Abstract
Background:
This study compared the efficacy of the EX-PRESS® glaucoma filtration device and trabeculectomy in primary open-angle glaucoma up to five years after surgery.
Methods:
Patients from a previously reported randomized, open-label, parallel-arm clinical trial in which 78 patients received either the EX-PRESS glaucoma filtration device or underwent a trabeculectomy were followed for up to an additional four years (five total) beyond the original study (39 eyes per treatment group). Risk-benefit data were obtained for up to five years after glaucoma surgery. Outcome variables were intraocular pressures and intraocular pressure medications. Complete success was denoted by intraocular pressure values ≤ 18 mmHg without medication.
Results:
The EX-PRESS glaucoma filtration device controlled intraocular pressure more effectively without medication for more patients from year 1 (86.8% versus 61.5%, P = 0.01) to year 3 (66.7% versus 41.0%, P = 0.02) than trabeculectomy. At year 1, only 12.8% of patients required intraocular pressure medication after EX-PRESS implantation, compared with 35.9% after trabeculectomy. The proportions became closer at year 5 (41% versus 53.9%). The responder rate was higher with EX-PRESS and time to failure was longer. In addition, surgical interventions for complications were fewer after EX-PRESS implantation.
Conclusion:
This five-year analysis confirmed and extended the results reported after one year. Compared with trabeculectomy, EX-PRESS provided better intraocular pressure control in the first three years, and patients required fewer intraocular pressure medications and fewer surgical interventions during the five-year study period. For patients with primary open-angle glaucoma, the EX-PRESS glaucoma filtration device, implanted under a superficial scleral flap, produced significantly higher success rates than trabeculectomy. EX-PRESS is an effective device for long-term treatment of primary open-angle glaucoma.
Introduction
Open-angle glaucoma is a progressive optic neuropathy, resulting in loss of retinal ganglion cells leading to progressive damage of the visual field. Glaucoma is a leading cause of blindness in Western developed countries.Citation1–Citation3 Glaucoma treatments are directed at reducing intraocular pressure,Citation4,Citation5 either pharmacologically or surgically. Surgery is performed when intraocular pressure medication and laser treatment cease to control intraocular pressure.Citation6 Trabeculectomy is the usual surgical procedure.Citation7
An alternative procedure is to implant the EX-PRESS® glaucoma filtration device (Alcon Inc, Forth Worth, TX). The device is a small stainless steel, nonvalved flow-restricting device, designed to lower intraocular pressure in glaucomatous eyes.Citation8 It was developed as a less invasive surgical procedureCitation9,Citation10 compared with conventional trabeculectomy, because it is inserted under a scleral flap to shunt aqueous humor from the anterior chamber to the subconjunctival space using a filtration bleb.Citation11
Several studies have reported on the efficacy of EX-PRESS,Citation9,Citation12–Citation15 but only one randomized the surgical procedures.Citation12 While the efficacy results consistently demonstrated significant intraocular pressure reductions, they differed in the frequency of complications, with more such events occurring with earlier surgical techniques in which the EX-PRESS device was implanted under a conjunctival flap.Citation9,Citation14,Citation15 Since then, the implantation procedure has evolved, and the EX-PRESS device is now implanted under a scleral flap with similar efficacy results,Citation12,Citation13 and the postoperative complication rates have improved considerably.
In the only prospective randomized trial published to date, de JongCitation12 randomized patients to either trabeculectomy or EX-PRESS and assessed the results one year after surgery. de Jong found better intraocular pressure control (P = 0.02) in patients implanted with EX-PRESS (12.0 mmHg) compared with trabeculectomy (13.9 mmHg), fewer prescriptions for intraocular pressure medications, and higher responder rates with intraocular pressure thresholds fixed at either 15 mmHg or 18 mmHg. Also, patients who received EX-PRESS implantation went significantly longer without the need for intraocular pressure medications.
Lifelong treatment of glaucoma is necessary to ensure intraocular pressure control and prevent disease progression. Hence documentation of treatment safety and long-term efficacy is essential. The present paper reports risk-benefit data at five years after glaucoma surgery from patients from the initial de Jong study.Citation12
Methods
The study was conducted according to Dutch law and adhered to the tenets of the Helsinki Declaration. It was approved by the International Review Board at the Academic Medical Centre, Amsterdam University. All patients gave written informed consent before participating in the study.
The present analysis concerns data from the prospective randomized trial performed by de Jong.Citation12 Patients were recruited at a single center (Amsterdam Academic Medical Center, Ophthalmology Department, The Netherlands). All glaucoma surgery was performed by one surgeon (LdJ) between October 2003 and November 2004.
Surgical details are described elsewhere.Citation12 All surgeries were performed under topical anesthesia with Xylocaine® gel. Eyes randomized to EX-PRESS received the EX-PRESS R50®, a device developed for implantation under a scleral flap. The surgical procedures were similar in both treatment arms. During the whole procedure, only balanced salt solution was used to maintain or restore the anterior chamber, by injection through an additional corneal incision. No anterior chamber maintainer was used. A limbus-based conjunctival flap was dissected, followed by a “4 × 4 mm × half the scleral thickness” scleral flap dissected up to the clear cornea. For the eyes randomized to EX-PRESS, a preincision was made into the anterior chamber using a 27G needle parallel to the iris, followed by EX-PRESS device implantation. In the eyes randomized to trabeculectomy, a sclerotomy was performed associated with a peripheral iridectomy. Sutures were identical for both procedures. The scleral flap was then sutured using 10-0 nylon sutures. The number of nylon sutures used to close the scleral flap depended on the judgment of the amount of filtration by the surgeon. The conjunctiva was sutured over the limbus with one uninterrupted, single-running Vicryl suture. Postoperatively, corticosteroids were given six times a day, and if pressures were below 5 mmHg or the anterior chamber flattened, atropine1% twice daily was added. The steroids were tapered over six weeks according to the extent of wound healing. During the first week, postoperative antibiotics four times a day were added.
All eligible patients were 18 years old or older, and all presented with primary open-angle glaucoma not controlled by maximally tolerated intraocular pressure medication. Patients with other ocular diseases or previous ocular surgery (apart from cataract extraction) were excluded. Eligible patients were assigned to receive either a unilateral EX-PRESS device inserted under a scleral flap or trabeculectomy, according to a computer-generated randomization list.
Optical assessments at baseline and at years 1, 2, 3, 4, and 5 after surgery included Goldmann tonometryCitation16 to measure intraocular pressure and the Early Treatment Diabetic Retinopathy Study chart for visual acuity.Citation17 In addition, a daytime intraocular pressure profile was performed six months after surgery at 9 am, 11 am, 1 pm, and 4 pm.
Patients’ responses were classified as: intraocular pressure values less than 15 mmHg and 18 mmHg thresholds; no subsequent intraocular pressure medication prescribed; and no further surgery performed for glaucoma control. Three response criteria were defined, ie, “marginal success” (outcome 1), “partial success” (outcomes 1 and 2), and “complete success” (outcomes 1, 2, and 3). Topical intraocular pressure medication was recorded at each visit. Overall intraocular pressure medication consumption and the average number of constituent medicinal entities (eg, DuoTrav® was counted twice, as travoprost and timolol) were determined at annual assessments. Unscheduled visits to other eye clinics and eye surgeries were also recorded.
Statistical analysis
According to the protocol, the primary response criterion was failure rate at one year, defined as intraocular pressure ≥ 18 mmHg or the addition of an intraocular pressure-lowering medication. A sample of 40 patients per treatment group was needed to show a 32% difference, ie, EX-PRESS 15% versus trabeculectomy 47%, with alpha fixed at 5% and beta at 20% for a two-sided test.
All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Between-group comparisons were performed using two-sample t-tests for normally distributed continuous variables. When the latter criteria did not apply, the Wilcoxon’s test, Fisher’s Exact test, or Chi-square adjusted test was performed, depending on the sample size.
Daytime intraocular pressure profiles were analyzed by a repeated mixed analysis of variance that included three factors, ie, treatment, time, and patients. All factors were fixed, except for patients (random), in a full two-order model. A similar model was constructed for the intraocular pressure analysis comparing baseline with year 5 measurements. Complete, partial, and marginal success rates were evaluated using Kaplan–Meier survival curves and the log-rank test. Age adjustment employed linear models. The analysis was conducted on patients given an EX-PRESS device or standard trabeculectomy to one eye, and followed up for five years. All tests were interpreted at 5%, two-sided. No alpha adjustment was made for test multiplicity.
Results
Seventy-eight patients (39 per treatment group) participated in the analysis. presents the baseline sociodemographic data. Both groups were comparable except for age, whereby patients receiving the EX-PRESS were younger than those undergoing trabeculectomy.
Table 1 Patient characteristics and study follow-up
Baseline mean intraocular pressure values did not differ significantly either with or without age adjustment (). After EX-PRESS devices were implanted, patients showed stable intraocular pressure values (P = 0.67) spanning year 1 (12.0 mmHg) to year 5 (11.5 mmHg), whereas after trabeculectomy, intraocular pressure values decreased (P < 0.0001) from year 3 (13.5 mmHg) to years 4 (11.8 mmHg) and 5 (11.3 mmHg). Up to the end of year 3, intraocular pressure remained better controlled (P = 0.04) by EX-PRESS devices (intraocular pressure 12 mmHg) than by trabeculectomy (intraocular pressure 13.5 mmHg). During the remaining two years (to end of year 5) differences were not significant. Adjustment of patient age did not modify these conclusions.
Table 2 Mean (± standard deviation) intraocular pressure values
presents daytime intraocular pressure profiles six months after surgery. Patients in the trabeculectomy group had two times more intraocular pressure-lowering drug prescriptions.Citation12 No time × treatment interaction was observed, indicating no corresponding time effect with either treatment. The differences between the EX-PRESS device and trabeculectomy did not reach statistical significance (no age adjustment, P = 0.075; age-adjusted, P = 0.056).
Table 3 Daytime intraocular pressure profiles at six months after surgery
shows intraocular pressure correlation coefficients for years 1–5 between intraocular pressure values at baseline and after cataract surgery. All correlation values were less than r = 0.4 (recognized as clinically significantCitation18,Citation19) and none was statistically significant. Hence, intraocular pressure control after glaucoma surgery was independent of initial intraocular pressure values.
Table 4 Correlations between baseline and postoperative intraocular pressure measurements
and compare the success rates after EX-PRESS implantation and trabeculectomy with two different intraocular pressure thresholds (ie, 15 mmHg and 18 mmHg). Success rates during years 1–3 after EX-PRESS were significantly higher for both thresholds, compared with trabeculectomy, without inclusion of medications. When medication efficacy was considered, differences did not reach statistical significance, although EX-PRESS implantations continued to show higher success rates up to year 3.
Table 5 Intraocular pressure success rates (≤18 mmHg)
Table 6 Intraocular pressure success rates (≤15 mmHg)
Time to treatment failure according to the three different response criteria is depicted in . With the 15 mmHg intraocular pressure threshold, EX-PRESS implantation produced significantly higher success rates than trabeculectomy with all three criteria (). When 18 mmHg was the intraocular pressure threshold, the success rate of EX-PRESS implantation was again higher with all criteria, and significantly so for two criteria ().
Figure 1 Survival curves comparing Ex-PRESS implants with trabeculectomy. A) Kaplan–Meier life table curves of complete success (IOP ≤ 18 mmHg): no IOP medication or subsequent glaucoma surgery (log-rank, p = 0.0049). B) Kaplan–Meier life table curves of partial success (IOP ≤ 18 mmHg), i.e. including IOP medication: no subsequent glaucoma surgery (log-rank, p = 0.0085). C) Kaplan–Meier life table curves for marginal success (IOP ≤ 18 mmHg) incorporating IOP medication and subsequent glaucoma surgery (log-rank, p = 0.18). D) Kaplan–Meier life table curves of complete success (IOP ≤ 15 mmHg): no IOP medication or subsequent glaucoma surgery (log-rank, p = 0.0014). E) Kaplan–Meier life table curves of partial success (IOP ≤ 15 mmHg) i.e. including IOP medication: no subsequent glaucoma surgery (log-rank, p = 0.0026). F) Kaplan–Meier life table curves of partial success (IOP ≤ 15 mmHg) i.e. including IOP medication: no subsequent glaucoma surgery (log-rank, p = 0.0026).
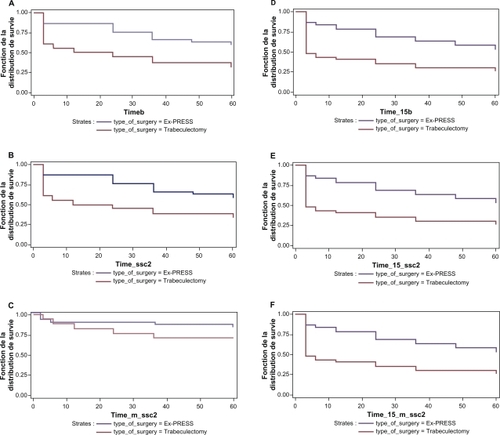
The use of topical intraocular pressure medication is shown in . At all follow-up assessments, the likelihood of being prescribed an intraocular pressure medication was less after EX-PRESS implantation than after trabeculectomy.
Table 7 Intraocular pressure drug prescriptions
Lastly, complications during the follow-up period indicated that more surgical interventions followed trabeculectomy than after EX-PRESS implantation. Trabeculectomy patients experienced more needling (9 versus 3) and cataract surgery than EX-PRESS patients (8 versus 5).
Discussion
The present report is the first long-term efficacy comparison of trabeculectomy and the EX-PRESS glaucoma filtration device based on a randomized clinical trial. Patients implanted with EX-PRESS were more frequent responders, had better intraocular pressure control during the first three years, and fewer intraocular pressure drug prescriptions, compared with standard trabeculectomy. Thus, the results reported at one year by de Jong et alCitation12 persisted beyond the initial follow-up period. The technical aspects of EX-PRESS implantation and its short-term complications were discussed extensively in the former paper. This extension report confirms those points, except that subsequent surgical interventions for glaucoma were more frequent in the trabeculectomy group than in the EX-PRESS group. However, the difference was not statistically significant because such events were rare and sample sizes were small.
The study was performed at a single center and all operations were by the same surgeon. Accordingly, the variance of our efficacy estimates may have been reduced by practice effects, thus increasing the sensitivity of the study to differences between EX-PRESS implantation and standard trabeculectomy. This would limit the extrapolation of our results to other centers and countries.
However, the study can be compared with others on the basis of its trabeculectomy control group. First, the intraocular pressure drug prescription rates after trabeculectomy were close to those of Papaconstantinou et al,Citation20 who reported a prescription rate of 0.5 items at six months, which is similar to the present rate of 0.74 items at one year. Second, Gedde et alCitation21 reported a mean intraocular pressure of 13.3 ± 6.8 mmHg three years after trabeculectomy, which compares with the present 13.5 ± 3.3 mmHg with a similar number of prescribed drugs. Third, according to Geffen et al,Citation22 intraocular pressure values ranged from 6 to 21 mmHg (mean decrease 20%) four years after subconjunctival topical lidocaine anesthesia and trabeculectomy without further glaucoma therapy or repeat filtration surgery, comparable with the present 46.2% after four years (although the success criteria differed slightly). Fourth, Gilmour et alCitation23 reported a 42% successful outcome (intraocular pressure < 18 mmHg, without intraocular pressure medication) at 40 months, similar to the present 41.0% success rate at three years. Lastly, Yalvac et alCitation24 observed complete success (intraocular pressure 6–21 mmHg, without medication) in 66.2% of eyes at six months after trabeculectomy and 55.1% at three years. Our corresponding findings were 61.5% at one year and 41.0% at three years, the latter discrepancy probably being explained by our 18 mmHg intraocular pressure threshold. The aforegoing results need to be confirmed by a multicenter, randomized, clinical trial for broader population inferences.
The present intraocular pressure effect of EX-PRESS implantation was approximately 1.5 mmHg greater than for trabeculectomy over three years, which is clinically relevant at a population level. Also, the standard deviation of intraocular pressure was reduced after glaucoma surgery, indicating that EX-PRESS implantations provided good intraocular pressure control for most patients (ie, no outliers) and that the procedure effectiveness is predictable. Furthermore, postoperative intraocular pressure values were independent of their baseline values, suggesting that even the most severe cases could benefit from such treatment and achieve intraocular pressure values of 11.5 mmHg at five years. Hence, intraocular pressure control using EX-PRESS is clinically relevant and predictable, and may be offered to even the most severe patients. Visual field measurements and quality of life effects should be investigated by future long-term trials.
The intraocular pressure benefits of EX-PRESS implantation, compared with standard trabeculectomy, persisted for three years and remained stable over five years. By contrast, standard trabeculectomy produced a lesser intraocular pressure reduction during the first three years, matching the EX-PRESS intraocular pressure effect only at four and five years, on average. The difference may be explained by trabeculectomy patients receiving more intraocular pressure medication, with prescription adjustments, before they matched the intraocular pressure effect. Moreover, trabeculectomy patients required more needling during follow-up visits. The overall implication is that patients given EX-PRESS implantation may call upon fewer medical resources, which may generate savings compared with standard trabeculectomy. A full health economics evaluation is needed for precise estimates.
Several limitations apply to this analysis. First, the physician knew which treatment was given when measuring intraocular pressure and writing intraocular pressure drug prescriptions, although intraocular pressure is a rather objective measure. Second, the study was limited to one center and surgeon, raising possible questions about practice effects, as discussed, and extrapolation to other populations. Third, patients were operated on one eye only, which introduces the possibility that intraocular pressure drugs instilled into the nonoperated eye might interact with the operated eye. Fourth, it may be questioned whether resources expended on intraocular pressure medication were truly stochastic variables, or driven by the protocol. Resources dedicated to control intraocular pressure after surgery might be different from those reported in this trial. Fifth, with the sample size fixed ad hoc, the observed number of patients does not allow reliable inferences concerning the incidence rates of long-term adverse events (eg, needling). Additional studies, recruiting larger samples and conducted at more centers, are needed to confirm the five-year results.
In conclusion, this five-year analysis confirmed the results presented at one year by de Jong et al.Citation12 EX-PRESS implantations are more effective than standard trabeculectomy in controlling intraocular pressure during the first three years. This result was obtained with fewer subsequent surgical interventions and less topical intraocular pressure medication.
Disclosure
This study was supported by a grant from Alcon Management SA, Geneva, Switzerland.
References
- HensonDBThampyRPreventing blindness from glaucomaBMJ2005331750912012116020831
- KingmanSGlaucoma is second leading cause of blindness globallyBull World Health Organ2004821188788815640929
- QuigleyHABromanATThe number of people with glaucoma worldwide in 2010 and 2020Br J Ophthalmol200690326226716488940
- CaprioliJColemanALIntraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the Advanced Glaucoma Intervention StudyOphthalmology200811571123112918082889
- KeltnerJLMillerJPParrishRK2ndWilsonMRKassMAThe Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucomaArch Ophthalmol2002120671472012049575
- European Glaucoma SocietyTerminology and Guidelines for Glaucoma3rd ed Available at: http://www.eugs.org/eng/EGS_guidelines.asp. Accessed January 21, 2011.
- MantravadiAVMyersJSReconsidering trabeculectomy’s strengths and weaknessesClin Experiment Ophthalmol201038982782821138509
- MarisPJJrIshidaKNetlandPAComparison of trabeculectomy with EX-PRESS miniature glaucoma device implanted under scleral flapJ Glaucoma2007161141917224744
- WamsleySMosterMRRaiSAlvimHSFontanarosaJResults of the use of the EX-PRESS miniature glaucoma implant in technically challenging, advanced glaucoma cases: A clinical pilot studyAm J Ophthalmol200413861049105115629303
- DahanECarmichaelTRImplantation of a miniature glaucoma device under a scleral flapJ Glaucoma20051429810215741808
- TraversoCEDe FeoFMessas-KaplanALong term effect on IOP of a stainless steel glaucoma drainage implant (EX-PRESS) in combined surgery with phacoemulsificationBr J Ophthalmol200589442542915774918
- De JongLThe EX-PRESS glaucoma shunt versus trabeculectomy in open-angle glaucoma: A prospective randomized studyAdv Ther200926333634519337705
- MarisPJGIshidaKNetlandPComparison of trabeculectomy with EX-PRESS miniature glaucoma device implanted under scleral flapJ Glaucoma2007161141917224744
- GandolfiSTraversoCFBronASellemEKaplan-MessasABelkinMShort-term results of a miniature draining implant for glaucoma in combined surgery with phacoemulsificationActa Ophthalmol Scand Suppl20022366612390153
- Kaplan-MessasATraversoCFSellemEZbigniewZBelkinMThe EX-PRESS miniature glaucoma implant in combined surgery with cataract extraction: Prospective studyInvest Ophthalmol Vis Sci2002433348A
- MosesRAThe Goldmann applanation tonometerAm J Ophthalmol195846686586913606204
- CampariniMCassinariPFerrignoLMacalusoCETDRS-fast: Implementing psychophysical adaptive methods to standardized visual acuity measurement with ETDRS chartsInvest Ophthalmol Vis Sci20014261226123111328731
- CampbellDTFiskeDWConvergent and discriminant validation by the multitrait-multimethod matrixPsychol Bull19595628110513634291
- HaysRHayashiTBeyond internal consistency reliability: Rationale and user’s guide for multitrait analysis program on the microcomputerBehav Res Methods Instrum Comput199022167175
- PapaconstantinouDGeorgalasIKarmirisETrabeculectomy with OloGen versus trabeculectomy for the treatment of glaucoma: A pilot studyActa Ophthalmol2010881808519900209
- GeddeSJSchiffmanJCFeuerWJHerndonLWBrandtJDBudenzDLTube Versus Trabeculectomy Study GroupThree-year follow-up of the tube versus trabeculectomy studyAm J Ophthalmol2009148567068419674729
- GeffenNCarrilloMMJinYTropeGEBuysYMEffect of local anesthesia on trabeculectomy successJ Glaucoma200817865866119092462
- GilmourDFMannersTDDevonportHVargaZSoleboALMilesJViscocanalostomy versus trabeculectomy for primary open angle glaucoma: 4-year prospective randomized clinical trialEye20092391802180717293790
- YalvacISSahinMEksiogluUMidilliogluIKAslanBSDumanSPrimary viscocanalostomy versus trabeculectomy for primary open-angle glaucoma: Three-year prospective randomized clinical trialJ Cataract Refract Surg200430102050205715474813