Abstract
Background
Clareon® is a new hydrophobic acrylic optic biomaterial designed for enhanced clarity and greater resistance to glistening. The present study evaluated the effectiveness and safety of a three-piece hydrophobic, monofocal intraocular lens (IOL) Model MA60NM, made of this new optic material.
Methods
In this prospective, multicenter, open-label study, eligible patients aged ≥60 years, underwent a unilateral implantation with IOL Model MA60NM following phacoemulsification. Patients were followed-up for up to 3 years after implantation. Visual outcome and serious adverse events (SAEs, cumulative and persistent) were compared to ISO grid rates (BS EN ISO 11979-7:2006). The primary effectiveness variable was Best Spectacle-Corrected Visual Acuity (BSCVA) at 1-year postoperative follow-up. In addition, posterior capsular opacification (PCO) was assessed qualitatively and graded by slit lamp exam on a 5-point scale at all visits.
Results
Overall, 179 and 138 patients completed the 1-year and 3-year postoperative follow-up, respectively. The BSCVA outcomes were better with IOL Model MA60NM than the ISO grid rates with 95.5% of patients at 1 year and 94.2% of patients at 3 years having achieved a BSCVA of 20/40 or better vs 92.5% in ISO grid. The incidence of cumulative or persistent SAEs was lower after Model MA60NM implantation than the ISO grid reference. The incidence of clinically significant PCO was 1.1% at the 1-year and 2.2% at the 3-year visit. Posterior capsulotomy rate was 1.1% at 1 year and 1.4% at 3 years.
Conclusion
The three-piece hydrophobic, monofocal IOL Model MA60NM was effective for the visual correction of aphakia and successfully met all the safety parameters as defined by the ISO criteria. PCO and posterior capsulotomy rates were low over the 3-year follow-up period. This study provides evidence and supports the long-term safety and effectiveness of the new optic biomaterial Clareon®.
Introduction
Posterior capsular opacification (PCO) is a relatively common complication after an uneventful cataract surgery.Citation1,Citation2 It occurs as a result of the growth and migration of the residual lens epithelial cells (LECs) from the peripheral posterior capsular bag into the space between the capsule and the optic of the intraocular lens (IOL) leading to opacification.Citation1–Citation5 It has an adverse effect on optic clarity, quality of vision, and visual function.Citation1–Citation5 It is a delayed manifestation that can appear months or years after cataract surgery, with an incidence of 28%–67% after IOL implantation in adults and 100% in children, and depends on several factors.Citation5–Citation7
IOL capsular biocompatibility is an important contributor to the development of PCO.Citation1,Citation2 Studies have shown that the IOL material and design have an impact on the rate of PCO development.Citation4,Citation8–Citation10 In a meta-analysis of 11 studies involving 889 eyes, hydrophobic IOLs were found to be associated with significantly lower subjective PCO scores in comparison with hydrophilic lenses.Citation11 Unlike hydrophobic IOLs that strongly adhere to the posterior capsule, hydrophilic polymers and silicone materials promote fibrosis, cell migration, and proliferation on the IOL surface and thus tend to lead to higher rates of PCO formation.Citation12,Citation13 In addition, lower PCO rates are observed with IOLs that have a sharp-edged design as it inhibits the adhesion of macrophages and LECs to IOL.Citation4,Citation10,Citation14 Therefore, apart from clinical performance, it is also essential to consider the capsular biocompatibility of any new IOL optic material.
Clareon® (Alcon Laboratories Inc., Fort Worth, TX, USA) is a new cross-linked acrylic optic biomaterial developed by combining a hydrophilic polymer (2-hydroxyethyl-methacrylate) and a hydrophobic component (phenylethyl acrylate) with a chemically bonded ultraviolet absorber. The present study describes the long-term effectiveness and safety of a three-piece, soft, foldable, acrylic, hydrophobic, monofocal lens Model MA60NM, made of Clareon® biomaterial, in patients implanted after cataract surgery by phacoemulsification.
Methods
Study design
This prospective, multicenter, open-label study was conducted from January 2000 to December 2004 at seven sites in four countries (three sites in Germany, two in UK, one site each in Spain and Italy). This study was performed in compliance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice, and IOL standards per the International Organization for Standardization (ISO).Citation14,Citation15 The protocol and its amendments were approved by institutional review boards at each participating center.
Eligible patients underwent a unilateral IOL implantation with the IOL Model MA60NM (Alcon Laboratories Inc.) following phacoemulsification. The MA60NM IOL had a three-piece construction, a 6.0-mm-diameter biconvex optic, and an overall length of 13.0 mm. The haptics were of blue core polymethylmethacrylate with a 10° angulation. The IOL was manufactured in powers of 15.0–29.0 diopter in 0.5-diopter increments. Each IOL had a unique serial number. Each IOL package contained a patient registration card (Lens Implant Card), a patient identification card, adhesive labels with IOL labels, and the IOL.
Postoperative visits were scheduled at days 1–2 (visit 1), days 7–14 (visit 2), days 30–60 (visit 3), days 90–119 (visit 4), days 120–180 (visit 5), and days 330–420 (visit 6/ 1-year) after surgery. Following a protocol amendment, the study was extended to include two additional postoperative visits during days 630–780 (visit 7/2-year) and days 990–1140 (visit 8/3-year). Patients who had exited the initial study at the end of 1 year were contacted and re-enrolled into the study for the extension phase.
The objective of this study was to compare visual acuity and serious adverse events (SAEs) in patients implanted with IOL Model MA60NM vs historical control incidence levels by using the rates and methods described in the BS EN ISO 11979-7:2006. The BS EN ISO 11979-7:2006 specifies particular requirements for clinical investigations for posterior and anterior chamber monofocal IOLs for the correction of aphakia.Citation15
Study population
Eligible patients were male or female patients aged ≥60 years, in good general and ocular health, with an age-related cataract that required extraction with IOL implantation, who expected to achieve a postoperative 20/40 or better visual acuity, and who were willing and able to complete all required postoperative visits.
Patients with any of the following conditions were excluded: amblyopia; clinically severe corneal dystrophy; congenital, traumatic, or complicated cataracts or rubella cataract; cornea plana; proliferative diabetic retinopathy; history of macular edema (ME) or retinal detachment or uveitis/ iritis; iris neovascularization; pupil diameter <5.0 mm after dilation; medically uncontrolled glaucoma; optic atrophy; previous corneal transplant or glaucoma filtering surgery; recurrent severe anterior or posterior segment inflammation due to unknown etiology; vitreous loss; significant anterior chamber hyphema; Alzheimer’s disease; terminal cancer; or any other condition/complication that, in the opinion of the surgeon, would compromise the stability of the IOL.
Endpoints
The primary effectiveness variable was Best Spectacle-Corrected Visual Acuity (BSCVA) of 20/40 or better at the 1-year postoperative follow-up. The primary safety variable was incidence of SAEs, both cumulative and persistent SAEs. In addition, postoperative clinical observations (ie, non-serious AEs, AEs associated with the surgical procedure or the IOL implantation), and other supportive safety observations (ie, IOL observations [phenomena such as debris on the surface of the IOL, forceps marks on the surface of the IOL, and glistenings], IOL position change, qualitative PCO, posterior capsulotomy, intraocular pressure [IOP]) were also monitored during the study.
Effectiveness assessments
BSCVA
BSCVA was assessed at all postoperative visits and was measured to the smallest line using a standard Snellen chart equal to a distance of 6 m testing distance.
Safety assessments
Cumulative and persistent SAEs
SAEs were occurrences considered to be potentially sight-threatening. Cumulative SAEs were those events that had occurred at any time during the clinical study. Cumulative SAEs defined in the protocol were hypopyon, intraocular infection/endophthalmitis, lens dislocation, cystoid ME, pupillary block, retinal detachment/repair, hyphema, any secondary surgical intervention (retinal detachment repair was not considered a secondary surgical intervention), and other unexpected, potentially sight-threatening clinical observations as assessed by the investigator. Persistent SAEs were defined as those events that were present at the 1-year postoperative visit (330–420 days after surgery) or later and specifically included corneal stromal edema, iritis, raised IOP requiring treatment and vitritis.
Postoperative clinical observations
Nonserious adverse events (AEs) were those that were not potentially sight threatening and were reported as clinical observations. In addition, postoperative clinical observations were monitored and included postoperative ocular events normally associated with the surgical procedure or IOL implantation, such as corneal edema, iritis, macular degeneration/drusen, membrane formation on IOL, pigment precipitation on IOL, transiently raised IOP requiring treatment, synechiae, vitreous detachment, vitritis, and any additional findings the investigator might observe. Some of the postoperative events were part of the protocol-defined cumulative or persistent SAEs and were required to be reported as such.
Other supportive safety observations
Other supportive observations evaluated in this study included IOL observations, IOL position change, posterior capsule opacification, posterior capsulotomy, and IOP.
PCO was qualitatively assessed at each scheduled and unscheduled visit during this clinical study. PCO was graded as none, clinically non-significant, or clinically significant requiring Neodymium:Yttrium Aluminum Garnet (Nd:YAG) laser capsulotomy.
Statistical analysis
All analyses were descriptive; data were summarized as n and percentage (%) per study visits. The primary effectiveness analysis was performed on the “all implanted” group, which included all patients who were successfully implanted with the study lens and had at least one postoperative visit. In addition, the effectiveness analysis was performed on “best case” group, that is, all patients implanted with a study lens, had at least one postoperative visit, and did not have any preoperative ocular pathology or macular degeneration at any postoperative visit. Patients with any violation of the inclusion/exclusion criteria or the surgery techniques defined in the investigational plan were excluded from the per-protocol analysis.
Safety results were summarized for the safety analysis set, which included all patients enrolled in this study with attempted IOL implantation (successful or aborted after contact with the eye).
Ethical approval and consent to participate
The study protocol was approved by the central Institutional Review Board/Ethics committee of each participating country (Saint-Thomas’ Hospital Research Ethics Committee, Medical Committee Office Saint-Thomas’s Hospital; East Berkshire Research Ethics Committee, John Lister Postgraduate Medical Center, Wexham Park Hospital; Ethical Committee at the Faculty of Medicine, RWTH – University of Aachen, Aachen, Germany; Ethikkomission Freiburg, Freiburg, Germany; Instituto Oftalmologico de, Alicante, Spain; Ufficio Sperimentazioni, Istituto Scientifico, Ospedale S Raffaele, Milan, Italy). All participants provided written informed consent before entering the study.
Results
In total, 189 patients underwent the unilateral implantation with the IOL Model MA60NM, and 179 (94.7%) patients completed the 1-year postoperative follow-up. In all, 10 patients discontinued the study and the major reasons were lost-to-follow-up (n=2), unable to attend follow-up visits (n=3), death (n=1), worsening health condition (n=2), and patient was uncooperative (n=2). In total, 152 patients were re-enrolled for the extension phase at the completion of 1 year, 138 patients were observed 2-year postoperatively, and 138 were evaluated at the 3-year visit. Of these, 124 represented the consistent cohort who attended both the 2- and 3-year follow-up visits.
The “all implanted” group included 179 and 138 patients for the 1- and 3-year analyses, respectively. The “best case” analysis included 139 and 110 patients from the “all implanted” group at the 1-year and 3-year postoperative visits, respectively.
The mean age of patients in the “all implanted” group was 74.1±7.5 (range 53–90) years, 61.9% (n=115) were female and 98.4% (n=186) were Caucasian.
Effectiveness
The proportion of patients in the “all implanted” group with a BSCVA of 20/40 or better at the 1-, 2- and 3-year postoperative follow-up visits is shown in . At 1 year, 95.5% of patients implanted with IOL MA60NM had BSCVA of 20/40 or better, which was favorable when compared with an ISO grid rate of 92.5%. Moreover, in the “best case” group analysis, 100% of patients had BSCVA of 20/40, which was favorable when compared with an ISO grid rate of 96.7%.
Figure 1 Best Spectacle-Corrected Visual Acuity status 20/40 or better at 1-, 2-and 3-year postoperative follow-up visits in patients implanted with IOL Model MA60NM – “all implanted” group and “best case” group.
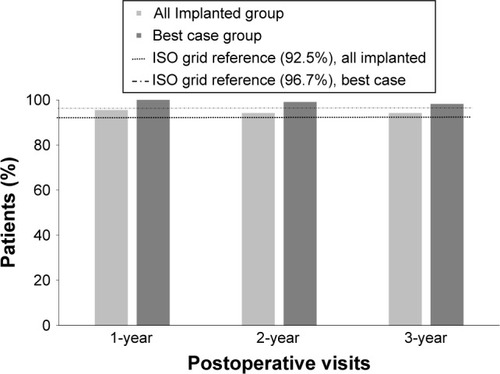
At the 3-year follow-up, 94.2% and 98.2% of patients had a BSCVA of 20/40 or better in the “all implanted” group and “best case groups,” respectively, and the proportion was higher than the ISO grid rate (). When analyzed by baseline age-category, BSCVA results were similar to overall group ().
Table 1 The Best Spectacle-Corrected Visual Acuity status 20/40 or better at 1-, 2- and 3-year postoperative follow-up visits with IOL Model MA60NM, by age category – “all implanted” group
Safety
No clinically significant differences were identified in the rate of SAEs, postoperative clinical observations, and other supportive safety observations between IOL Model MA60NM and ISO reference grid rates. No safety issues were observed, based on age, gender, or best-corrected distance visual acuity.
The incidence of cumulative or persistent SAEs following IOL Model MA60NM implantation was lower than the ISO grid reference (). Overall, three SAEs were observed in IOL Model MA60NM group: two occurrences of cystoid ME and one occurrence of a secondary surgical intervention (correction of residual refraction by LASIK); neither of the SAEs were considered IOL related. There were no reports of persistent SAEs.
Table 2 Cumulative serious adverse events incidence rates through 1 year with IOL Model MA60NM vs ISO Grid Rates – “all implanted” group
The postoperative clinical observations observed following IOL implantation are shown in . Most frequently reported postoperative non-serious AEs were corneal edema, macular degeneration, pigment precipitation on lens and “other.” Some of the clinical observations were present only at one visit, while others persisted across multiple visits. The presence of ocular inflammation (corneal edema and iritis) was not observed beyond the days 30–60 postoperative visit. Vitreous detachment and macular degeneration were not associated with cataract surgery. No clinically relevant sequelae were associated with pigment precipitation.
Table 3 Incidence of postoperative non-serious AEs through 1 year with IOL Model MA60NM – “all implanted” group
The trend was similar with other supportive safety observations. Four IOL observations were assessed as clinically significant by the investigator. These included two reports of debris on the IOL and two reports of forceps marks on the surface. Overall, the incidence of trace glistening was low: 6.1% (n=11) at the 1-year postoperative visit, 1.4% (n=2) at the 2- and 3-year follow-up visits. In one patient, three occurrences of IOL tilt (due to optic not positioned in the bag) were noted in consecutive visits (starting from day 90 to day 119 visit onward). Also, there was one occurrence of IOL decentration (2.0 mm; the reason given by the investigator was that the haptics could not be seen). Raised IOP requiring treatment was not observed at the 1-year follow-up visit or later.
Clinically significant PCO was reported in six (3.2%) eyes (eleven reports of clinically significant PCO in five eyes and five reports of clinically significant PCO requiring YAG in two eyes). By follow-up period, the incidence of clinically significant PCO was 1.1%, 2.2%, and 2.2% at 1-, 2-, and 3-year postoperative visits, respectively (). Clinically non-significant PCO was reported in 27.9%, 18.8%, and 21.7% of patients at 1-, 2-, and 3-year postoperative visits, respectively (). The rate of clinically significant PCO requiring Nd:YAG laser capsulotomy was very low, 1.1% and 1.4%, at the end of 1- and 3-years, respectively.
Table 4 Incidence of PCO at 1-, 2-, and 3-year postoperative follow-up visits with IOL Model MA60NM – “all implanted” group
Discussion
The study demonstrated that the three-piece foldable IOL Model MA60NM made of optic biomaterial Clareon® was effective for visual correction of aphakia when implanted following cataract extraction by phacoemulsification. The primary effectiveness objective was met; at the 1-year postoperative follow-up, a relatively higher proportion of patients implanted with IOL Model MA60NM achieved excellent BSCVA than the historical control value. These visual outcomes were maintained at 3 years. Furthermore, 100% of “best case” patients achieved BSCVA of 20/40 or better 1 year after surgery. Based on the assessment of SAEs, postoperative clinical observations and other supportive safety observations, no unexpected or unwarranted safety issues were observed during the 3-year study period. In particular, we also assessed PCO rates for up to 3 years after surgery following implantation with the Clareon®-based IOL. Overall, in the present study, reports of clinically significant PCO and PCO requiring Nd:YAG laser capsulotomy were very low.
PCO continues to be an undesirable outcome after IOL implantation. In addition, the treatment of PCO, Nd:YAG laser capsulotomy, is associated with significant risks such as IOL damage, cystoid ME, IOP elevation, posterior segment complications and is also an additional financial burden on the health care systems.Citation16 Across studies, the reported incidence of Nd:YAG laser capsulotomy is between 2% and 67%, and the reported rates vary depending on the follow-up period and the type of IOL.Citation17–Citation22 The risk of Nd:YAG laser capsulotomy is reported to be sevenfold higher with hydrophilic lenses than with the hydrophobic lenses.Citation11
The Nd:YAG laser capsulotomy rate is considerably lower with Acrysof® IOLs (Alcon Laboratories Inc.) than other IOL lens materials.Citation17–Citation19,Citation23–Citation26 The Acrysof® IOLs are a series of acrylic, foldable, hydrophobic, single-, and multi-piece lenses with established clinical performance and safety for over two decades.Citation17–Citation19,Citation23–Citation28 AcrySof® IOLs are very stable, have high fibronectin binding, and provide enhanced capsular adhesion, thus providing maximal IOL optic-posterior capsule contact acting as an excellent barrier to prevent cellular proliferation.Citation5,Citation12 In addition, the AcrySof® IOLs present minimal to absent Soemmering’s ring formation (precursor to PCO development), PCO and anterior capsular opacification, decentration, and minimal tilt.Citation5,Citation26,Citation28 In a 7-year follow-up study, the cumulative Nd:YAG laser posterior capsulotomy rate after implantation of AcrySof® IOLs was observed to be ~1%–2% of new cases per year, reaching a plateau at 6 years.Citation27 In the present study, the posterior capsulotomy rates with Clareon®-based IOL Model MA60NM was <1.5% during the 3-year period, which is closer to the lower end of the range of 0%–10% reported in studies with AcrySof® IOLs during 2- to 5-year follow-up period.Citation9,Citation17,Citation19,Citation29,Citation30
Clareon® is built on the same proven fundamental attributes of AcrySof® technology and is a modified advanced cross-linked optic biomaterial designed for enhanced clarity, a low level of surface haze, and a resistance to the formation of glistening compared to other IOL materials (data on file). The introduction of a hydrophilic copolymer, 2-hydroxyethyl- methacrylate, in the acrylic backbone of Clareon®, creates an interface for the interaction of water molecules in the optic, preventing microvacuoles formation, thereby reducing the chances of glistening.Citation31,Citation32 Biomechanical characteristics of the IOL and its interaction with the lens capsule also has an effect on the ease of implantation, mechanical stability, and clinical outcomes.Citation33
In the present study, overall no safety concerns were reported with regard to IOL observation and IOL position (only one case each of IOL decentration and IOL tilt were reported) with Model MA60NM.
The open-label design and descriptive analyses of data are some of the limitations of the present study. Furthermore, considering the multifactorial etiology, other potential factors can also influence PCO rates in a given population. At the time of the study completion, BS EN ISO 11979-7:2006 was applicable and was thus used as a standard reference. Since then, updated ISO guidelines are available and should be considered for future studies.
This is the first prospective trial that provides valuable information on the long-term safety of the new optic biomaterial Clareon®. The Clareon® aspheric single-piece hydrophobic acrylic IOL Model SY60WF (Alcon Laboratories Inc.) has recently received CE mark approval in the EU and is made of the same polymeric biomaterial as the three-piece IOL used in this study. Some previous studies have shown that there is no difference in the PCO/YAG rates of single- and multi-piece IOLs that are made from the same biomaterial.Citation28,Citation34 However, this needs to be confirmed in future studies with the one-piece IOL Model SY60WF.
Conclusion
Patients had excellent visual outcomes and a low incidence of PCO, Nd:YAG laser capsulotomy, and glistening with the three-piece IOL Model MA60NM implantation during a 3-year follow-up period. Results from this study support the long-term effectiveness and safety of the optic biomaterial Clareon®.
Author contributions
Both the authors were involved in the management of the study on behalf of the sponsor, interpretation of analyzed data, development of the manuscript, and have approved the final version for submission.
Acknowledgments
The study was funded by Alcon Laboratories Inc. (a Novartis company), Fort Worth, TX, USA. Medical writing support for the development of this manuscript was provided by Shivani Vadapalli (Novartis Healthcare Pvt Ltd, Hyderabad, India).
Disclosure
At time of the study, Andrew Maxwell was a medical monitor and a consultant for Alcon. Rajaraman Suryakumar is an employee of Alcon Laboratories Inc., Fort Worth, TX, USA. The authors report no other conflicts of interest in this work.
References
- AwasthiNGuoSWagnerBJPosterior capsular opacification: a problem reduced but not yet eradicatedArch Ophthalmol2009127455556219365040
- ÖzyolPÖzyolEKarelFBiocompatibility of Intraocular LensesTurk J Ophthalmol201747422122528845327
- AppleDJSolomonKDTetzMRPosterior capsule opacificationSurv Ophthalmol1992372731161455302
- NishiONishiKOsakabeYEffect of intraocular lenses on preventing posterior capsule opacification: design versus materialJ Cataract Refract Surg200430102170217615474832
- PandeySKAppleDJWernerLMaloofAJMilvertonEJPosterior capsule opacification: a review of the aetiopathogenesis, experimental and clinical studies and factors for preventionIndian J Ophthalmol20045229911215283214
- JorgePAJorgeDVenturaCVIncidence of posterior capsule opacification following the implantation of a foldable hydrophilic acrylic intraocular lens: a 4 year follow-up studyArq Bras Oftalmol201477422222425410172
- PandeySKWilsonMETrivediRHPediatric cataract surgery and intraocular lens implantation: current techniques, complications, and managementInt Ophthalmol Clin200141317519611481546
- BuehlWFindlOEffect of intraocular lens design on posterior capsule opacificationJ Cataract Refract Surg200834111976198519006748
- HollickEJSpaltonDJUrsellPGThe effect of polymethylmethacrylate, silicone, and polyacrylic intraocular lenses on posterior capsular opacification 3 years after cataract surgeryOphthalmology1999106149559917780
- ChengJWWeiRLCaiJPEfficacy of different intraocular lens materials and optic edge designs in preventing posterior capsular opacification: a meta-analysisAm J Ophthalmol2007143342843617224119
- ZhaoYYangKLiJHuangYZhuSComparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: An updated meta-analysisMedicine20179644e830129095259
- LinnolaRJSundMYlönenRPihlajaniemiTAdhesion of soluble fibronectin, laminin, and collagen type IV to intraocular lens materialsJ Cataract Refract Surg199925111486149110569163
- SchauersbergerJAmonMKrugerAAbelaCSchildGKolodjaschnaJLens epithelial cell outgrowth on 3 types of intraocular lensesJ Cataract Refract Surg200127685085411408130
- NishiONishiKAkuraJNagataTEffect of round-edged acrylic intraocular lenses on preventing posterior capsule opacificationJ Cataract Refract Surg200127460861311311632
- BN EN – ISO 11979-7Ophthalmic implants – intraocular lenses – Part 7Clinical Investigations2006 Available from: https://www.iso.org/standard/36409.htmAccessed September 24, 2018
- KarahanETuncerIZenginMOThe Effect of ND:YAG laser posterior capsulotomy size on refraction, intraocular pressure, and macular thicknessJ Ophthalmol20142014846385524724016
- RönbeckMZetterströmCWejdeGKugelbergMComparison of posterior capsule opacification development with 3 intraocular lens types: five-year prospective studyJ Cataract Refract Surg200935111935194019878826
- IwaseTNishiYOvesonBCJoYJYjJHydrophobic versus double-square-edged hydrophilic foldable acrylic intraocular lens: effect on posterior capsule opacificationJ Cataract Refract Surg20113761060106821596248
- CullinFBuschTLundströmMEconomic considerations related to choice of intraocular lens (IOL) and posterior capsule opacification frequency – a comparison of three different IOLsActa Ophthalmol201492217918323280286
- ChangABehndigARønbeckMKugelbergMComparison of posterior capsule opacification and glistenings with 2 hydrophobic acrylic intraocular lenses: 5- to 7-year follow-upJ Cataract Refract Surg201339569469823499068
- ChangAKugelbergMPosterior capsule opacification 9 years after phacoemulsification with a hydrophobic and a hydrophilic intraocular lensEur J Ophthalmol201727216416827445063
- SundelinKAlmarzoukiNSoltanpourYPetersenAZetterbergMFive-year incidence of Nd:YAG laser capsulotomy and association with in vitro proliferation of lens epithelial cells from individual specimens: a case control studyBMC Ophthalmol20141411625274548
- AppleDJPengQVisessokNEradication of posterior capsule opacification. Documentation of a marked decreased in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortemOphthalmol2001108505518
- BeltrameGSalvetatMLChizzoliniMPosterior capsule opacification and Nd:YAG capsulotomy rates after implantation of silicone, hydrogel and soft acrylic intraocular lenses: a two-year follow-up studyEur J Ophthalmol200212538839412474921
- MissierKAANuijtsRMTjiaKFPosterior capsule opacification: silicone plate-haptic versus AcrySof intraocular lensesJ Cataract Refract Surg20032981569157412954308
- MengualEGarcíaJElviraJCRamónHueso JClinical results of AcrySof intraocular lens implantationJ Cataract Refract Surg19982411141179494908
- DavisonJANeodymium:YAG laser posterior capsulotomy after implantation of AcrySof intraocular lensesJ Cataract Refract Surg20043071492150015210228
- NejimaRMiyataKHonbouMA prospective, randomised comparison of single and three piece acrylic foldable intraocular lensesBr J Ophthalmol200488674674915148204
- KugelbergMWejdeGJayaramHZetterströmCTwo-year follow-up of posterior capsule opacification after implantation of a hydrophilic or hydrophobic acrylic intraocular lensActa Ophthalmol200886553353618081899
- SchmidbauerJMVargasLGAppleDJEvaluation of neodymium:yttrium-aluminum-garnet capsulotomies in eyes implanted with AcrySof intraocular lensesOphthalmology200210981421142612153790
- vander Mooren MFranssenLPiersPEffects of glistenings in intraocular lensesBiomed Opt Express2013481294130424009993
- TetzMJorgensenMRNew Hydrophobic IOL Materials and Understanding the Science of GlisteningsCurr Eye Res2015401096998125621973
- LaneSSBurgiPMiliosGSOrchowskiMWVaughanMSchwarteEComparison of the biomechanical behavior of foldable intraocular lensesJ Cataract Refract Surg200430112397240215519095
- ZemaitieneRJasinskasVAuffarthGUInfluence of three-piece and single-piece designs of two sharp-edge optic hydrophobic acrylic intraocular lenses on the prevention of posterior capsule opacification: a prospective, randomised, long-term clinical trialBr J Ophthalmol200791564464817124239
