Abstract
The main treatment for pterygium is surgical removal. However, pterygium surgery is concerned with high rates of postoperative recurrence. Predicting factors of recurrence are not fully understood, yet, but they probably depend on a multitude of patient-related, clinical, and/or surgical factors. Several adjuvant treatments have been proposed to reduce postoperative pterygium recurrence, including different antimetabolites, antiangiogenetic factors, and radiation therapy. The purpose of this review is to collect the current evidence regarding application and limits of different therapeutic approaches for preventing postoperative recurrence of pterygium, giving insights and perspectives for better management of this disease. In the light of the current evidence, pterygium surgery cannot disregard wound coverage with conjunctival autografting or rotational flap combined with adjuvant treatments. The rotational flap technique is associated with shorter surgical time rates and prevents graft displacement and necrosis, given its vascular pedicle. Amniotic membrane may still be reserved in case of great conjunctival defects or insufficient conjunctiva. Repeated subconjunctival antivascular endothelial growth factor injections can be considered as an effective and safe adjuvant treatment. Moreover, management of postoperative pain is crucial. Innovative treatment strategies will probably target different molecular pathways, considering recent findings regarding pterygium pathogenesis, to improve better understanding and develop universally shared guidelines. Great importance shall be dedicated to the identification of novel molecular biomarkers and favoring factors of recurrence, in order to achieve a customized surgical treatment for each patient and obtain maximal reduction of postoperative recurrence.
Introduction
Pterygium is an abnormal growth of epithelial and fibrovascular tissue invading the cornea across the limbus and can lead to impaired vision (due to excessive dimensions or induced astigmatism) or recurrent inflammation. Pterygium formation is thought to be the result of altered epithelial cell proliferation and altered vascularization.Citation1–Citation5 However, the precise pathogenesis of this disease is still unclear.
Pathogenic factors related to pterygium formation include ultraviolet (UV) radiation (as observed in numerous studies, probably through damage to DNA, proteins and lipids, both direct or induced by reactive oxygen species),Citation6–Citation10 viral infections (mainly herpes simplex virus, cytomegalovirus, and human papillomavirus),Citation11–Citation13 epigenetic aberrations, transition from epithelial to mesenchymal tissue,Citation14 inflammatory and anti-apoptotic mechanisms,Citation15–Citation18 neoangiogenic upregulation,Citation2,Citation19 stimulation of lymphangiogenic response,Citation20 deregulation of extracellular matrix modulators,Citation21 growth factors,Citation22–Citation25 recruitment of bone marrow-derived stem and progenitor cells,Citation26,Citation27 and modifications in cholesterol metabolism.Citation28 Moreover, Anguria et al reported that hereditary predisposition may be fundamental for the onset and persistence of pterygium.Citation29
The main treatment for pterygium is surgical removal, which has to be taken into consideration in case of reduced visual acuity due to visual axis involvement, induced astigmatism, or frequent inflammation and discomfort. Surgical techniques include bare sclera excision, conjunctival autograft, conjunctival transpositional flap, and amniotic membrane grafting.Citation30 However, pterygium surgery is concerned by postoperative recurrence (whose rate can be up to 89% and its severity may vary according to the adopted approach and preoperative conditions) because fibrovascular growth may occur also with greater extension than its primary presentation.Citation31
Predicting factors of recurrence are not fully understood, yet, but they probably depend on a multitude of patient-related (genetics, environment) and/or surgical factors.Citation32 Among patient-related factors, ethnicity is considered a significant predisposing feature (Hispanics and dark-skinned individuals are the mostly affected).Citation33 Other patients’ characteristics associated with higher recurrence are young age, current active growth, preexisting disfiguration of lacrimal caruncle, ocular motility restriction, concurrent ocular surface inflammation, fibrogenic constitution, and family history.Citation34
According to a grading system developed by Tan et al, a fleshy-like aspect of the pterygium is correlated with high recurrence rates, especially after bare sclera excision.Citation35 Greater vertical size of pterygium was also associated with a higher recurrence rate.Citation34 Moreover, different biomarkers (which are involved in reduced inhibition of epithelial and fibroblastic proliferation) are associated with higher recurrence rate. For example, excessive levels of stromal cell-derived factor 1 (which promotes TGF-β expression) and angiogenin were observed in pterygium fibroblasts.Citation36 As of today, additional studies are needed to further evaluate biomarkers before their application in clinical practice because histological and immunohistological features are still not sufficient to reliably predict recurrence predisposition.
Several adjuvant treatments have been proposed to reduce postoperative pterygium recurrence, including different anti-metabolites, antiangiogenetic factors, radiations, as well as other novel materials and administration methods. The purpose of this review is to collect the current evidence regarding application and limits of different therapeutic approach for preventing postoperative recurrence of pterygium, giving insights and perspectives for better management of this disease.
Surgical treatment
Bare sclera technique
The bare sclera technique is the first technique adopted for pterygium removal and is characterized by simple excision, allowing the scleral bed to re-epithelialize. However, this technique tends to favor postoperative pterygium proliferation because small tissue residues may be left in the scleral bed, resulting in high recurrence rates (24%–89%).Citation37 A reduction of recurrence rates – as suggested by several authors – can be obtained by combination of excision with accurate Tenon and fibrotic tissue removal.Citation32 Nevertheless, a recent research reported that bare sclera technique was associated with higher recurrence rates, which became lower when adjuvant treatments were used.Citation37 The bare sclera technique can be easily performed with short surgical time (depending on pterygium size), and it is appealing for trainee surgeons, but it is currently rarely applied, given the high risk of recurrence. In fact, a meta-analysis of randomized controlled clinical trials comparing bare sclera technique and conjunctival autograft demonstrated that the risk of recurrence following surgical treatment is significantly higher (ranging from 6 to 25 times) when no graft placement is performed.Citation38 In order to achieve better postsurgical outcomes and lesser recurrences, bare sclera technique must be associated with adjuvant treatments or alternative surgical techniques featuring complete coverage of the conjunctival defect.
Conjunctival autograft
Conjunctival autografting was first described in 1985 by Kenyon et al. This technique involves the realization of a free autograft from nearby conjunctiva that will be applied over the exposed scleral bed once pterygium excision has been performed.Citation39 This procedure is associated with lower recurrence rates when compared with the bare sclera excision alone, with more long-term efficacy. Even if recurrence rates after conjunctival autograft vary among different clinical studies, this technique is often considered to be the most effective method for pterygium treatment.
Syam et al reported a recurrence rate of 3.3% in their study, similar to the results obtained in a case series conducted by Bilge,Citation40 while Koranyi et al, Fernandes et al, Ma et al, and Al Fayez reported 13.5%, 12.2%, 5.4%, and 8.3% recurrence rates, respectively.Citation31,Citation41–Citation43 However, much higher recurrence rates have been reported (ranging from 31.3% to 33.3%), in case of recurrent pterygium excision.Citation40 A novel technical variant named “pterygium extended removal followed by extended conjunctival transplantation” (P.E.R.F.E.C.T.) was conducted in Australia, with reports of patients’ follow-up longer than 1 year and a recurrence rate of 1.6%.Citation44
Although conjunctival autografting is effective in preventing pterygium recurrence, this technique requires technical expertise and extended operative time due to fixation of conjunctival autograft, especially when sutures are used. In fact, because of the need for graft fixation, the surgical time dedicated to conjunctival autograft can be longer than the one needed for simple bare sclera excision. Moreover, although rarely, the graft can be displaced or lost. Conjunctival autografts can be fixated at the level of the scleral bed through different methods. Usually, sutures are associated with postoperative discomfort, chronic inflammation, and granuloma formation.Citation45 Fibrin glue is an alternative, synthetic adhesive (prepared from a donor plasma), first described for pterygium surgery by Cohen and McDonald in 1993.Citation46 Koranyi et al confronted conjunctival autograft fixation using fibrin glue adhesive with suture-assisted fixation, reporting that fibrin glue had a 5.3% recurrence rate while suture-assisted procedures were associated with a recurrence rate of 13.5%.Citation47 Fibrin glue fixation requires a shorter operation time, but its drawbacks include potential risk of infections, hypersensitivity reactions, potential risk for dehiscence, and higher costs.Citation48–Citation50 Another fixation method that has been proposed is the in situ blood coagulum technique. This method has the advantages of eliminating the risk of transmitted infections and hypersensitivity reactions by using the patient’s own clotting factors. Several studies have reported that recurrence rates of fibrin glue-assisted conjunctival autografting and in situ coagulum were similar; however, the use of autologous blood was associated with higher risk of graft displacement and retractions.Citation51–Citation53 In situ blood coagulum has similar recurrence rates and lesser postoperative discomfort when compared with suture-assisted procedures, but complications related to graft failure and graft retractions were still more common after the use of in situ blood coagulum, although the difference was not statistically significant.Citation51 Kumar and Singh concluded that fibrin glue remains the most effective method for conjunctival autograft fixation in pterygium surgery with least surgical time and postoperative discomfort.Citation45 The use of sutures is related to maximal surgical time and postoperative discomfort while providing better graft stability, but recurrence rates are lower with fibrin glue-assisted fixation.Citation54 Cauterization is another surgical option, but it is still necessary to assess whether this method may be superior to fibrin glue. A clinical trial has been recently presented in order to evaluate the feasibility of this method in comparison with fibrin glue.Citation55
Currently, graft edema, graft necrosis, graft displacement or loss, inclusion cysts, subconjunctival hematoma, Tenon’s granuloma, giant papillary conjunctivitis, corneal narrowing, and Dellen ulcers have been reported to be the most common postoperative complications of primary pterygium surgery.Citation56,Citation57 Conjunctival granuloma (CG) is an uncommon complication of pterygium excision combined with a conjunctival autograft. Mullins et al reported a prevalence of 2% of postoperative granulomas in their study.Citation58 In a case series of 100 patients who underwent surgical treatment, a total of 52 eyes developed CGs after pterygium surgery, of which 3 were recurrent pterygium eyes.Citation59 Zhang et al reported that the incidence of CGs in primary pterygium eyes was 1.3% and 2.2% in the case of recurrent pterygia surgery.Citation60 Common causes of granulation tissue development are foreign bodies of several origins (suture material or filaments); laxly sutured conjunctival wounds with exposition of sclera and fascia tissue; uneven graft tissue edges; irregular conjunctival stitches; lack of local blood supply; inflammation, infection, or other minor factors (such as suture type and other foreign bodies).Citation60,Citation61 When conjunctival autograft is associated with an excessive use of mitomycin C, avascular scleral stromalysis may occur. However, even if recurrent cases with cicatricial strabismus may occur, conjunctival autograft in combination with mitomycin C presents low recurrence rates and must be taken into consideration.Citation62
It has been shown that after a very long follow-up of almost two decades there are no significant differences regarding postoperative recurrences or complications when using upper or lower conjunctiva for grafting, even if larger studies are required in order to confirm these results.Citation63,Citation64 Moreover, it is possible that patients who underwent excision with lower flap reported less postoperative discomfort because the upper eyelid has a greater range of motion than lower eyelid, which might lead to a more intense inflammation, tear film instability, dry eye symptoms, and longer corneal epithelial healing time.Citation64 In the light of these considerations, conjunctival autograft in pterygium surgery can be associated with potential drawbacks. Closure of large defects is difficult, and the conjunctiva must be preserved for the possibility of glaucoma filtering surgery. Syam et al found that 36.66% of patients developed conjunctival scarring at the site of the donor conjunctiva.Citation65 For this reason, in case of patients who may benefit from glaucoma filtration surgery, it is more advisable to utilize inferior conjunctival portions for autografting. In addition, it is not feasible to use this technique to cover wide ocular surface defects occurring in cases of large or double-headed pterygia.
According to Paracha et al, recurrence rates of the conjunctival autograft method were similar to those achieved when mitomycin was used in association with the bare sclera technique.Citation66 Moreover, this technique has a lower recurrence rate when compared with the application of amniotic membrane or simple bare sclera excision.Citation67,Citation68 Finally, combination of conjunctival autograft with intraoperative mitomycin C proved to be more effective in reducing recurrence rates than cases in which the two techniques are separately applied.Citation69,Citation70
Amniotic membrane
Amniotic membrane is the innermost layer of the placenta (featuring a thick basement membrane and an avascular stromal matrix), which can be used as a graft with anti-inflammatory and antifibrotic properties, capable of providing numerous growth factors, and promoting proliferation and differentiation of epithelial cells without the risk of immunological reactions. Amniotic membrane stromal matrix is effective in suppressing the expression of TGF-β signaling and myofibroblast transformation in pterygium.Citation71,Citation72 Other studies have shown that amniotic membrane facilitates epithelialization, maintains normal epithelial phenotype, and reduces inflammation, scarring, and vascularization.Citation37,Citation73,Citation74 Given these features, human amniotic membrane has been considered useful in several ophthalmic surgeries, including pterygium and other conjunctival diseases.Citation75 Typically, it must be placed over the bare sclera, with the basement membrane facing up and the stroma facing down. Fibrin glue may be also used to promote amniotic membrane graft fixation to the underlying sclera.
The application of amniotic membrane appears to be safe and effective, and it is associated with lower recurrence rates when compared with the bare sclera technique.Citation76,Citation77 The reported recurrence rates with amniotic membrane graft vary between 3.8% and 40.9%. In a prospective study, Prabhasawat et al reported a recurrence rate of 10.9% after amniotic membrane apposition.Citation73 Solomon et al subsequently modified the technique achieving a lower recurrence rate of 3%.Citation78 However, when compared with conjunctival autografts, amniotic membrane efficacy remains controversial.Citation79
In fact, four randomized clinical trials examined pterygium recurrence rates after amniotic membrane grafts compared with conjunctival autograft procedures. All four studies showed a lower pterygium recurrence rate in conjunctival or limbal autograft groups (P=0.05).Citation80–Citation83 Ma et al retrospectively evaluated the efficacy and safety of amniotic membrane compared with conjunctival autograft and topical 0.02% mitomycin C, after excision of primary pterygium, with no significant difference in recurrence rates among the groups under study.Citation42 Given these considerations and comparable recurrence rates in the literature, pterygium excision associated with amniotic membrane is a less tedious and less time-consuming method, providing the possibility of conjunctival sparing, especially needed in case of future glaucoma surgery.
Inhibition of aberrant neovascularization, prevention of inflammation, and promotion of conjunctival re-epithelialization are the main reasons for amniotic membrane effects in pterygium surgery.Citation84–Citation86 Conjunctival autografting may provide a source of conjunctival epithelium, whereas amniotic membrane seems to play a role in inhibiting the development of progenitor cells involved in pterygium recurrence.Citation87 The procedural time of this technique can be potentially shorter, and it is technically easier to perform than conjunctival autografting. Even if results presented in the literature indicated similar visual acuity changes and epithelial healing with the amniotic membrane technique when compared with conjunctival autografting, greater inflammation and higher recurrence rates were seen in the amniotic membrane group.Citation88 Moreover, conjunctival autografting was more effective than amniotic membrane to prevent pterygium recurrence after a 6-month follow-up, especially in recurrent pterygia.Citation79 Even so, amniotic membrane application may still be useful to cover large conjunctival defects after pterygium excision and to preserve conjunctiva in glaucomatous patients. Future studies should assess changes in patient-reported discomfort and visual acuity, evaluating the effects of different surgical variations.
Amano et al showed a recurrence rate of 8.7% when intraoperative 0.04% mitomycin C was associated.Citation89 This approach reduced mitomycin C dosage to avoid overspill to the entire ocular surface. Coupled with short exposure to mitomycin C, amniotic membrane can be considered as a feasible alternative for pterygium surgery, as shown in a case series by Rosen.Citation90 Amniotic membrane grafts have been also used as an adjuvant procedure in combination with conjunctival autografting, in order to improve recurrence rates reduction. To date, the combined use of these two techniques has been reserved for large or severely inflamed lesions or in case of persistent recurrence.Citation91 The safety and effectiveness of this combined method have been emphasized by several studies. Conjunctival autografting in combination with amniotic membrane further reduces recurrence rates, dry eye, and conjunctival inflammation compared with both techniques alone, with better clinical outcomes.Citation92,Citation93
In another study, the use of hyperdry amniotic membrane (HD-AM) has been evaluated in comparison with conjunctival autograft.Citation94 HD-AM is made with fresh human amniotic membrane using the hyperdrying method and returns to a layered structure after absorbing water. Okabe et al found that the structures of collagen fibers in the connective tissues were not destroyed in the hyperdry state and were more stable than cryopreserved amniotic membrane.Citation95 Allen et al also showed that the biochemical composition of HD-AM, including the number of factors such as epidermal growth factor and TGF-β1, was similar to fresh amniotic membrane.Citation96 HD-AM is useful in the covering of wide ocular surface defects such as in the case of large or double-headed pterygium. Moreover, management of HD-AM is simple and its use may lead to shorter operating times. It has been reported that recurrence rates in patients receiving HD-AM were significantly lower (5.06%) than recurrence rates in conjunctival autografting (20.97%; P=0.003).Citation94
Conjunctival transpositional flap
Rotational conjunctival flaps have been carried out since 1940s with different recurrence rates. McCoombes et al reported a 3.2% recurrence rate, whereas Alpay et al reported a recurrence rate of 33.33%.Citation97,Citation98 Bilge compared conjunctival transpositional flap with conjunctival autografting, evaluating its efficacy, safety, and operating time. Both procedures seemed to provide low recurrence rates, without severe complications. Conjunctival transpositional flap has less torsion effects on tissues and has better cosmetic results in an early and late postoperative period when compared with conjunctival autografting.Citation40 This technique can be used as an acceptable method for pterygium surgery, especially in patients with insufficient conjunctiva.Citation99 In general, conjunctival transpositional flap is a more challenging surgery than conjunctival autografting, but once mastered, it needs less surgery time when compared with conjunctival autografting. It has been reported that the average surgery duration for both methods can be equal, at around 50 minutes, although it varies among different studies. This was attributed to the difficulty of separating the fibrovascular tissue from a small graft, the smaller size of the graft in relation with the bare sclera, and the need for more sutures to hold the graft in the transpositional method.Citation99 Nevertheless, Dadeya et al have observed that the duration of transpositional flap is shorter than the autografting method by 20 minutes, saving a significant amount of surgical time with recurrence rates of 5.58% for rotational flap and 5.55% for autografting.Citation100 However, Wu et al reported that the recurrence rate after the transpositional flap technique was 35%.Citation101 Jap et al used the rotational flap method when autografting was contraindicated and recorded a recurrent rate of 4% with an average follow-up of 12 months.Citation102 Combination of transpositional conjunctival flap and intraoperative 0.02% mitomycin C for 5 minutes provided a recurrence rate of 3% after 1 year.Citation103
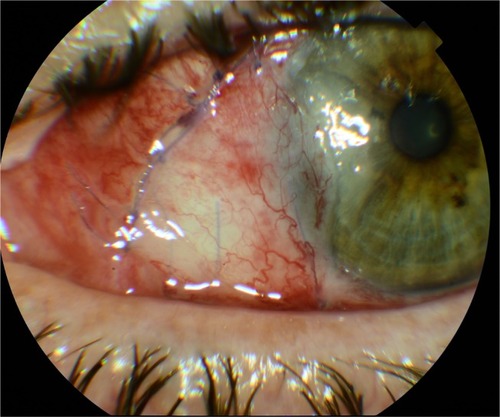
All these findings suggest that the transpositional method is similar to conjunctival autografting in terms of recurrence rates, which become significantly lower when intraoperative mitomycin C is applied, an effective separation of the fibrovascular tissue is performed, and corticosteroid eye drops are used for a longer postoperative time. Given that complications are more frequent in the autografting method, it is better to use this type of surgery when suitable or sufficient conjunctiva for surgery is observed.Citation100 Transpositional flap can be safely used when the conjunctival features do not allow conjunctival autografting, with similar recurrence rates and significantly shorter surgical time, even if more studies with a greater number of patients should be performed to get statistically significant results. In addition, conjunctival autografting may need peribulbar anesthesia and tractioning sutures, while it is nearly unnecessary for the transpositional flap technique.Citation40 Also, in transpositional graft technique, there is no risk of graft loss and inversion, and the vessel structure is preserved, with better healing process and reduced risk of graft necrosis (). However, transpositional flap cannot be taken into consideration in case of large pterygia in which a wider grafting is needed.
Adjuvant therapies
Mitomycin C
Mitomycin C, a substance isolated from Streptomyces caespitosus, is an antibiotic and antineoplastic drug that has been suggested as an adjuvant treatment for pterygium surgery in the early 1960s.Citation104 Positive outcomes have been reported after an intraoperative application of 0.02% mitomycin C. In fact, recurrence after mitomycin C use in primary pterygium has been reported to be as low as 7%, while rates after recurrent pterygia were reduced to 9%.Citation105 The association of 5-minute application of 0.02% mitomycin C with bare sclera excision showed to reduce the recurrence rate to 5%.Citation106 Moreover, no substantial differences have been observed between postoperative 0.02% mitomycin C for 5 days or intraoperative mitomycin C at different dosages when combined with rotational conjunctival flap.Citation107–Citation112 However, it has been shown that conjunctival autografting is associated with significantly lower recurrence rates when compared with excision combined with intraoperative mitomycin C.Citation69,Citation113 Nonetheless, two studies reported that the combination of conjunctival autograft with mitomycin C, regardless of the application dosage or method, resulted in significantly lower pterygium recurrence.Citation69,Citation114 Moreover, Cardillo et al have reported recurrence rates ranging from 4% to 6% with mitomycin C of 0.02% and 0.04%. Given the similar efficacy, it is recommended to use intraoperative mitomycin C at the lowest dose.Citation113 The most commonly used concentration of mitomycin C is 0.02% (0.2 mg/mL), and the most common application time length is 3 minutes. However, further studies are still needed to determine the optimal concentration and exposure duration.Citation37
Several randomized controlled clinical trials compared recurrence rates using various protocols that incorporated intraoperative or postoperative mitomycin C with different exposure times. Studies regarding primary pterygia alone or combined with recurrent pterygia reported no significant differences in recurrence rates in case of intraoperative or postoperative mitomycin C.Citation115–Citation117 Currently, there are no data suggesting whether intraoperative or postoperative mitomycin C is more efficacious when combined with conjunctival autograft surgery.
Common complications after mitomycin C application are usually limited to photophobia, postoperative irritation, and ocular discomfort with increased tears, especially if used at low dosages.Citation118 However, severe complication can still occur, even after several months from initial treatment. Such adverse events include cataract, symblepharon, anterior uveitis, iritis, scleral thinning or necrosis, corneal opacification and ulceration, prolonged pain, and persistent conjunctival and scleral defects.Citation37 High doses and patients’ predisposition (eg, dry eye, ichthyosis, acne rosacea) are probably the main reason for mitomycin C-related adverse events.Citation32 Hayasaka et al reported late complications featuring calcified plaques at the level of the excision site. When these plaques were removed, scleral thinning was observed, with the need for additional patching grafts in some cases.Citation105 Finally, Dougherty et al described a case of severe corneoscleral melting in a patient receiving application of 0.02% mitomycin C for 3 minutes followed by closure with a conjunctival rotational flap.Citation119
5-Fluorouracil (5-FU)
5-FU is an antimetabolite first synthesized in 1957 by Dushinsky et al,Citation120 with inhibiting effects on the proliferation of corneal epithelial cells and fibroblasts located in conjunctiva and Tenon’s capsule. 5-FU has been used as an adjuvant in pterygium surgery with recurrence rates ranging from 11.4% to 60.0% after bare sclera technique.Citation121 According to a series of 125 eyes receiving intraoperative 5-FU (at a dose of 25 mg/mL), pterygia recurred in 36%.Citation122 Higher doses of 5-FU (50 mg/mL) lead to a recurrence decrease at around 11%, with no statistically significant advantages when compared with conjunctival autografting alone.Citation121 Although there is little evidence in the literature to encourage a routinely 5-FU use for this surgery, this adjuvant treatment appears to maintain a role in treating recurrent pterygia.Citation123–Citation125 In fact, Prabhasawat et al reported recurrence rates of 7.7% when 5-FU was used for impending recurrent pterygium.Citation73
Only minor or temporary adverse events have been reported after 5-FU use in pterygium surgery.Citation32 However, a case of cicatricial ectropion after topical 5-FU and a case of punctal–canalicular stenosis after systemic administration have been described.Citation126,Citation127 Adverse events after 5-FU application are more commonly reported in its employment for glaucoma surgery, including persistent epithelial defects, spontaneous bleb rupture, and bacterial ulcerations. However, 5-FU doses used in these cases are five to ten times higher than those suggested for pterygium surgery (10/20 mg). Because toxicity is still possible even at lower doses, the use of 5-FU is not recommended in patients with a history of corneal diseases.Citation128,Citation129
Radiation
The use of radiotherapy as an adjuvant treatment for pterygium has been described since 1912.Citation130 Since 1950, the radioactive material used for pterygium treatment through irradiation has been strontium-90 (88Sr). This element seems suitable for the treatment of ocular surface diseases because it emits β-rays whose energy decrease rapidly as they penetrate in the underlying tissues (dropping off to 41% at 1 mm depth, 19% at 2 mm, 9% at 3 mm, and 1% at 5 mm), preventing damage to deeper structures.Citation131 Currently, there exists a wide variety of dose fractionation schemes, ranging from 20 Grays (Gy) in single fraction in the immediate postoperative period to 60 Gy in 6 weekly fractions or 24–30 Gy in 3 weekly fractions.Citation132–Citation134 Positive outcomes were also proven in case of immediate postoperative radiotherapy of 25 Gy in a single fraction, after excision with bare sclera technique (6.82% recurrence rate).Citation132 Brenner and Merriam implied that fractionation rather than single dose gives an increased therapeutic ratio between absence of recurrences and late side effects.Citation135 Nonetheless, as supported by the North Florida Pterygium Study Group and other authors, radiotherapy gives best results when started within 24 hours of complete excision.Citation134
Adverse events following radiotherapy include ocular burning, foreign body sensation, photophobia, conjunctival scarring, corneal opacities, cataracts, grauloma formation, and scleral atrophy. Severe late adverse events that may occur years after surgery are represented by bacterial corneoscleritis, scleral ulceration, and symblepharon.Citation136–Citation139 However, as of today, there is still no definitive consensus between single dose vs total dose. Moreover, the most effective time of exposure needs to be further assessed.
Antivascular endothelial growth factor (anti-VEGF)
Khalfaoui et al found that VEGF is overexpressed in recurrent pterygium and suggested an important role of angiogenesis and neovascularization in pterygium recurrence.Citation140 The first study to report a case of subconjunctival injection of bevacizumab in primary pterygium was conducted by Teng et al. A subconjunctival injection of bevacizumab was performed into an inflamed pterygium at the level of the limbus, with rapid regression of neovascularization signs, and inflammation, although the effects were only short term.Citation141 This transient effect is probably related to a limited bioavailability in a framework of continuous VEGF expression. An intensive high-dose anti-VEGF therapy with ziv-aflibercept can lead to resolution of inflammation and new vessel growth in inflamed pterygia, but current evidence does not support intralesional VEGF antagonists in the surgical management of noninflamed pterygia.Citation142
Shenasi et al, in their study evaluating subconjunctival bevacizumab injection immediately after pterygium excision, found that the recurrence rate was lower in patients receiving the adjuvant treatment.Citation143 Razeghinejad and Banifatemi reported that subconjunctival bevacizumab injected immediately after rotational conjunctival flap and 4 days after surgery was efficient in preventing pterygia recurrence, even if the difference with the control group failed to reach a statistically significant level.Citation144 Shahin et al found that a subconjunctival injection of 1.25 mg/0.05 mL of bevacizumab at the end of the surgery did not result in a statistically significant difference in the recurrence rates.Citation145
Even if a single postoperative administration of subconjunctival bevacizumab is well tolerated and decreases the number and caliber of corneal blood vessels, this favorable effect is incomplete and temporary.Citation146 Repeated injections in the first year after surgery may help prevent the high recurrence rate; however, side effects of multiple or increased doses must be addressed, and further evaluations are needed to be prove bevacizumab as a good adjuvant. In fact, considering its high costs, bevacizumab has not presented fully satisfactory results. In a previous study, we evaluated the application of subconjunctival bevacizumab injections at the dosage of 2.5 mg/0.1 mL, before and after surgical pterygium excision with bare sclera technique (1 week before and 15 days after surgery). It was the only study with this particular injection timing, proving that this repeated modality may be useful in preventing lesion recurrence after bare scleral procedures and that it can lead to more satisfactory benefit–cost ratio. Furthermore, bevacizumab subconjunctival administration is well tolerated and may represent a safer alternative when compared with other surgical techniques and adjunctive drugs.Citation147 Finally, a recent meta-analysis by Sun et al confirmed a statistically significant effect of bevacizumab in preventing primary pterygium recurrence, without significant increase in adverse reactions.Citation148
Preoperative subpterygium-combined injection of bevacizumab and mitomycin C is safe and effective in reducing the postoperative recurrence of primary pterygium, especially if applied 1 month before surgery. A study evaluating histological and immunohistological changes reported a decreased fibrovascular activity and degeneration of the extracellular matrix and nerve axons. Moreover, combination of mitomycin C and bevacizumab led to a significant decrease in CD31-positive cells, with low levels of inflammatory cellular infiltration, fibroblasts, and goblet cells, in association with a significant increase in collagen fibers. This could be secondary to the combined anti-VEGF action of bevacizumab and the antifibroblastic activity of mitomycin C.Citation149
Topical cyclosporine A (CsA)
CsA is an anti-inflammatory and immunosuppressive drug, which can help to significantly reduce pterygium recurrence when used in the postoperative time.Citation150,Citation151 Ibáñez et al used adjuvant CsA at a 0.10% concentration, which differed from other studies that used CsA at a 0.05% concentration with no significant differences.Citation152 Moreover, adjuvant use of CsA in the treatment of pterygium was superior when compared with patients receiving only excisional surgery.Citation151,Citation153,Citation154
In the study by Aydin et al, only one case of recurrence occurred in the CsA group after conjunctival autografting, with a recurrence rate five times lower than the control group.Citation153 However, because of the small sample size, the study failed to reach statistical significance. Similarly, in the study by Ibáñez et al, the recurrence rate was two times higher in the control group than in the CsA group.Citation152
It is possible that surgical techniques as conjunctival autograft or conjunctival flap rotation could themselves decrease the incidence of pterygium recurrence, regardless of the adjuvant use of CsA. Thus, additional studies with larger sample sizes and longer follow-up are needed in order to draw more definitive conclusions. Possible mechanisms explaining the adjuvant use of CsA are related to the higher expression of IL-6 and IL-8 in pterygia epithelium leading to angiogenesis increase through VEGF.Citation16,Citation155,Citation156 Similarly, T-lymphocyte-mediated cellular immunity seems to play a key role in pterygium pathogenesis. Moreover, Lin et al showed that lymphatic microvessel density was a predictive factor for pterygium recurrence.Citation157,Citation158 CsA can selectively suppress T-lymphocytes, which can produce inflammatory cytokines and mediators.Citation159 In addition, CsA can also inhibit the angiogenesis triggered by VEGF. Therefore, inhibition of both these pathways provided by CsA might be an effective method for preventing postoperative recurrence.
Adjuvant use of CsA is relatively safe in comparison with other adjuvant treatment, except in the case of preexisting scleral thinning. Nevertheless, CsA administration can still be considered a well-tolerated adjuvant substance for pterygium treatment. However, the current limited evidence can only suggest that CsA is useful to significantly reduce recurrence rates when compared with simple pterygium excision, whereas the adjuvant use of CsA may not reduce the risk of pterygium recurrence when combined with conjunctival autografting or rotational flap.Citation151
Clinical perspectives
Key features of surgical techniques and adjuvant treatments are summarized in and , respectively. Meticulous excision of pterygium stromal tissue is the first step that must be performed to achieve favorable surgical outcomes because the presence of fibroblasts is probably related to postoperative recurrence. However, this is not sufficient to prevent recurrence events. In order to minimize the risk of postoperative recurrence, it is necessary to perform a complete fibroblast ablation at the level of the surgical wound through accurate removal of subconjunctival fibrovascular tissue. In addition, to promote rapid epithelialization, the bare scleral area should always be covered using a conjunctival rotational flap, amniotic membrane, or a conjunctival autograft. In fact, bare sclera technique is characterized by high recurrence rates ranging from 33% to 88%.Citation35
Table 1 Summary of main characteristics of different surgical techniques for pterygium excision
Table 2 Summary of characteristics of different adjuvant treatments in pterygium surgery
Moreover, a residual large defect after excision induces severe pain leading to wound hypertrophy and higher risk of recurrence, according to our and other authors’ experience.Citation160 It has been reported that neuropeptides involved in pain sensitivity, such as substance P, stimulates chemotaxis of injury-inducible stromal-like cells from the bone marrow to the site of injury.Citation161 For these reasons, techniques leading to high perioperative pain should be avoided if possible. In addition, epithelial wound healing should be promoted and accelerated in order to prevent prolonged stromal overgrowth that may occur when the overlying epithelium (which provides inhibition of stromal proliferation through contact) is absent. Postoperative pain management is crucial in reducing the risk of recurrence and should be performed through either bandage patching or therapeutic contact lenses (TCLs). Despite the fact that bandage patching may interfere with visual field (thus contraindicated in case of monocular vision), it has been reported that this method procures better pain relief and sleep quality in the first 24 hours after pterygium excision when compared with TCLs.Citation162 For this reason, tight bandage patching should be preferred over contact lenses if no contraindications to ocular occlusion are observed. TCLs can still be applied in case of persistent pain or corneal defects after the first postoperative day. Anyways, both methods proved to be efficient in controlling postoperative pain and favoring corneal reepithelization.Citation163–Citation165 Anti-inflammatory eye drops, such as corticosteroids and NSAID, and artificial tears represent commonly used molecules for controlling pain and favoring corneal healing after pterygium surgery. It has been observed that nepafenac 0.1% eye drops administered thrice a day for 3 days (in association with postoperative therapy with topical ciprofloxacin, fluorometholone, and artificial tears) provided a significant pain reduction when compared with placebo after pterygium excision with autologous conjunctival graft.Citation166 An alternative treatment for rapid corneal epithelial healing and pain reduction after pterygium surgery is represented by autologous serum eye drops, which led to favorable results, as reported in a study by Sul et al.Citation167 However, the evidence of autologous serum eye drops in pterygium surgery is currently limited. In addition, systemic analgesic drugs should be considered in the management of postoperative pain, especially if intense or persistent despite adequate topical treatment.
Immediate coverage of large defects with conjunctival autograft, rotational flap, or amniotic membrane is recommended. Healthy conjunctival tissue should be preserved if possible, especially in case of patients who may require surgery for glaucoma. As of today, conjunctival autografting is considered the most effective technique for pterygium treatment and proved to provide better outcomes with respect to amniotic membrane transplantation. Moreover, conjunctival autografting operating time can be importantly reduced, thanks to sutureless fixating techniques. According to the literature, amniotic membrane transplantation can still be reserved in case of unhealthy or insufficient conjunctival tissue. In our experience, in case of recurrence after conjunctival rotational flap technique, it is advisable to apply a conjunctival or amniotic membrane patch associated with long-lasting treatment with corticosteroid eye drops in order to reduce postsurgical flogosis. Superior or inferior conjunctival tissue did not prove to provide better results when compared with each other.
Several adjuvant options to reduce the proliferative activity of stromal fibroblasts during the postoperative recovery phase have been proposed. Fonseca et al suggested that bare sclera excision + β therapy 25 Gy in single dose, bare sclera technique + mitomycin C 0.02%, and conjunctival autograft + cyclosporine 0.05% eye drops are the best strategies to prevent recurrence after pterygium surgery.Citation168 However, randomized clinical trials to evaluate efficacy of adjuvant therapies for pterygium are relatively few and with small sample size. In the light of the current evidence, mitomycin C, β-radiotherapy, and bevacizumab were significantly more effective than placebo for reducing recurrence following pterygium excision, with mitomycin C having the higher efficacy.Citation118,Citation169–Citation173 Although bevacizumab ranked higher than β-radiotherapy for its efficacy, this difference was not statistically significant. However, bevacizumab is associated with less severe adverse events when compared with mitomycin C or radiation therapy; hence, repeated injections (which are needed to avoid the transient effects of anti-VEGF drugs) are safe and possible. Topical postoperative CsA gives appealing hopes for adjuvant therapy, but current evidence is still poor to evaluate its safety and efficacy in pterygium surgery. 5-FU did not show a statistically significant efficacy in preventing postoperative recurrence.
As of today, optimal dose, duration, and administration protocol for adjuvant treatments are still not fully defined. With regard to radiotherapy, the commonly used dose is 25 Gy with bare sclera technique, which is decreased to 10 Gy when combined with conjunctival autografting.Citation174 Administration and dose of 5-FU were consistent among trials; however, duration of administration varied from 3 to 5 minutes. Bevacizumab dosage ranged from 1.25 to 7.5 mg in different studies, and its administration approach can be manifold among different studies.Citation174 The most common way of bevacizumab administration is subconjunctival at the time of surgery, associated with pre- or postoperative injections. We have reported that recurrence after bare sclera technique was reduced with both pre- and postoperative subconjunctival injections of bevacizumab and that this administration protocol is safe and feasible.Citation147 Administration protocols of mitomycin C are inconsistent as well, with different dose, durations, and approaches. Adjuvant treatments still need standardization regarding dosage, time, and ways of administration because no definitive recommendations or guidelines are being produced. Another issue that must be taken into consideration is the side effects associated with adjuvant therapies, which alter the benefit–risk ratio. Therefore, the detection of patients who will benefit from adjuvant treatments is most relevant in the preoperative setting and risk factors for recurrence must be addressed. Reports showed that 50% of recurrences might occur within 4 months and 97% might occur within 12 months. Thus, follow-up of at least 1 year is appropriate.
Some studies reported that age, race, morphology, and increased inflammation after surgery are related to a greater risk of recurrence.Citation34,Citation175–Citation180 Therefore, accurate collection of patients’ clinical information is fundamental to better predict the recurrence risk. However, actual assessment tools for routine clinical practice have not yet been developed for this disease. In order to find out reliable strategies for pterygium treatment, better understanding of molecular mechanisms involved in its pathogenesis is necessary. Several molecular pathways have been evaluated, and different biomarkers have been proposed. It is known that pterygium features altered proliferation of basal epithelial cells and neovascularization, with invasion of corneal epithelium. UV radiation, together with epigenetic aberrations, is strongly related to pterygium development and progression. However, because viral infections have been reported as a potential factor for pterygium pathogenesis, deeper understanding of their role may be crucial in the detection of subjects at risk of recurrence.Citation14 Pterygium fibrovascular redness has been proposed as a novel parameter for pterygium grading. In fact, an automated redness analysis proposed by Hilmi et al showed a significant correlation with visual acuity and contrast sensitivity reduction, suggesting that pterygium morphology must be considered for the evaluation of clinical decisions and postoperative recurrence prediction.Citation181
Several growth factors and cytokines involved in angiogenesis and lymphangiogenesis, and many different proteinases as well, are related to pterygium development and persistence. Another promising field of research for pterygium pathogenesis is represented by modifications of cholesterol metabolism, even if further studies are needed.Citation14 Transition from epithelial to mesenchymal cells in pterygium has suggested that this pathology shares molecular basis with tumors. In fact, molecular pathways involved in increased proliferative activity (Ki-67, proliferation cell nuclear antigen, and erythropoietin), inhibition of apoptosis (Bcl-2 and p53), and malignancy (Hsp90) are overexpressed in the epithelial pterygium cells when compared with normal conjunctiva.Citation182–Citation184 In addition, abnormal methylation of genes related to extracellular matrix modulation 2, tumor suppression (p16), and cell adhesion (TGM-2 and CD24) has been correlated to pterygium invasiveness and uncontrolled proliferation.Citation185,Citation186 Oxidative stress caused by UV radiation may be responsible for these abnormal methylation patterns, although not confirmed yet.
Several studies have demonstrated a significant improvement in induced astigmatism after pterygium surgery.Citation187–Citation192 Kheirkhah et al evaluated corneal astigmatism with a Scheimpflug imaging system, concluding that pterygium surgery was associated with significant changes in curvature of front and back corneal surfaces, especially in case of advanced pterygia.Citation193 However, it has been observed that no surgical technique or fixating method was superior in terms of postoperative astigmatism changes.Citation194 Pterygium, especially with large size, is correlated with optical aberrations, and several studies have demonstrated that surgery with conjunctival autograft leads to significant improvements in corneal wave front aberrations (including high-order aberrations, trefoil, and coma), visual acuity, refractive errors, and different corneal topographic values.Citation195–Citation198 However, there are no comparison studies regarding optical aberration modifications after adjuvant techniques different from conjunctival autografting.
Conclusion
Bare sclera technique is associated with worst outcomes, with higher recurrence risk. Pterygium surgery cannot disregard wound coverage with conjunctival autografting or rotational flap combined with adjuvant treatments. According to our experience, the conjunctival rotational flap technique is associated with low recurrence rates and the vascular pedicle prevents graft displacement and necrosis. Despite their high cost, subconjunctival anti-VEGF injections (which can be performed before and after surgery) are a safe and efficient adjuvant treatment. However, future treatment strategies will probably target multiple pathways, taking into consideration the novel findings related to pathogenesis. Additional studies should consider recurrence rates in accordance with geographic regions and long-term follow-ups to improve the understanding of pterygium treatment and develop universal guidelines. Great importance should be dedicated to the identification of novel molecular biomarkers of recurrence, in order to perform a customized surgical treatment for each patient and achieve maximal reduction of postoperative recurrence.
Disclosure
The authors report no conflicts of interest in this work.
References
- DushkuNJohnMKSchultzGSReidTWPterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cellsArch Ophthalmol2001119569570611346397
- AspiotisMTsanouEGorezisSAngiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1Eye20072181095110116823458
- KaseSOsakiMJinXHIncreased expression of erythropoietin receptor in human pterygial tissuesInt J Mol Med200720569970217912463
- TsaiYYChiangCCYehKTLeeHChengYWEffect of TIMP-1 and MMP in pterygium invasionInvest Ophthalmol Vis Sci20105173462346720207965
- LiangKJiangZDingBQChengPHuangDKTaoLMExpression of cell proliferation and apoptosis biomarkers in pterygia and normal conjunctivaMol Vis2011171687169321738398
- CoroneoMTPterygium as an early indicator of ultraviolet insolation: a hypothesisBr J Ophthalmol199377117347398280691
- HilgersJHPterygium: its incidence, heredity and etiologyAm J Ophthalmol196050463564413714249
- MarchettiCSidahmed-AdrarNCollinFJoreDGardès-AlbertMBonnefont-RousselotDMelatonin protects PLPC liposomes and LDL towards radical-induced oxidationJ Pineal Res201151328629621545523
- KauHCTsaiCCLeeCFIncreased oxidative DNA damage, 8-hydroxydeoxy-guanosine, in human pterygiumEye200620782683116113633
- BalciMSahinSMutluFMYağciRKaranciPYildizMInvestigation of oxidative stress in pterygium tissueMol Vis20111744344721321673
- ReidTWDushkuNDoes human papillomavirus cause pterygium?Br J Ophthalmol200387780680812812871
- Di GirolamoNAssociation of human papilloma virus with pterygia and ocular-surface squamous neoplasiaEye201226220221122134594
- DetorakisETDrakonakiEESpandidosDAMolecular genetic alterations and viral presence in ophthalmic pterygiumInt J Mol Med200061354110851263
- Cárdenas-CantúEZavalaJValenzuelaJValdez-GarcíaJEMolecular basis of pterygium developmentSemin Ophthalmol201631656758325415268
- Di GirolamoNChuiJCoroneoMTWakefieldDPathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinasesProg Retin Eye Res200423219522815094131
- Di GirolamoNKumarRKCoroneoMTWakefieldDUVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cellsInvest Ophthalmol Vis Sci200243113430343712407153
- SiakJJKNgSLSeetLFBeuermanRWTongLThe nuclear-factor κB pathway is activated in pterygiumInvest Ophthalmol Vis Sci201152123023620811049
- PinkertonODHokamaYShigemuraLAImmunologic basis for the pathogenesis of pterygiumAm J Ophthalmol19849822252286383051
- LingSLiangLLinHLiWXuJIncreasing lymphatic microvessel density in primary pterygiaArch Ophthalmol2012130673574222801834
- JinJGuanMSimaJDecreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygiaCornea200322547347712827055
- RiauAKWongTTLanWAberrant DNA methylation of matrix remodeling and cell adhesion related genes in pterygiumPLoS One201162e1468721359202
- KriaLOhiraAAmemiyaTImmunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygiumActa Histochem19969821952018739304
- HanahanDFolkmanJPatterns and emerging mechanisms of the angiogenic switch during tumorigenesisCell19968633533648756718
- ParkCYChoiJSLeeSJHwangSWKimEJChuckRSCyclooxygenase-2-expressing macrophages in human pterygium co-express vascular endothelial growth factorMol Vis2011173468348022219642
- SolomonAGrueterichMLiDQMellerDLeeSBTsengSCOverexpression of insulin-like growth factor-binding protein-2 in pterygium body fibroblastsInvest Ophthalmol Vis Sci200344257358012556385
- YeJSongYSKangSHYaoKKimJCInvolvement of bone marrow-derived stem and progenitor cells in the pathogenesis of pterygiumEye200418883984315002023
- SongYSRyuYHChoiSRKimJCThe involvement of adult stem cells originated from bone marrow in the pathogenesis of pterygiaYonsei Med J200546568769216259068
- PeirettiEDessìSMulasCModulation of cholesterol homeostasis by antiproliferative drugs in human pterygium fibroblastsInvest Ophthalmol Vis Sci20074883450345817652712
- AnguriaPKitinyaJNtuliSCarmichaelTThe role of heredity in pterygium developmentInt J Ophthalmol20147356357324967209
- MohammedITreatment of pterygiumAnn Afr Med201110319720321912002
- FernandesMSangwanVSBansalAKOutcome of pterygium surgery: analysis over 14 yearsEye200519111182119015543190
- HovanesianJAStarrCEVromanDTThe ASCRS Cornea Clinical CommitteeSurgical techniques and adjuvants for the management of primary and recurrent pterygiaJ Cataract Refract Surg201743340541928410726
- RohrbachIMStarcSKnorrMVorhersage von Pterygiumrezidiven Aufgrund Morphologischer und Immunhistologischer Parameter [Predicting recurrent pterygium based on morphologic and immunohistologic parameters]Ophthalmologe19959244634687549330
- KimKWKimJCCurrent approaches and future directions in the management of pterygiumInt J Ophthalmol201811570971129862166
- TanDTCheeSPDearKBLimASEffect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excisionArch Ophthalmol199711510123512409338666
- KimKWParkSHKimJCFibroblast biology in pterygiaExp Eye Res2016142323926675401
- KaufmanSCJacobsDSLeeWBDengSXRosenblattMIShteinRMOptions and adjuvants in surgery for pterygium: a report by the American Academy of OphthalmologyOphthalmology2013120120120823062647
- Sánchez-ThorinJCRochaGYelinJBMeta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygiumBr J Ophthalmol19988266616659797669
- KenyonKRWagonerMDHettingerMEConjunctival autograft transplantation for advanced and recurrent pterygiumOphthalmology19859211146114704080320
- BilgeADComparison of conjunctival autograft and conjunctival transposition flap techniques in primary pterygium surgerySaudi J Ophthalmol201832211011329942178
- KoranyiGSeregardSKoppEDThe cut-and-paste method for primary pterygium surgery: long-term follow-upActa Ophthalmol Scand200583329830115948780
- MaDHSeeLCLiauSBTsaiRJAmniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatmentBr J Ophthalmol200084997397810966947
- Al FayezMFLimbal versus conjunctival autograft transplantation for advanced and recurrent pterygiumOphthalmology200210991752175512208727
- CorneliusCRRecurrence rate and complications of pterygium extended removal followed by extended conjunctival transplantCornea201736110110327749451
- KumarSSinghRPterygium excision and conjunctival autograft: a comparative study of techniquesOman J Ophthalmol201811212412829930445
- CohenRAMcDonaldMBFixation of conjunctival autografts with an organic tissue adhesiveArch Ophthalmol19931119116711688363455
- KoranyiGSeregardSKoppEDCut and paste: a no suture, small incision approach to pterygium surgeryBr J Ophthalmol200488791191415205236
- RomanoVCrucianiMContiLFontanaLFibrin glue versus sutures for conjunctival autografting in primary pterygium surgeryCochrane Database Syst Rev2016124CD01130827911983
- RatnalingamVEuALNgGLTaharinRJohnEFibrin adhesive is better than sutures in pterygium surgeryCornea201029548548920308876
- UyHSReyesJMFloresJDLim-Bon-SiongRComparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excisionOphthalmology2005112466767115808260
- CelikTIn situ blood coagulum versus sutures for autograft fixation after pterygium excisionCurr Eye Res201843897798029708441
- KurianAReghunadhanINairKGAutologous blood versus fibrin glue for conjunctival autograft adherence in sutureless pterygium surgery: a randomised controlled trialBr J Ophthalmol201599446447025326519
- ChoudhurySDuttaJMukhopadhyaySComparison of autologous in situ blood coagulum versus sutures for conjunctival autografting after pterygium excisionInt Ophthalmol2014341414823733278
- NatungTKeditsuAShullaiWGoswamiPKSuturelessGPKSutureless, glue-less conjunctival autograft versus conjunctival autograft with sutures for primary, advanced pterygia: an interventional pilot studyJ Clin Diagn Res2017118NC04NC0728969169
- LešinMParadžikMMarin LovrićJCauterisation versus fibrin glue for conjunctival autografting in primary pterygium surgery (CAGE CUP): study protocol of a randomised controlled trialBMJ Open201886e020714
- KüçükerdönmezCAkovaYAAltinörsDDComparison of conjunctival autograft with amniotic membrane transplantation for pterygium surgery: surgical and cosmetic outcomeCornea200726440741317457187
- VrabecMPWeisenthalRWElsingSHSubconjunctival fibrosis after conjunctival autograftCornea19931221811838500328
- MullinsJBHoldsJBBranhamGHThomasJRComplications of the transconjunctival approach. A review of 400 casesArch Otolaryngol Head Neck Surg199712343853889109784
- FerryAPPyogenic granulomas of the eye and ocular adnexa: a study of 100 casesTrans Am Ophthalmol Soc1989873273432562522
- ZhangZYangZPanQChenPGuoLClinicopathologic characteristics and the surgical outcome of conjunctival granulomas after pterygium surgeryCornea20183781008101229877925
- KapadiaSBHeffnerDKPitfalls in the histopathologic diagnosis of pyogenic granulomaEur Arch Otorhinolaryngol199224941952001642875
- LindquistTPLeeWBMitomycin C-associated scleral stromalysis after pterygium surgeryCornea201534439840125719251
- ZlotoORosenNLeshnoARosnerMVery long term success of pterygium surgery with conjunctival graftCont Lens Anterior Eye201740426726928522251
- KoçFDemirbayPTekeMYPrimer ve rekürren pterygiumda konjonktival otogreftlemeT Oft Gaz2002583588
- SyamPPEleftheriadisHLiuCSInferior conjunctival autograft for primary pterygiaOphthalmology2003110480681012689907
- ParachaQAyoobMDawoodZMirzaSARecurrence rate with use of intraoperative mitomycin C versus conjunctival autograft following pterygium excisionPak J Med Sci20143061243124625674116
- KeklikciUCelikYCakmakSSUnluMKBilekBConjunctival-limbal autograft, amniotic membrane transplantation, and intraoperative mitomycin C for primary pterygiumAnn Ophthalmol2007394296301
- TananuvatNMartinTThe results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograftCornea200423545846315220729
- Frucht-PeryJRaiskupFIlsarMLandauDOrucovFSolomonAConjunctival autografting combined with low-dose mitomycin C for prevention of primary pterygium recurrenceAm J Ophthalmol200614161044105016546105
- SegevFJaeger-RoshuSGefen-CarmiNAssiaEICombined mitomycin C application and free flap conjunctival autograft in pterygium surgeryCornea200322759860314508255
- TsengSCLiDQMaXSuppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrixJ Cell Physiol1999179332533510228951
- LeeSBLiDQTanDTMellerDCTsengSCSuppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membraneCurr Eye Res200020432533410806447
- PrabhasawatPBartonKBurkettGTsengSCComparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excisionOphthalmology199710469749859186439
- SangwanVSBurmanSTejwaniSMaheshSPMurthyRAmniotic membrane transplantation: a review of current indications in the management of ophthalmic disordersIndian J Ophthalmol200755425126017595472
- ThatteSAmniotic membrane transplantation: an option for ocular surface disordersOman J Ophthalmol201142677221897621
- ArainMAYaqubMAAmeenSSIqbalZNaqviAHNiaziMKAmniotic membrane transplantation in primary pterygium compared with bare sclera techniqueJ Coll Physicians Surg Pak201222744044322747864
- Moreno-LópezREstudio comparativo entre escisión de pterigión primario con autoinjerto conjuntival, membrana amniótica y cierre primario [Comparative study between primary pterygium excision using conjunctival autograft, amniotic membrane, and primary closure]Rev Mex Oftalmol200478291297
- SolomonAPiresRTTsengSCAmniotic membrane transplantation after extensive removal of primary and recurrent pterygiaOphthalmology2001108344946011237898
- ClearfieldEHawkinsBSKuoICConjunctival autograft versus amniotic membrane transplantation for treatment of pterygium: findings from a Cochrane systematic reviewAm J Ophthalmol201718281728734814
- TananuvatNMartinTThe results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograftCornea200423545846315220729
- ÖzerAYıldırımNErolNYurdakulSLong-term results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft techniques in primary pterygium excisionsOphthalmologica2009223426927319339811
- KeklikciUCelikYCakmakSSUnluMKBilekBConjunctival-limbal autograft, amniotic membrane transplantation, and intraoperative mitomycin C for primary pterygiumAnn Ophthalmol2007394296301
- BesharatiMRMiratashiSAAhmadiABPterygium surgery: amniotic membrane or conjunctival autograft transplantationInt J Ophthalmol2006612581262
- HaoYMaDHHwangDGKimWSZhangFIdentification of antiangiogenic and antiinflammatory proteins in human amniotic membraneCornea200019334835210832697
- BultmannSYouLSpandauUAmniotic membrane downregulates chemokine expression in human keratocytesInvestig Ophthalmol Vis Sci199940S578
- TsengSCLiDQMaXSuppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrixJ Cell Physiol1999179332533510228951
- YeJKookKHYaoKTemporary amniotic membrane patch for the treatment of primary pterygium: mechanisms of reducing the recurrence rateGraefes Arch Clin Exp Ophthalmol2006244558358816170535
- KatırcıogluYAAltiparmakUEngur GoktasSCakirBSingarEOrnekFComparison of two techniques for the treatment of recurrent pterygium: amniotic membrane vs conjunctival autograft combined with mitomycin CSemin Ophthalmol2015305–632132724506693
- AmanoSMotoyamaYOshikaTEguchiSEguchiKComparative study of intraoperative mitomycin C and beta irradiation in pterygium surgeryBr J Ophthalmol200084661862110837388
- RosenRAmniotic membrane grafts to reduce pterygium recurrenceCornea201837218919328976415
- ChoHChuckRSPterygium excision and placement of amniotic membrane graftsHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Inc201291100
- GhanavatiSZShoushaMABetancurtCPerezVLCombined conjunctival autograft and overlay amniotic membrane transplantation; a novel surgical treatment for pterygiumJ Ophthalmic Vis Res20149339940325667744
- ShimazakiJKosakaKShimmuraSTsubotaKAmniotic membrane transplantation with conjunctival autograft for recurrent pterygiumOphthalmology2003110111912412511356
- PanXZhangDJiaZChenZSuYComparison of hyperdry amniotic membrane transplantation and conjunctival autografting for primary pterygiumBMC Ophthalmol201818111929764389
- OkabeMKitagawaKYoshidaTHyperdry human amniotic membrane is useful material for tissue engineering: physical, morphological properties, and safety as the new biological materialJ Biomed Mater Res A2014102386287023589398
- AllenCLClareGStewartEAAugmented dried versus cryopreserved amniotic membrane as an ocular surface dressingPLoS One2013810e7844124205233
- McCoombesJAHirstLWIsbellGPSliding conjunctival flap for the treatment of primary pterygiumOphthalmology199410111691738302551
- AlpayAUğurbasSHErdoganBComparing techniques for pterygium surgeryClin Ophthalmol20093697419668546
- BamdadSKooshkiASYasemiMSurgical outcome of conjunctival rotational autograft-mitomycin C (MMC) versus free conjunctival autograft-MMC for pterygium removal: a randomized clinical trialElectron Physician20179125877588429560137
- DadeyaSMalikKPGullianiBPPterygium surgery: conjunctival rotation autograft versus conjunctival autograftOphthalmic Surg Lasers20023326927412134984
- WuWKWongVWChiSCLamDSSurgical management of double-head pterygium by using a novel technique: conjunctival rotational autograft combined with conjunctival autograftCornea20072691056105917893533
- JapAChanCLimLTanDTConjunctival rotation autograft for pterygium. An alternative to conjunctival autograftingOphthalmology1999106167719917783
- YoungALTamPMLeungGYChengLLLamPTLamDSProspective study on the safety and efficacy of combined conjunctival rotational autograft with intraoperative 0.02% mitomycin C in primary pterygium excisionCornea200928216616919158559
- AlmondMCDastrupBTKaufmanSC5-Fluorouracil and mitomycin C: adjuncts to pterygium surgeryHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Inc20125564
- HayasakaSNodaSYamamotoYSetogawaTPostoperative instillation of low-dose mitomycin C in the treatment of primary pterygiumAm J Ophthalmol198810667157183143266
- Frucht-PeryJIlsarMHemoISingle dosage of mitomycin C for prevention of recurrent pterygium: preliminary reportCornea19941354114137995063
- MaharPSConjunctival autograft versus topical mitomycin C in treatment of pterygiumEye199711Pt 67907929537132
- ManningCAKloessPMDiazMDYeeRWIntraoperative mitomycin in primary pterygium excision. A prospective, randomized trialOphthalmology199710458448489160032
- SharmaAGuptaARamJGuptaALow-dose intraoperative mitomycin-C versus conjunctival autograft in primary pterygium surgery: long term follow-upOphthalmic Surg Lasers200031430130710928667
- KeklikciUCelikYCakmakSSUnluMKBilekBConjunctival-limbal autograft, amniotic membrane transplantation, and intraoperative mitomycin C for primary pterygiumAnn Ophthalmol2007394296301
- AriSCacaIYildizZÖSakalarYBDoganEComparison of mitomycin C and limbal-conjunctival autograft in the prevention of pterygial recurrence in Turkish patients: a one-year, randomized, assessor-masked, controlled trialCurr Ther Res Clin Exp200970427428124683237
- BiswasMCShawCMandalRTreatment of pterygium with conjunctival limbal autograft and mitomycin C – a comparative studyJ Indian Med Assoc2007105420020220417822189
- KoranyiGArtzénDSeregardSKoppEDIntraoperative mitomycin C versus autologous conjunctival autograft in surgery of primary pterygium with four-year follow-upActa Ophthalmol201290326627020528781
- CardilloJAAlvesMRAmbrosioLEPoterioMBJoseNKSingle intraoperative application versus postoperative mitomycin C eye drops in pterygium surgeryOphthalmology199510212194919529098301
- HelalMMessihaNAmayemAEl-MaghrabyAElsherifZDabeesMIntraoperative mitomycin-C versus postoperative topical mitomycin-C drops for the treatment of pterygiumOphthalmic Surg Lasers19962786746788858633
- HosalBMGürselEMitomycin-C for prevention of recurrent pterygiumAnn Ophthalmol2000322107109
- OguzHBasarEGurlerBIntraoperative application versus postoperative mitomycin C eye drops in pterygium surgeryActa Ophthalmol Scand199977214715010321528
- Frucht-PeryJIlsarMThe use of low-dose mitomycin C for prevention of recurrent pterygiumOphthalmology199410147597628152772
- DoughertyPJHardtenDRLindstromRLCorneoscleral melt after pterygium surgery using a single intraoperative application of mitomycin-CCornea19961555375408862932
- DushinskyRPlevenEHeidelbergerCThe synthesis of 5-fluoropy-rimidinesJ Am Chem Soc19577945594560
- SmithSD’AmorePADreyerEBComparative toxicity of mitomycin C and 5-fluorouracil in vitroAm J Ophthalmol199411833323378085590
- ValeziVGSchelliniSAHata ViveirosMMPadovaniCRSegurança e efetividade no tratamento do pterígio usando infiltração de 5-fluoruracila no intraoperatorio [Safety and efficacy of intraoperative 5-fluorouracil infiltration in pterygium treatment]Arq Bras Oftalmol200972169173 Portuguese19466323
- AkarsuCTanerPErginA5-Fluorouracil as chemoadjuvant for primary pterygium surgery: preliminary reportCornea200322652252612883344
- PikkelJPorgesYOphirAHalting pterygium recurrence by postoperative 5-fluorouracilCornea200120216817111248822
- PrabhasawatPTesavibulNLeelapatranuraKPhonjanTEfficacy of subconjunctival 5-fluorouracil and triamcinolone injection in impending recurrent pterygiumOphthalmology200611371102110916730066
- GalentinePSloasHHargettNCupplesHPBilateral cicatricial ectropion following topical administration of 5-fluorouracilAnn Ophthalmol19811355755776455082
- CaravellaLPBurnsJAZangmeisterMPunctal-canalicular stenosis related to systemic fluorouracil therapyArch Ophthalmol19819922842867469866
- Hickey-DwyerMWishartPKSerious corneal complication of 5-fluorouracilBr J Ophthalmol19937742502518494864
- WeinrebRNAdjusting the dose of 5-fluorouracil after filtration surgery to minimize side effectsOphthalmology19879455645702439972
- BurkADie Behandlung der Homhaut epitheliome durch Rontgen-strahlenStrahlenther Onkol19121168171
- JarosPADeluiseVPPingueculae and pterygiumSurv Ophthalmol1988324149
- Jürgenliemk-SchulzIMHartmanLJRoesinkJMPrevention of pterygium recurrence by postoperative single-dose beta-irradiation: a prospective randomized clinical double-blind trialInt J Radiat Oncol Biol Phys20045941138114715234049
- De KeizerRJPterygium excision with or without postoperative irradiation, a double-blind studyDoc Ophthalmol1982523–43093157040006
- ParyaniSBScottWPWellsJWManagement of pterygium with surgery and radiation therapy. The North Florida Pterygium Study GroupInt J Radiat Oncol Biol Phys19942811011038270429
- BrennerDJMerriamGRPostoperative irradiation for pterygium: guidelines for optimal treatmentInt J Radiat Oncol Biol Phys19943037217257928506
- BahrassaFDattaRPostoperative beta radiation treatment of pterygiumInt J Radiat Oncol Biol Phys1983956796846853267
- BeyerDCPterygia: single-fraction postoperative beta irradiationRadiology199117825695711987626
- CooperJSPostoperative irradiation of pterygia: ten more years of experienceRadiology19781283753756674650
- WilderRBBuattiJMKittelsonJMPterygium treated with excision and postoperative beta irradiationInt J Radiat Oncol Biol Phys19922335335371612953
- KhalfaouiTMkannezGColinDImmunohistochemical analysis of vascular endothelial growth factor (VEGF) and p53 expression in pterygium from Tunisian patientsPathol Biol201159313714119481369
- TengCCPatelNNJacobsonLEffect of subconjunctival bevacizumab on primary pterygiumCornea200928446847019411971
- MansourAMRegression of inflamed pterygia by frequent high-dose intralesional ziv-afliberceptCornea20173681002100528614154
- ShenasiAMousaviFShoa-AhariSRahimi-ArdabiliBFouladiRFSubconjunctival bevacizumab immediately after excision of primary pterygium: the first clinical trialCornea201130111219122221955635
- RazeghinejadMRBanifatemiMSubconjunctival bevacizumab for primary pterygium excision; a randomized clinical trialJ Ophthalmic Vis Res201491223024982728
- ShahinMMElbendaryAMElwanMMMahaMAmalMMohamedMIntraoperative subconjunctival bevacizumab as an adjunctive treatment in primary pterygium: a preliminary reportOphthalmic Surg Lasers Imaging201243645946622882007
- StivalLRLagoAMFigueiredoMNBittarRHMachadoMLNassaralla JuniorJJEfficacy and safety of subconjunctival bevacizumab for recurrent pterygiumArq Bras Oftalmol20147714725076364
- NuzziRTridicoFEfficacy of subconjunctival bevacizumab injections before and after surgical excision in preventing pterygium recurrenceJ Ophthalmol20172017116824670682467728634544
- SunYZhangBJiaXLingSDengJEfficacy and safety of bevacizumab in the treatment of pterygium: an updated meta-analysis of randomized controlled trialsJ Ophthalmol201820184598173459817930254755
- AlsmmanAHRadwanGAbozaidMAMohammedUAAbd ElhaleimNGPreoperative subconjunctival combined injection of bevacizumab and mitomycin C before the surgical excision of primary pterygium: clinical and histological resultsClin Ophthalmol20171149350128331283
- Yalcin TokOBurcu NurozlerAErgunGAkbas KocaogluFDumanSTopical cyclosporine A in the prevention of pterygium recurrenceOphthalmologica2008222639139618765950
- ZhangQBaoNLiangKTaoLAdjuvant use of cyclosporine a in the treatment of primary pterygium: a systematic review and meta-analysisCornea20183781000100729601365
- IbáñezMEugarriosMFCalderónDITopical cyclosporin A and mitomycin C injection as adjunctive therapy for prevention of primary pterygium recurrenceOphthalmic Surg Lasers Imaging200940323924419485286
- AydinAKaradayiKAykanUCanGColakogluKBilgeAHEffectiveness of topical ciclosporin A treatment after excision of primary pterygium and limbal conjunctival autograftJ Fr Ophtalmol200831769970418971855
- RenYWangCLinYStudy on topical cyclosporine A in the prevention of pterygium recurrenceInt J Ophthalmol2009922402241
- WangIJLaiWTLiouSWImpression cytology of pterygiumJ Ocul Pharmacol Ther200016651952811132899
- NakamuraMNishidaTDifferential effects of epidermal growth factor and interleukin 6 on corneal epithelial cells and vascular endothelial cellsCornea199918445245810422859
- EbrahimiMEKordi-TamandaniDMArishMA novel approach to investigation of the pathogenesis of pterygium based on assessment of promoter hyper-methylation and expression profile of CTLA4 gene: a credible report of CTLA4 gene expression in human eye tissueGene2016583213013326899867
- LinHLuoLLingSLymphatic microvessel density as a predictive marker for the recurrence time of pterygium: a three-year follow-up studyMol Vis20131916617323378730
- TangBRenHLiuHCCR5 blockade combined with cyclosporine A attenuates liver GVHD by impairing T cells functionInflamm Res2016651191792427423909
- KimKWKimJCCurrent approaches and future directions in the management of pterygiumInt J Ophthalmol201811570971129862166
- HongHSLeeJLeeEA new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cellsNat Med200915442543519270709
- PratDZlotoOBen ArtsiEBen SimonGJTherapeutic contact lenses vs tight bandage patching and pain following pterygium excision: a prospective randomized controlled studyGraefes Arch Clin Exp Ophthalmol2018256112143214830173337
- YeungSNLichtingerAKimPEfficacy and safety of patching vs bandage lens on postoperative pain following pterygium surgeryEye201529229529625475236
- DagliogluMCCoskunMIlhanNThe effects of soft contact lens use on cornea and patient’s recovery after autograft pterygium surgeryCont Lens Anterior Eye201437317517724172652
- ArenasEGarciaSA scleral soft contact lens designed for the postoperative management of pterygium surgeryEye Contact Lens200733191217224673
- OzcimenMSakaryaYGoktasSEffect of nepafenac eye drops on pain associated with pterygium surgeryEye Contact Lens201541318718925603438
- SulSKorkmazSAlacamliGOzyolPOzyolEApplication of autologous serum eye drops after pterygium surgery: a prospective studyGraefes Arch Clin Exp Ophthalmol2018256101939194330022252
- FonsecaECRochaEMArrudaGVComparison among adjuvant treatments for primary pterygium: a network meta-analysisBr J Ophthalmol2018102674875629146761
- BekibeleCOBaiyerojuAMOlusanyaBAAshayeAOOluleyeTSPterygium treatment using 5-FU as adjuvant treatment compared to conjunctiva autograftEye2008221313416778821
- PherwaniAVakilVEatamadiHSinghRDuaHSPostoperative subconjunctival 5-fluorouracil in the management of recurring pterygiumBr J Ophthalmol200791339839917322471
- RachmielRLeibaHLevartovskySResults of treatment with topical mitomycin C 0.02% following excision of primary pterygiumBr J Ophthalmol19957932332367703200
- VianiGAStefanoEJde FendiLIFonsecaECLong-term results and prognostic factors of fractionated strontium-90 eye applicator for pterygiumInt J Radiat Oncol Biol Phys20087241174117918632216
- Jürgenliemk-SchulzIMHartmanLJRoesinkJMPrevention of pterygium recurrence by postoperative single-dose beta-irradiation: a prospective randomized clinical double-blind trialInt J Radiat Oncol Biol Phys20045941138114715234049
- ZengWLiuZDaiHAnti-fibrotic, anti-VEGF or radiotherapy treatments as adjuvants for pterygium excision: a systematic review and network meta-analysisBMC Ophthalmol201717121129178848
- LiuJFuYXuYTsengSCNew grading system to improve the surgical outcome of multirecurrent pterygiaArch Ophthalmol20121301394922232474
- OlusanyaBAOgunOABekibeleCORisk factors for pterygium recurrence after surgical excision with combined conjunctival autograft (CAG) and intraoperative antimetabolite useAfr J Med Med Sci2014431354025335376
- HaSWParkJHShinIHKimHKClinical analysis of risk factors contributing to recurrence of pterygium after excision and graft surgeryInt J Ophthalmol20158352252726086001
- SandraSZeljkaJZeljkaVAKristianSIvanaAThe influence of pterygium morphology on fibrin glue conjunctival autografting pterygium surgeryInt Ophthalmol2014341757923733279
- VarssanoDShalevHLazarMFischerNPterygium excision with conjunctival autograft: true survival rate statisticsCornea20133291243125023594771
- KheirkhahANazariRNikdelMGhassemiHHashemiHBehrouzMJPostoperative conjunctival inflammation after pterygium surgery with amniotic membrane transplantation versus conjunctival autograftAm J Ophthalmol2011152573373821742306
- HilmiMRChe AzeminMZMohd KamalKMohd TamrinMIAbdul GaffurNTengku SembokTMPrediction of changes in visual acuity and contrast sensitivity function by tissue redness after pterygium surgeryCurr Eye Res201742685285628118054
- ChowersIPe’erJZamirELivniNIlsarMFrucht-PeryJProliferative activity and p53 expression in primary and recurrent pterygiaOphthalmology2001108598598811320032
- KaseSTakahashiSSatoINakanishiKYoshidaKOhnoSExpression of p27(KIP1) and cyclin D1, and cell proliferation in human pterygiumBr J Ophthalmol200791795896117179165
- KimKWParkSHWeeSWKimJCOverexpression of angiogenin in pterygium body fibroblasts and its association with proliferative potencyInvest Ophthalmol Vis Sci20135496355636223989187
- RiauAKWongTTLanWFingerSNAberrant DNA methylation of matrix remodeling and cell adhesion related genes in pterygiumPLoS One201162e1468721359202
- ChenKHHsuWMIntraoperative ethanol treatment as an adjuvant therapy of pterygium excisionInt J Biomed Sci20062441442123675010
- AshayeAORefractive astigmatism and pterygiumAfr J Med Med Sci19901932252282120924
- SorianoJMJanknechtPWitschelHEffect of pterygium operation on preoperative astigmatism. Prospective studyOphthalmologe19939066886908124034
- AvisarRMeklerSSavirHEffect of pterygium excision on keratometric readingsHarefuah1994126263658144083
- SternGALinAEffect of pterygium excision on induced corneal topographic abnormalitiesCornea199817123279436876
- FongKSBalakrishnanVCheeSPTanDTRefractive change following pterygium surgeryClao J19982421151179571272
- TomidokoroAOshikaTAmanoSEguchiKEguchiSQuantitative analysis of regular and irregular astigmatism induced by pterygiumCornea199918441241510422852
- KheirkhahASafiHMolaeiSNazariRBehrouzMJRajuVKEffects of pterygium surgery on front and back corneal astigmatismCan J Ophthalmol201247542342823036543
- Altan-YayciogluRKucukerdonmezCKaralezliACorakFAkovaYAAstigmatic changes following pterygium removal: comparison of 5 different methodsIndian J Ophthalmol201361310410823514644
- GumusKErkilicKTopaktasDColinJEffect of pterygia on refractive indices, corneal topography, and ocular aberrationsCornea2011301242920861731
- GumusKTopaktasDGöktaşAKarakucukSOnerAMirzaGEThe change in ocular higher-order aberrations after pterygium excision with conjunctival autograft: a 1-year prospective clinical trialCornea201231121428143122495030
- OzgurhanEBKaraNCankayaKICorneal wavefront aberrations after primary and recurrent pterygium surgeryEye Contact Lens201541637838125839342
- RazmjooHVaeziM-HPeymanAKooshaNMohammadiZAlaviradMThe effect of pterygium surgery on wavefront analysisAdv Biomed Res2014319625337526