Abstract
Purpose:
Tumor necrosis factor alpha (TNF-α), a macrophage/monocyte derived pluripotent cytokine is associated with tissue ischemia, neuronal damage and remodeling. The physiological level of TNF-α in aqueous humor of normal and glaucomatous eyes is unknown. In this study, we evaluated the TNF-α levels in aqueous in patients with primary open angle glaucoma (POAG) and compared them to controls.
Methods:
50–100 μL of undiluted aqueous humor samples were obtained from eyes of 32 POAG patients who underwent cataract extraction, trabeculectomy or aqueous shunt implantation. Controls were obtained from 32 normal subjects who underwent routine cataract surgery. TNF-α levels were quantified using singleplex bead immunoassay analysis.
Results:
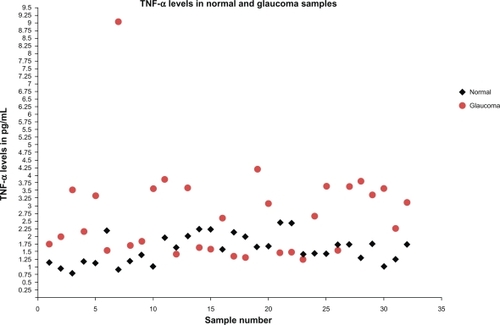
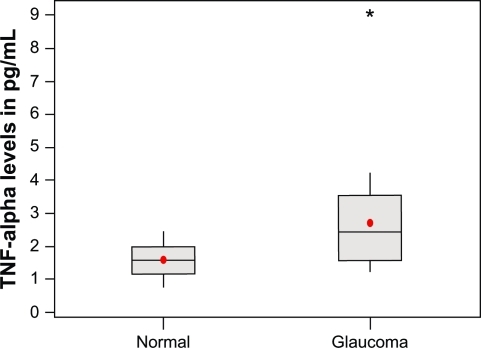
The average TNF-α level in POAG samples was 2.72 ± 1.5 pg/mL (mean ± SD). The average TNF-α level in normal samples was 1.59 ± 0.46 pg/mL (mean ± SD). Significant increase of TNF-α levels in POAG samples was noted in comparison to normal samples (P < 0.001).
Conclusion:
TNF-α levels are elevated in aqueous in patients with POAG compared to normal subjects based on highly sensitive Luminex® bead immunoassay and may be a reliable biomarker in the progression of glaucoma.
Keywords:
Introduction
Tumor necrosis factor alpha (TNF-α), a macrophage/monocyte derived pluripotent cytokine, is associated with tissue ischemia, neuronal damage, and remodeling,Citation1 and increased levels signify neuronal damage after brain trauma.Citation2,Citation3
TNF-α mRNA is upregulated in the retina, with retinal ganglion cell and oligodendrite cell loss and consecutive optic atrophy, in a mouse model of glaucoma.Citation4 Elevated TNF-α levels in aqueous in this model was attributed to release from damaged retinal ganglion cells (RGC) and oligodendrocytes secondary to high intraocular pressure.Citation5 In humans, immunohistochemical analysis revealed elevated TNF-α expression in the optic nerve and the retina of glaucomatous eyes.Citation6,Citation7 However, the physiological level of TNF-α in aqueous humor of normal and glaucomatous eyes is unknown.
Estimation of TNF-α may serve as a biomarker in both undiagnosed early glaucoma as well as in primary open angle glaucoma (POAG). Additionally, evaluating TNF-α levels in glaucoma patients may also help monitor disease progression and the effectiveness of therapy. We evaluated TNF-α levels in aqueous samples of patients with POAG and compared them to controls with singleplex bead immunoassay.
Methods
Subjects
Our study group consisted of 32 eyes belonging to 32 POAG patients (mean age ± SD, 68.6 ± 10.7 years; male/female, 17/15) who underwent cataract extraction, trabeculectomy, or aqueous shunt implantation at the University of Florida, Jacksonville. Patients with secondary etiologies, such as pseudoexfoliation or pigmentary glaucoma, were excluded from the study. Thirty-two normal subjects (mean age ± SD, 70.3 ± 10.3 years; male/female, 13/19), who underwent routine cataract surgery, acted as controls. Subjects in both groups with diabetes, macular degeneration, or systemic inflammatory conditions were excluded. All study participants underwent a comprehensive ophthalmic examination, including visual acuity, measurement of intraocular pressure, slit lamp examination, and fundus biomicroscopy. At the time of surgery, all patients with glaucoma were being treated with intraocular pressure lowering agents.
Aqueous sample collection
Aqueous sample collections were performed using a standard sterilization procedure. Sample collection protocol was approved by the institutional review board at the University of Florida, Jacksonville. Fifty to 100 μL of undiluted aqueous humor samples were collected, from each eye, in a tuberculin syringe prior to the beginning of surgery. Inadvertent touching of intraocular tissues was avoided to prevent contamination of aqueous samples with blood. After collection, samples were stored at −80°C.
Use of immunobead assay for TNF-α analysis
Samples were diluted 1:2 and processed for TNF-α analysis using singleplex bead immunoassay (Invitrogen Corporation, USA). Standards were reconstituted and serially diluted, per the manufacturer’s instructions. To each well of a 96-well microtiter plate, 25 μL of bead and wash solutions were added and incubated for 30 seconds. After the incubation time, supernatant was aspirated, using vacuum manifold; 50 μL of incubation buffer were added to each well, followed by 50 μL of assay diluent and 50 μL of the aqueous sample. Diluted standards were added to the designated wells and the plate was incubated for 2 hours at room temperature on an orbital shaker at 250–500 rpm. After incubation, the liquid was removed and the excess beads were washed off. Furthermore, 100 μL of detector antibody was added to each well and incubated on an orbital shaker at room temperature for 1 hour.
Excess detector antibodies were removed, followed by the addition of substrate complex (S-RPE, Streptavidin R-Phycoerythrin), and the fluorescence was measured using the Luminex 100™ IS fluoroanalyzer (Luminex Inc., Austin, TX) after 30 minutes of incubation at room temperature. For each reaction, both negative and positive controls were included on the microtiter plate. To ensure proper assay, a known concentration of human recombinant TNF-α was included in each run as a positive control.
Analysis of TNF-α levels
A graphical plot was constructed, using standard concentration against standard fluorescence. The curve fitting linear regression algorithm was performed using GraphPad InStat data analysis software (Graphpad, San Diego, CA). The concentration of TNF-α level was extracted from standard curve and expressed as pg/mL. The effect of dilution was considered in determining the final values.
Statistical analysis
Statistical analysis was performed with GraphPad InStat and Minitab® 13 (Minitab, State College, PA) statistical packages. A t-test was used to compare the intergroup variation in TNF-α levels between normal and glaucoma samples. A P-value of <0.05 was considered statistically significant.
Results
TNF-α levels were determined in aqueous of 32 glaucomatous eyes and 32 control eyes using a highly sensitive microparticle bead based immunoassay. To ensure the accuracy of the assay, 25 to 100 pg/mL of known concentration of human recombinant TNF-α was used in each assay as internal control.
The mean age of glaucoma patients was 68.6 ± 10.7 years and that of the controls was 70.3 ± 10.3 years. Among the glaucoma samples, 17 patients were male and 15 patients were female and in the controls, 13 were male and 19 were female. All glaucoma samples were from POAG patients with varying disease severity. The glaucoma samples were well matched to control samples in age and gender with no significant difference ().
Table 1 Demographic data of subjects with glaucoma and normal samples
The average TNF-α level observed in glaucoma samples was 2.72 ± 1.5 pg/mL (mean ± SD), with a range of 1.25 to 9.0 pg/mL. The average TNF-α level observed in normal samples was 1.59 ± 0.46 pg/mL (mean ± SD), with a range of 0.806 to 2.456 pg/mL (). We observed a significant increase in TNF-α levels in the glaucomatous samples in comparison to the normal samples (P < 0.001, t-test) ().
Discussion
Glaucoma, a common cause of blindness that affects millions of people, is characterized by optic nerve atrophy from progressive apoptotic neuronal degeneration secondary to elevated intraocular pressure (IOP) over a long period of time. Retinal ganglion cells, in response to stress mediated through elevated IOP, undergo apoptosis with consecutive atrophy.
Cytokine release and oxidative stress contribute to glaucomatous degeneration.Citation8 TNF-α, a potent immunomodulatory cytokine, is upregulated in a patient with posttraumatic brain injury.Citation1,Citation2 Poor outcome is associated with elevated levels, while inhibition of the TNF-α release limits brain damage.Citation3 TNF-α acts on TNF-α receptor 1 (p55) and mediates apoptosis through a caspase-induced pathway. The addition of exogenous TNF-α following ischemic cerebral injury exacerbated neuronal damage, while blocking TNF-α was found to be neuroprotective in a rat model.Citation3
In the glaucomatous eye, an upregulation of TNF-α protein, an elevated TNF-α gene expression in glial cells, and a TNF-α receptor 1 in retinal ganglion cells, were noted.Citation7 Intraocular pressure induced apoptosis of retinal ganglion cells (RGC), through caspase-3 and caspase-8 activation.Citation9 In animal models, TNF-α induced apoptosis was inhibited with a TNF-α neutralizing antibody.Citation5
Neurodegenerative disorders, such as Parkinson’s disease,Citation10 Alzheimer’s disease,Citation11 and multiple sclerosis,Citation12 have elevated levels of TNF-α. The pathogenesis of these disorders, as well as glaucoma, is a result of a neuroinflammatory process.Citation13–Citation15 Pro-inflammatory cytokines, such as TNF-α, may contribute to this process.
In a previous study by Sawada et al,Citation16 TNF-α was evaluated in aqueous samples of 84 glaucoma-affected eyes and 79 eyes from the control group, using an enzyme-linked immunosorbent assay (ELISA). TNF-α was detected in only 19 eyes, with an overall increased rate of detection in glaucomatous eyes compared to control eyes. However, the slightly elevated TNF-α aqueous levels in POAG and normal tension glaucoma were not found to be statistically significant, while those with exfoliation glaucoma showed a statistically significant increase compared to control eyes. Kuchtey et alCitation17 further elucidated TNF-α levels in aqueous humor, with multiplex microparticle-based immunoassay, but were unable to detect TNF-α in glaucomatous eyes.
In the present study, the more sensitive singleplex bead immunoassay was used to determine values in normal eyes as well as eyes with primary open-angle glaucoma. We found the average level of TNF-α in normal samples to be 1.09 pg/mL, compared to 3.35 pg/mL in glaucomatous eyes. Though most of the patients in this study had well-controlled IOP, seven patients had advanced glaucoma and underwent trabeculectomy, or valve placement, for IOP refractory to medical therapy.
Though vitreous levels of TNF-α are likely to be higher, due to its proximity to the retina, a small proportion of TNF-α diffuses to the aqueous humor. Sensitive immunoassay that detects these levels may identify the presence of glaucomatous damage in a quantitative manner. Using this bead based immunoassay, the TNF-α levels can be estimated even at low levels. Factors such as severity of glaucoma and use of topical medications may also influence TNF-α levels. This aspect of the study must be further evaluated in a larger cohort study.
In summary, TNF-α levels were elevated in aqueous in patients with POAG compared to normal subjects, based on highly sensitive Luminex® bead immunoassay.
Disclosure
The authors report no conflicts of interest in this work.
References
- Muñoz-FernándezMAFresnoMThe role of tumor necrosis factor, interleukin 6, interferon-gamma, and inducible nitric oxide synthase in the development and pathology of the nervous systemProg Neurobiol19985633073409770242
- ErtelWKeelMBonaccioMRelease of anti-inflammatory mediators after mechanical trauma correlates with severity of injury and clinical outcomeJ Trauma19953958798877474003
- ShohamiEBassRWallachDYaminAGallilyRInhibition of tumor necrosis factor alpha (TNF-α) activity in rat brain is associated with cerebroprotection after closed head injuryJ Cerebr Blood Flow Metab1996163378384
- NakazawaTNakazawaCMatsubaraATumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucomaJ Neurosci20062649126331264117151265
- TezelGWaxMBIncreased production of tumor necrosis factor-alpha by glial cells exposed to stimulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cellsJ Neurosci200020238693870011102475
- YuanLNeufeldAHTumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve headGlia2000321425010975909
- TezelGLiLYPatilRVWaxMBTNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyesInvest Ophthalmol Vis Sci20014281787179411431443
- TezelGOxidative stress in glaucomatous neurodegeneration: mechanisms and consequencesProg Retin Eye Res200625549051316962364
- McKinnonSJLehmanDMKerrigan-BaumrindLACaspase activation and amyloid precursor protein cleavage in rat ocular hypertensionInvest Ophthalmol Vis Sci20024341077108711923249
- ScalzoPKümmerACardosoFTeixeiraALIncreased serum levels of soluble tumor necrosis factor-alpha receptor-1 in patients with Parkinson’s diseaseJ Neuroimmunol200911302161–212212519732964
- AlvarezACacabelosRSanpedroDGarcía-FantiniMAleixandreMSerum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer diseaseNeurobiol Aging200728453353616569464
- TsukadaNMiyagiKMatsudaMYanagisawaNYoneKTumor necrosis factor and interleukin-1 in the CSF and sera of patients with multiple sclerosisJ Neurol Sci199110422302341940977
- AhmedFBrownKMStephanDAMorrisonJCJohnsonECTomarevSIMicroarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressureInvest Ophthalmol Vis Sci20044541247125815037594
- HunotSHirschECNeuroinflammatory processes in Parkinson’s diseaseAnn Neurol200353Suppl 3S49S58 discussion S58–S6012666098
- HenekaMTO’BanionMKInflammatory processes in Alzheimer’s diseaseJ Neuroimmunol20071841–2699117222916
- SawadaHFukuchiTTanakaTAbeHTumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucomaInvest Ophthalmol Vis Sci201051290390619737888
- KuchteyJRezaeiKAJaru-AmpornpanPMultiplex Cytokine Analysis Reveals Elevated Concentration of Interleukin-8 in Glaucomatous Aqueous HumorInvest Ophthalmol Vis Sci201051126441644720592224