Nichols KK, Holland E, Toyos MM, et al. Ocular comfort assessment of lifitegrast ophthalmic solution 5.0% in OPUS-3, a Phase III randomized controlled trial. Clin Ophthalmol. 2018;12:263–270.
On page 266, both mentions of Figure 3, should be .
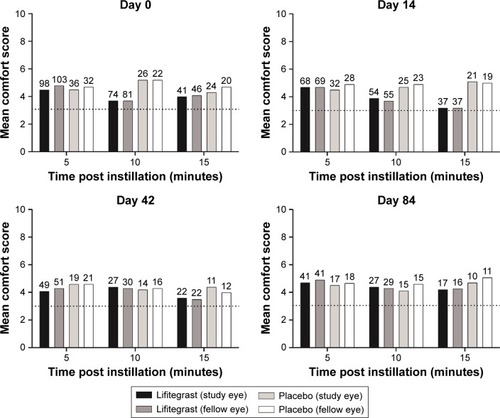
Figure 4 Drop comfort scores at 5, 10, and 15 minutes postinstillation among participants with drop comfort score >3 at 3-minute assessment (safety population; numbers above bars indicate participant numbers).
On page 267, is drawn incorrectly, the correct figure appears below:
