Abstract
Purpose
Retinal ganglion cell (RGC) death is a key feature of glaucoma. Elevated levels of tumor necrosis factor alpha (TNF-α), a pro-inflammatory cytokine, can induce RGC apoptosis and play a critical role in glaucomatous neurodegeneration. Based on the possible role of inflammation and oxidative stress in the pathogenesis of primary open-angle glaucoma (POAG), we investigated the association between plasma levels of TNF-α and POAG or its clinical indices in comparison to non-glaucomatous controls.
Patients and methods
In a case–control retrospective cohort of 51 POAG cases and 88 controls, plasma TNF-α levels were measured using an enzyme-linked immunosorbent assay (ELISA). The assay was performed in duplicates on an automated ELISA analyzer.
Results
Mean TNF-α level was significantly elevated in POAG cases (1.88 ± 2.17 pg/mL) than the controls (0.93 ± 1.49 pg/mL; p = 0.003). The overall dose–response trend was significant (χ2 = 6.12, df = 2; p = 0.047). No statistical difference was seen in age, gender and systemic disease distribution. A modest negative and significant correlation was seen between TNF-α level and number of antiglaucoma medications, an important clinical index of POAG severity. Moreover, logistic regression analysis showed that the risk of POAG was most significantly affected by TNF-α level and not by age and sex.
Conclusion
High systemic level of an inflammatory cytokine, TNF-α, is associated with POAG; however, its possible use as a biomarker for early glaucoma diagnosis and/or disease severity needs further investigation.
Introduction
Glaucoma is a group of optic neuropathies characterized by progressive damage to retinal ganglion cells (RGCs), resulting in characteristic cupping of the optic nerve head and loss of peripheral vision.Citation1 Primary open-angle glaucoma (POAG) is the second most common form of glaucoma in Saudi Arabia, which is clinically characterized by an open and normal anterior iridocorneal chamber angle.Citation2 Although POAG is clinically well defined, the underlying pathological mechanism(s) leading to the loss of RGCs is not yet completely understood. Aging, gender, African ancestry, family history, high intraocular pressure (IOP), central corneal thickness, myopia and genetic predisposition are some of the well-recognized risk factors associated with POAG pathogenesis.Citation3 In addition, there is evidence of significant oxidative damage in human trabecular meshwork cells of patients with glaucoma, causing elevated IOP and visual field damage.Citation4 Although IOP is considered a major risk factor for glaucoma, the role of other concomitant factors affecting the pathophysiology of glaucomatous RGC apoptosis, such as retinal ischemia,Citation5 antioxidant statusCitation6 and several cytokines like interleukins, interferon-gamma and tumor necrosis factor alpha (TNF-α), has also been extensively investigated.Citation7,Citation8
Cytokine release and oxidative stress can contribute to the pathogenesis of glaucomatous degeneration.Citation9 TNF-α is a pro-inflammatory and neuroprotective cytokine that plays an important role in various immunological and physiological processes associated with retinal ischemia, neuronal damage and tissue regeneration.Citation10 The diverse roles of TNF-α are mediated via two distinct receptors – a 55 kDa receptor 1 (TNF-R1) and a 75 kDa receptor 2 (TNF-R2) – and depend on which receptor is activated.Citation11 Stimulation via TNF-R2 is neuroprotective and promotes tissue regeneration, whereas activation via TNF-R1 leads to recruitment of immune cells causing inflammation. Moreover, TNF-R1 can directly induce oxidative stress by the activation of reactive oxygen and nitrogen species-producing enzymes.Citation12 Both, TNF-α-induced oxidative stress and inflammation interact and cooperate to promote neurodegeneration, suggesting a causative role of TNF-α in neurodegenerative disorders, including glaucoma.Citation10,Citation12
Previous studies have demonstrated an increased concentration of cytokine TNF-α in glaucomatous aqueous humor (AH)Citation13,Citation14 or serum cytokinesCitation15 in patients with POAG and have shown that TNF-α is capable of inducing RGC apoptosis, suggesting a critical role of TNF-α in glaucomatous degeneration.Citation12,Citation16 TNF-α is expressed in optic nerve and retina of human glaucomatous eyes which parallels with the progression of optic nerve damage.Citation17 Moreover, we recently showed elevated levels of plasma TNF-α in a group of patients with pseudoexfoliation glaucoma.Citation18
Since inflammation is an important aspect of various age-related disorders, including POAG, cytokine levels can serve as potential biomarkers in clinical practice. Based on the role of inflammation and oxidative stress in the pathogenesis of POAG, we investigated the relationship between plasma levels of TNF-α and POAG or its clinical indices as compared to non-glaucomatous controls.
Patients and methods
Study population
The study followed the Declaration of Helsinki guidelines for conducting research involving humans and was approved by the Institutional Review Board and Ethics Committee of College of Medicine, King Saud University (approval number 08-657). Written informed consent was obtained from all participants. A case–control retrospective cohort was selected to perform the investigation. Individuals of Saudi origin with clinical diagnosis of POAG and non-glaucomatous controls of the same ethnicity were recruited at King Abdulaziz University Hospital in Riyadh, Saudi Arabia. The inclusion and exclusion criteria have been described previously.Citation6 In the POAG patients (n = 51), the disc or retinal nerve fiber layer showed the following characteristics: thinning or notching of disc rim, progressive changes, nerve fiber layer defect; presence of characteristic abnormalities in visual field (eg, arcuate scotoma, nasal step, paracentral scotoma, generalized depression) in the absence of other causes or explanation and open anterior chamber angles bilaterally on gonioscopy. Cases with evidence of secondary glaucoma, for example, pigmentary dispersion syndrome, pseudoexfoliation, history of steroid use or ocular trauma were excluded. Individuals (n = 88) with normal IOP, open angles on gonioscopy and healthy optic disc on examination served as controls. None of the participants included in this study were reported to have any infectious or immunomodulating diseases (rheumatoid arthritis, lupus, Crohn’s disease). Subjects refusing to participate were also excluded.
Sample collection and TNF-α estimation by enzyme-linked immunosorbent assay (ELISA)
Plasma samples were obtained from ethylenediaminetetraacetic acid blood and stored at −80°C until use. Plasma TNF-α level was measured using an ELISA (Catalog No STA00C, Quantikine® ELISA; R&D Systems Inc., Minneapolis, MN, USA) in 51 POAG cases and 88 non-glaucomatous controls as described previously.Citation18 The assay utilizes a monoclonal antibody specific for human TNF-α that is precoated onto a microplate and was performed in duplicate on a ChemWell-T bioanalyzer (Awareness Technology Inc., Palm City, FL, USA), as per the manufacturer’s instructions (R&D Systems Inc.). The steps were programmed using a ChemWell-T Assay Editor in the standard ELISA assay mode. The steps included addition of 50 μL of assay diluent and 200 μL of plasma sample/standard with mixing for 1 min and 2 h incubation at room temperature (RT). This was followed by washing (4× with a soak time of 30 s), addition of TNF-α conjugate (200 μL), mixing for 1 min and incubation for 2 h at RT. A 200 μL of substrate, prepared immediately before use by mixing equal volumes of stabilized hydrogen peroxide and tetramethylbenzidine, was added after washing (4× with a soak time of 30 s). The plate was incubated in dark at RT for 20 min with continuous mixing for 1 min. The final reaction was terminated with 50 μL of stop solution (2 N sulfuric acid), and absorbance was measured at 450 nm with wavelength correction set at 540 nm. The TNF-α concentration was estimated from the calibrated standard curve and expressed in picograms per milliliter.
Statistical analysis
Data were presented as mean ± SD for TNF-α level and other clinical variables. Analyses were performed using Student’s t-test (two tailed), Pearson’s chi-square test and nonparametric Mann–Whitney U-test to evaluate the differences between the two study groups. Correlation testing was done using Spearman’s rho test. Logistic regression analysis was performed to estimate the impact and direction of the effect of other risk factors and mean TNF-α level in patients with POAG. A receiver operating characteristic (ROC) curve was generated, and the area under the curve (AUC) value was calculated using Mann–Whitney U-test. The confidence interval (CI) was set to 95%, and p < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS, version 19.0 (IBM Corp., Armonk, NY, USA).
Results
shows the demographic characteristics, status of systemic conditions and TNF-α level in the POAG cases and control groups included in this study. The two study groups were similar for age (p = 0.409), gender (p = 0.697) and distribution of other systemic conditions (p > 0.05). The mean TNF-α level was significantly higher in the POAG patients (1.88 ± 2.17 pg/mL) than the control subjects (0.93 ± 1.49 pg/mL; 95% CI = 0.33–1.56; p = 0.003). The median values for TNF-α level among the cases and the control subjects were 0.90 pg/mL (range = 0–6.8) and 0 pg/mL (range = 0–8), respectively, and were also significantly different (p = 0.008) ().
Figure 1 Box plot showing the distribution of TNF-α levels in the POAG cases and control group.
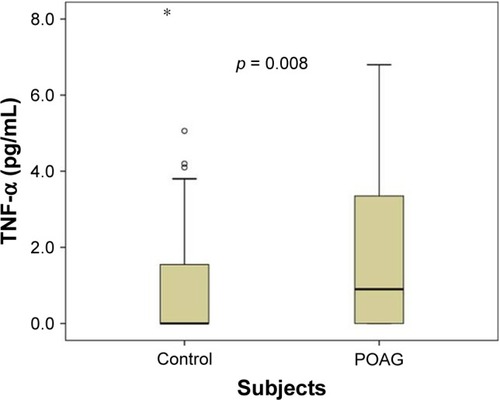
Table 1 Demographics, clinical characteristics and TNF-α level in POAG cases and controls
To assess any dose–response trend, the data was dichotomized (uncategorized) into quartiles, and two cutoff points were identified at 0.0 pg/mL (first quartile or the 25th percentile) and 2.3 pg/mL (third quartile or the 75th percentile). Subjects were then categorized into three groups by using these two cutoff points. The overall dose–response trend was statistically significant (χ2 = 6.12, df = 2; p = 0.047). Comparison between individuals where TNF-α was absent or its concentration was undetectable (0 pg/mL; first quartile) and individuals in the medium category (0.1–2.3 pg/mL; interquartile) did not yield any significant association; however, individuals in the highest category (>2.3 pg/mL; third quartile) showed increased risk of the disease (odds ratio = 3.54, 95% CI = 1.51–8.27; p = 0.0028; ).
Table 2 Quartile distribution of TNF-α level to assess the risk of POAG
A correlation testing between TNF-α levels and age and other clinical indices relevant to POAG, such as IOP, cup-to-disc ratio and number of antiglaucoma medications, was performed using Spearman’s correlation. A modest negative correlation was observed with the number of antiglaucoma medications (p = 0.042), but there was no significant correlation with the age of onset, IOP and cup-to-disc ratio (). Logistic regression analysis showed that the risk of disease outcome (POAG) was most significantly affected by TNF-α level (p = 0.004) but not by age and sex ().
Table 3 Correlation between TNF-α level and other clinical variables
Table 4 Binary logistic regression analysis to determine the effect of age, sex and TNF-α level on disease outcome
Moreover, to determine whether the levels of plasma TNF-α can be used to discriminate between POAG cases and controls, we generated an ROC curve. Comparison of POAG patients with controls yielded an ROC curve area (AUC) of 0.625 (95% CI = 0.526–0.724) that was statistically significant (p = 0.014) but exhibited a poor discriminatory power to distinguish between POAG cases and controls.
Discussion
Glaucoma is characterized by the progressive loss of RGCs resulting in gradual loss of vision and blindness. However, the underlying mechanism(s) promoting RGC damage is still unclear. Accumulating evidence suggests a key role for inflammatory cytokine in glaucomatous degeneration.Citation9–Citation11 Glaucomatous insults, such as axonal injury, elevated IOP, ischemia or obstruction by lamina cribrosa, trigger apoptosis and increased oxidative stress leading to the formation of reactive oxygen and nitrogen species at the localized tissues initiating cytokine secretion in various inflammatory cells.Citation9,Citation10 In this study, we demonstrate a clear association between increased levels of plasma TNF-α and POAG.
Since cytokines are important mediators of the inflammatory process, multiple studies have assessed the local and systemic profile of cytokine TNF-α levels in patients with POAG using different approaches and variable end points. Sawada et al reported elevated levels of TNF-α in the AH of patients with pseudoexfoliation glaucoma and POAG.Citation13 However, the increase in POAG cases was not significant. Similarly, Balaiya et al reported significant elevation of AH TNF-α in POAG.Citation14 Using multiplex cytokine analysis, several other investigators reported there was no change in aqueous TNF-α in POAGCitation19–Citation24 compared to other cytokines. However, using multiplex approach, Tong et al reported significant elevation in vitreous TNF-α in POAG patients.Citation25 Similar to our finding of increased plasma cytokine, TNF-α, in POAG patients, Huang et al reported significant alterations of serum TH1 and TH2 cytokines in POAG as compared to non-glaucomatous controls, suggesting a plausibility of abnormal immune environments contributing to the glaucomatous neuropathy in POAG.Citation15 In addition, based on a similar mechanism of an activated immune response, we recently demonstrated an association between plasma TNF-α and pseudoexfoliation glaucoma.Citation18
As compared to most studies reported in the literature (discussed above) that utilized AH to estimate TNF-α level in POAG, we estimated TNF-α level from plasma in a group of clinically well-defined POAG patients and compared it to non-glaucomatous controls devoid of any ophthalmic disorders. TNF-α levels are known to be affected by age, infection and other systemic conditions such as diabetes, hypertension and atherosclerosis.Citation26 Therefore, care was taken to exclude individuals with infectious/autoimmune disease and select matching controls in terms of age, ethnicity, gender and systemic conditions. Both the mean and median TNF-α levels were significantly increased in our POAG cohort, and the effect was dose dependent. Besides, since the two study groups were similar for systemic diseases, the increase in TNF-α in the plasma could be more plausibly related to POAG condition rather than to any of the systemic disorders. Furthermore, TNF-α level exhibited a modest negative correlation with the number of antiglaucoma medications, a clinical index to assess severity of the disease, suggesting its utility as a marker of disease severity. In contrast, however, the AUC analysis indicated that TNF-α was a poor marker to discriminate between POAG cases and non-glaucomatous controls. Clearly, further investigations are needed to substantiate these findings in a much larger cohort. However, the elevated level of TNF-α in the plasma (in this study) and the AH (previous studies) is a clear indication of an activated immune response triggered by inflammation and its plausible association with glaucomatous degeneration,Citation12 which is in accordance to our previous observation in pseudoexfoliation glaucoma.Citation18
A number of previous studies have provided evidence for a role of TNF-α in glaucomatous degeneration. An increased expression of retinal TNF-α mRNA has been reported to be associated with RGC loss and optic aptropy.Citation16 Similarly, administration of exogenous TNF-α has been demonstrated to promote RGC loss and optic nerve degeneration, whereas genetic or pharmacological depletion of TNF-α or its receptors has been shown to stimulate RGC survival.Citation12 Although the specific reason and mechanism of the relationship between high levels of TNF-α (or cytokines in general) and glaucoma is still uncertain, in vitro experiments have demonstrated that TNF-α can induce RGC death through caspase-3 and caspase-8 activation and/or via the mitochondrial cell death pathway by inducing oxidative damage.Citation12 The observation that the mean TNF-α level was elevated in POAG patients in comparison to age-, gender- and ethnicity-matched non-glaucomatous controls certainly suggests increased inflammation that may contribute to the creation of an oxidative stress status and, in a mechanism(s) described above, such a situation may contribute to glaucoma development and/or progression. Besides, an SNP (G-308A of rs1800629) upstream of the promoter site of TNF-α has been reported to cause elevation of TNF-α and is positively associated with POAG in the Turkish, Egyptian and Iranian populations.Citation27–Citation29 Similarly, there is also some evidence for the role of mitochondrial abnormalities in patients with POAG from Saudi Arabia.Citation30 A plausibility of similar mechanism(s) or as of yet unrecognized gene–gene or gene–environment interaction by elevated systemic levels of this marker contributing to glaucoma in our patients cannot be ruled out in this study.
There are certain limitations to this study. Primarily, we acknowledge the fact that the systemic increase in TNF-α level may not represent the exact intraocular microenvironment at the anterior segment structures, which are directly exposed to free radicals and are thus more pathophysiologically relevant in the development and progression of glaucoma through the oxidative stress or other mechanism(s). In the absence of any evidence of a direct correlation between cytokine alteration in the serum or plasma and cytokine levels in the eyes, this would require assessment of TNF-α level in AH samples. The study was limited to estimation of only a single cytokine. In addition, the sample size investigated was small, especially of POAG cases; thus, the statistical power of the study may be limited.
Conclusion
We found increased levels of plasma TNF-α in patients with POAG as compared to non-glaucomatous controls, indicating that high systemic levels of this inflammatory marker might be associated with POAG. However, further investigations in a large cohort and different ethnicities are needed to substantiate our findings.
Author contributions
All authors contributed toward data analysis, drafting and revising the article and agreed to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research and Glaucoma Research Chair of Department of Ophthalmology, College of Medicine, King Saud University, for their support and use of laboratory facilities. The funder had no role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript. The article has not been presented in any previous conference or scientific meeting.
Disclosure
The authors have no conflict of interests in this work, and the study was not supported or funded by any drug company.
References
- KwonYHFingertJHKuehnMHAlwardWLPrimary open-angle glaucomaN Engl J Med2009360111113112419279343
- Al ObeidanSADewedarAOsmanEAMousaAThe profile of glaucoma in a Tertiary Ophthalmic University Center in Riyadh, Saudi ArabiaSaudi J Ophthalmol201125437337923960951
- Abu-AmeroKKondkarAAChalamKVAn updated review on the genetics of primary open angle glaucomaInt J Mol Sci20151612288862891126690118
- IzzottiACartigliaCDe FloraSSaccaSMethodology for evaluating oxidative DNA damage and metabolic genotypes in human trabecular meshworkToxicol Mech Methods200313316116820021156
- ButtZO’BrienCMcKillopGAspinallPAllanPColor Doppler imaging in untreated high- and normal-pressure open-angle glaucomaInvest Ophthalmol Vis Sci19973836906969071223
- Abu-AmeroKKKondkarAAMousaAOsmanEAAl-ObeidanSADecreased total antioxidants in patients with primary open angle glaucomaCurr Eye Res201338995996423651069
- TezelGOxidative stress in glaucomatous neurodegeneration: mechanisms and consequencesProg Retin Eye Res200625549051316962364
- Munoz-FernandezMAFresnoMThe role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous systemProg Neurobiol19985633073409770242
- NitaMGrzybowskiAThe role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adultsOxid Med Cell Longev20162016316473426881021
- FischerRMaierOInterrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNFOxid Med Cell Longev2015201561081325834699
- AgarwalRAgarwalPGlaucomatous neurodegeneration: an eye on tumor necrosis factor-alphaIndian J Ophthalmol201260425526122824592
- TezelGTNF-alpha signaling in glaucomatous neurodegenerationProg Brain Res200817340942118929124
- SawadaHFukuchiTTanakaTAbeHTumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucomaInvest Ophthalmol Vis Sci201051290390619737888
- BalaiyaSEdwardsJTillisTKhetpalVChalamKVTumor necrosis factor-alpha (TNF-alpha) levels in aqueous humor of primary open angle glaucomaClin Ophthalmol2011555355621607023
- HuangPQiYXuYSSerum cytokine alteration is associated with optic neuropathy in human primary open angle glaucomaJ Glaucoma201019532433019730118
- NakazawaTNakazawaCMatsubaraATumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucomaJ Neurosci20062649126331264117151265
- YuanLNeufeldAHTumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve headGlia2000321425010975909
- KondkarAAAzadTAAlmobarakFAKalantanHAl-ObeidanSAAbu-AmeroKKElevated levels of plasma tumor necrosis factor alpha in patients with pseudoexfoliation glaucomaClin Ophthalmol20181215315929398902
- TakaiYTanitoMOhiraAMultiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataractInvest Ophthalmol Vis Sci201253124124722159018
- OhiraSInoueTIwaoKTakahashiETaniharaHFactors influencing aqueous proinflammatory cytokines and growth factors in uveitic glaucomaPLoS One2016111e014708026771310
- OhiraSInoueTShobayashiKIwaoKFukushimaMTaniharaHSimultaneous increase in multiple proinflammatory cytokines in the aqueous humor in neovascular glaucoma with and without intravitreal bevacizumab injectionInvest Ophthalmol Vis Sci20155663541354826030108
- KuchteyJRezaeiKAJaru-AmpornpanPSternbergPJrKuchteyRWMultiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humorInvest Ophthalmol Vis Sci201051126441644720592224
- ChuaJVaniaMCheungCMExpression profile of inflammatory cytokines in aqueous from glaucomatous eyesMol Vis20121843143822355254
- BorkensteinAFaschingerCMaierRMeasurement of tumor necrosis factor-alpha, interleukin-6, Fas ligand, interleukin-1alpha, and interleukin-1beta in the aqueous humor of patients with open angle glaucoma using multiplex bead analysisMol Vis2013192306231124265545
- TongYZhouYLZhengYBiswalMZhaoPQWangZYAnalyzing cytokines as biomarkers to evaluate severity of glaucomaInt J Ophthalmol201710692593028730084
- BruunsgaardHPedersenBKAge-related inflammatory cytokines and diseaseImmunol Allergy Clin North Am2003231153912645876
- BozkurtBMesciLIrkecMAssociation of tumour necrosis factor-alpha-308 G/A polymorphism with primary open-angle glaucomaClin Exp Ophthalmol2012404e156e16221575121
- HamidMAMoemenLLabibHHelmyHElserganyTRisk of open angle glaucoma due to tumor necrosis factor alpha gene polymorphismsElectron Physician2016821978198327054008
- RazeghinejadMRRahatFKamali-SarvestaniEAssociation of TNFA -308 G/A and TNFRI +36 A/G gene polymorphisms with glaucomaOphthalmic Res200942311812419556827
- Abu-AmeroKKMoralesJBosleyTMMitochondrial abnormalities in patients with primary open-angle glaucomaInvest Ophthalmol Vis Sci20064762533254116723467
