inc. Miami, FL.
Disclosure
Dr McDonald and Dr Nanda are consultants and members of the speaker bureau of Bio-Tissue Inc., that distributes PROKERA®. Dr Sheha and Mr Tighe are employees of TissueTech Inc. The authors report no other conflicts of interest in this work.
References
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShopOcul Surf20075759217508116
- GalorAFeuerWLeeDJDepression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative databaseAm J Ophthalmol2012154340.e23466.e222541654
- SchaumbergDADanaRBuringJESullivanDAPrevalence of dry eye disease among US men: estimates from the Physicians’ Health StudiesArch Ophthalmol200912776376819506195
- SchaumbergDASullivanDABuringJEDanaMRPrevalence of dry eye syndrome among US womenAm J Ophthalmol200313631832612888056
- ShehaHTsengSCGThe role of amniotic membrane for managing dry eye diseaseOcular Surface Disorder39th edLondonJP Medical2013325329
- ChengAMZhaoDChenRAccelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membraneOcul Surf201614566326387870
- JohnTTigheSShehaHCorneal nerve regeneration after self-retained amniotic membrane in dry eye diseaseJ Ophthalmol2017640491828894606
- LambiaseASacchettiMBoniniSNerve growth factor therapy for corneal diseaseCurr Opin Ophthalmol20122329630222543481
- JainPLiRLamaTSaragoviHUCumberlidgeGMeerovitchKAn NGF mimetic, MIM-D3, stimulates conjunctival cell glycoconjugate secretion and demonstrates therapeutic efficacy in a rat model of dry eyeExp Eye Res20119350351221726552
- LabbeALiangQWangZCorneal nerve structure and function in patients with non-Sjogren dry eye: clinical correlationsInvest Ophthalmol Vis Sci2013545144515023833066
- TouhamiAGrueterichMTsengSCThe role of NGF signaling in human limbal epithelium expanded by amniotic membrane cultureInvest Ophthalmol Vis Sci20024398799411923238
- BanerjeeANurnbergerSHennerbichlerSIn toto differentiation of human amniotic membrane towards the Schwann cell lineageCell Tissue Bank20141522723924166477
- AloeLTirassaPLambiaseAThe topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcersPharmacol Res20085725325818329283
- MaychukDYPrevalence and severity of dry eye in candidates for laser in situ keratomileusis for myopia in RussiaJ Cataract Refract Surg20164242743427063524
- WeiYAsbellPAThe core mechanism of dry eye disease is inflammationEye Contact Lens20144024825625390549
- YagciAGurdalCThe role and treatment of inflammation in dry eye diseaseInt Ophthalmol2014341291130125416345
- ParkWCTsengSCGModulation of acute inflammation and keratocyte death by suturing, blood and amniotic membrane in PRKInvest Ophthalmol Vis Sci2000412906291410967044
- WangMXGrayTBParksWCCorneal haze and apoptosis is reduced by amniotic membrane matrix in excimer laser photoablation in rabbitsJ Cat Refract Surg200127310319
- ShimmuraSShimazakiJOhashiYTsubotaKAntiinflammatory effects of amniotic membrane transplantation in ocular surface disordersCornea20012040841311333331
- BauerDWasmuthSHermansPOn the influence of neutrophils in corneas with necrotizing HSV-1 keratitis following amniotic membrane transplantationExp Eye Res20078533534517637463
- HeiligenhausABauerDMellerDSteuhlKPTsengSCImprovement of HSV-1 necrotizing keratitis with amniotic membrane transplantationInvest Ophthalmol Vis Sci2001421969197411481259
- BauerDWasmuthSHennigMBaehlerHSteuhlKPHeiligenhausAAmniotic membrane transplantation induces apoptosis in T lymphocytes in murine corneas with experimental herpetic stromal keratitisInvest Ophthalmol Vis Sci2009503188319819255156
- BauerDHennigMWasmuthSAmniotic membrane induces peroxisome proliferator-activated receptor-gamma positive alternatively activated macrophagesInvest Ophthalmol Vis Sci20125379981022222510
- LiWHeHKawakitaTEspanaEMTsengSCGAmniotic membrane induces apoptosis of interferon-gamma-activated macrophages in vitroExp Eye Res20068228229216109408
- HeHLiWChenSYSuppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extractInvest Ophthalmol Vis Sci2008494468447518614802
- MorkinMIHamrahPEfficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal painOcul Surf201816113213829032001
- LambiaseAMantelliFSacchettiMRossiSAloeLBoniniSClinical applications of NGF in ocular diseasesArch Ital Biol201114928329221702001
- NamavariAChaudharySChangJHCyclosporine immunomodulation retards regeneration of surgically transected corneal nervesInvest Ophthalmol Vis Sci20125373274022205605
- LeeHKRyuIHSeoKYHongSKimHCKimEKTopical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patientsOphthalmology200611319820516360211
- GaynesBIOnyekwulujeATopical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspensionClin Ophthalmol2008235536819668727
- SuriKKoskerMRaberIMSutureless amniotic membrane prokera for ocular surface disorders: short-term resultsEye Contact Lens20133934134723945524
- BrewittHSistaniFDry eye disease: the scale of the problemSurv Ophthalmol200145Suppl 2S199S20211587143
- The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShopOcul Surf200759310717508117
