Abstract
Purpose:
To determine the pattern of electroretinographic change after an intravitreal ranibizumab (Lucentis®) injection for the treatment of choroidal neovascular membrane (CNVM).
Patients and methods:
A prospective interventional case series of patients treated by intravitreal ranibizumab injection. Best corrected visual acuity, optical coherence tomography (OCT), and multifocal electroretinography (mfERG) were assessed prior to treatment, and 2 weeks, 1 and 3 months after treatment. Primary outcome was the functional change in amplitude and implicit time by mfERG and secondary outcome was the structural change in macular thickness by optical coherence tomography (OCT).
Results:
Twenty-six eyes from 25 consecutive patients were enrolled. At 3 months after treatment, the mean visual acuity (VA) improved from 1.06 to 0.84 logMAR (P = 0.034) and the mean macular thickness decreased from 389.7 to 264.4 microns (P = 0.003). The mean implicit time of the central zone showed an improvement at 3 months after treatment when compared with the response at baseline (P = 0.024) and at 1 month (P = 0.013) but the mean amplitude showed no significant change. In subgroup analysis, the eyes with initial visual acuity (VA) ≥ 20/200 had a significant improvement in mean implicit time of the peripheral zone at 2 weeks after treatment (P = 0.028). The OCT revealed a significant decrease (P < 0.003) in macular thickness at 1 and 3 months postoperatively.
Conclusion:
The mean implicit time of the central zone improved significantly at 3 months after treatment, whereas the mean amplitude showed no significant change. The macular thickness decreased significantly after the treatment, while VA improved to a lesser extent.
Introduction
Choroidal neovascular membrane (CNVM) is an abnormal vascular proliferation from beneath the retina and is related to many different underlying retinal conditions, especially age-related macular degeneration. Over the past few years, the introduction of anti-vascular endothelial growth factor (anti-VEGF) drugs has changed the way CNVM is treated. Ranibizumab is one of the novel anti-VEGF drugs that has been proved to be efficient and safe for intravitreal injection.Citation1 However, there are few reports of retinal function changes by electroretinography (ERG) after treatment with anti-VEGF agents.Citation2–Citation4 The multifocal ERG allows the simultaneous derivation of 103 local ERG signals in a central visual field of about 50–60° diameter around the fovea. The impairment of macular function due to regional disorders in the outer retinal layers can be described in detail by this technique, which allows the functional mapping of the retina.Citation5
The objective of the study was to determine the pattern of ERG change after an intravitreal ranibizumab injection, for patients with CNVM.
Materials and methods
The study was a prospective consecutive interventional study, under the approval of the Ethics Committee of Songklanagarind Hospital, Songkhla, Thailand. Patients were recruited from August 2007 to August 2008. The patients received a 0.5 mg intravitreal ranibizumab monotherapy for any type of CNVM confirmed by fundus fluorescein angiography (FFA). The method relies upon the results of the superiority of ranibizumab to photodynamic therapy (PDT) in all subgroups evaluated from the ANCHOR study.Citation6
The informed consents of all subjects who participated in the study were obtained after the nature of the procedure and possible discomforts and risks had been fully explained. All patients received their first intravitreal injection in an operating room under an aseptic preparation technique within 8 days after an angiographic study. Ranibizumab 0.5 mg (Lucentis®; Novartis Pharma AG, Basel, Switzerland) was injected intravitreally at 4.0 mm from the limbus in phakic eyes and at 3.5 mm in pseudophakic eyes. The patients were then instructed to take an antibiotic for 1 week and were scheduled for further postoperative visits at 2 weeks, 1 month, and 3 months after the injection. Retreatment with an interval of a month was considered if the retinal condition was still active. Any post-operative complications such as prolonged inflammation, endophthalmitis and rapid cataract progression were recorded.
The primary outcome was a change in functional response of the signal amplitude or response density and the implicit time or P1 latency which were shown by 103 numeric values in a multifocal ERG (mfERG). The ERG (VERIS Science 6.0.7 d15, EDI, Redwood City, CA, USA), as well as the patients, were prepared following the International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines.Citation7 Each patient was light adapted for at least 15 minutes with room lights or at least two hours after an exposure to a bright light. The patients’ pupils were fully dilated before the test to maximize the retinal illumination. The mfERG response signals were classified into 3 zones for analysis of localized alteration (). A central zone represented signals from 7 central hexagons, a mid zone represented a middle ring of 30 hexagons, and a peripheral zone represented 66 signals from hexagons in the peripheral macular area.
A structural change in central macular thickness was considered as a secondary outcome. The thickness was measured by optical coherence tomography (OCT; Stratus OCT III, v.4, Zeiss, Oberkochen, Germany). Fundus angiography was performed after informed consent was provided and the angiographic photos were captured by a Kowa VX-10i fundus camera (Kowa Co Ltd, Tokyo, Japan). Eyes that had been previously treated by laser photocoagulation, photodynamic therapy, or intravitreal injection of any therapeutic agent were excluded from the study.
The program SPSS version 11.5 was used for data processing. Statistical analyses included Student’s t-test for parametric data, Mann–Whitney U test for non-parametric data and Pearson’s correlation coefficient for a measure of association between two variables. A statistical difference was considered significant when the P-value was ≤0.05.
Results
The baseline demographic data are shown in . Twenty-seven eyes from 26 consecutive patients were initially enrolled in the study but 1 patient (1 eye) was excluded at the end of the study due to a protocol violation. The average age of the remaining 25 patients was 66.5 ± 9.3 years. Seventeen patients were non-smokers, 3 were occasional smoking males, and 5 were prolonged (>1 year) ex-smoking males. The mean baseline best corrected visual acuity (VA) was 1.06 ± 0.51 logMAR (logarithm of Minimum Angle of Resolution). Pre-operative FFA revealed 6 eyes (23.1%) with predominantly classic CNVM, 1 eye (3.8%) with minimally classic CNVM, and 19 eyes (73.1%) with occult CNVM. The greatest linear diameter (GLD) of the eyes with predominantly classic CNVM varied from 878 to 4,944 microns (mean 2,453.67 ± 1,454.81).
Table 1 Baseline clinical characteristics of the patients
The studied eyes were divided into 2 groups post hoc; 14 of 26 eyes (54%) in subgroup-A with initial VA > 1 logMAR (VA < 20/200) and 12 eyes (46%) in subgroup-B with initial VA ≤ 1 logMAR (VA ≥ 20/200). The average age in subgroup-A was 69.9 years and in subgroup-B was 62.4 years (P = 0.035). The preoperative mean macular thickness and intraocular pressure (IOP) were insignificantly different between subgroups (P = 0.451 and P = 0.754 respectively).
Twelve eyes received only one injection of intravitreal ranibizumab. Two eyes received their re-treatment once at a month apart. Eleven eyes received their re-treatment twice, in which case the second and the third ranibizumab injections were performed at one and two months after an initial treatment. A patient who had bilateral active disease began her second eye treatment a month later after she had finished the third injection for the first eye. There was neither a serious postoperative complication nor a need for cataract surgery reported within 6 months after an intravitreal injection. One patient had chest discomfort and chest muscle twitching 6 hours after his second intravitreal injection that was relieved by medications. He refused a third injection.
The mean baseline IOP was 12.5 ± 2.5 mmHg. The mean IOPs at 2 weeks, 1 month, and 3 months after treatment were 12.6, 12.3, and 10.9 mmHg, respectively. A statistically significant difference in IOP change was seen at the third month (P = 0.779, 0.922, and 0.011, respectively).
Primary outcome: functional change
The mean values of baseline amplitude or response density (nV/deg2) and implicit time (mseconds) of all patients and of both subgroups are shown in . A significant difference between subgroups appeared only in the amplitude of the mid zone. The overall mean implicit time of the central zone clearly demonstrated an improvement at 3 months after treatment when compared with the response at baseline (P = 0.024) and at 1 month (P = 0.013). There was no significant change in mean amplitude between visits, compared to the baseline value. In a subgroup analysis (), the significant change in subgroup-A was the amplitude in the mid zone at 1 month after treatment (P = 0.033). Only the mean implicit time of the peripheral zone in subgroup-B clearly showed an improvement at 2 weeks after treatment (P = 0.028).
Figure 2 A–C) A change from baseline in nanovolts/deg2 of the mean amplitude over time in the central, mid, and peripheral zones. D–F) A change from baseline in milliseconds of the mean implicit time over time in the central, mid, and peripheral zones. The vertical line = 1 standard error.
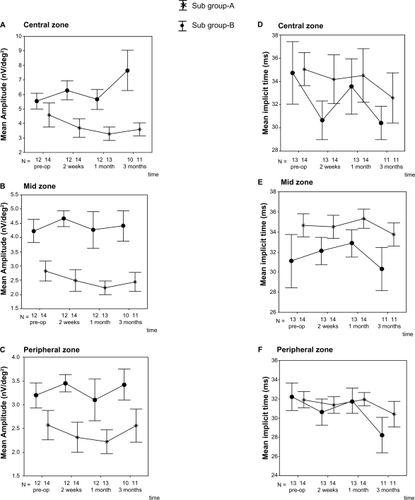
Table 2 Baseline amplitude and implicit time
A subgroup comparison showed that the postoperative mean amplitude in the central and mid zones of subgroup-B was better than that of subgroup-A at every follow-up visit (P < 0.02), whereas the mean implicit time had no significant difference between groups.
The mean postoperative VAs at 2 weeks, 1 month, and 3 months were 1.03, 0.96, and 0.84 logMAR respectively. The significant difference of VA change was found at 1 (P = 0.021) and 3 months (P = 0.034) after treatment. The correlation analysis also found a significant reversed association between changes in amplitude and logMAR VA in the central and mid zones from 2 weeks onwards (P < 0.002), however, there was no correlation between the changes in implicit time and logMAR VA.
Secondary outcome: structural change
The mean macular thicknesses were 389.7 ± 151.3 microns preoperatively, 290.0 microns at 1 month (P < 0.001) and 264.4 microns at 3 months (P = 0.003) postoperatively. Postoperative changes in macular thickness showed no significant difference among subgroups and had no correlation with either amplitude or implicit time.
Discussion
The present pilot study of an electrophysiologic response after an intravitreal ranibizumab injection included all ranges of initial VA in order to compare the pattern of electrophysiologic changes regardless of the visual outcome. Nevertheless, we performed a subgroup analysis to test the effect of disease severity, indicated by the level of vision, upon the response of mfERG.
The overall results showed a prominent improvement in the implicit time rather than the amplitude at 3 months after treatment, although there were a few significant postoperative differences within each subgroup. The patients in subgroup-B who had a better baseline VA were also significantly younger than the patients in subgroup-A (P = 0.035). The mean amplitude of subgroup-B was also better than that of subgroup-A on every visit, while the mean implicit time was not significantly different. This markedly showed a moderate to high statistical correlation of the signal amplitude with a patient’s VA (P < 0.02). Thus, age may have an effect on an amplitude difference between groups, but from the result of this study it has no effect on the implicit time. Moreover, the improvement in the implicit time after the treatment indicates that the potency of functional recovery does not depend on age.
A significant reversed association between the amplitude and logMAR of VA indicates that the higher the rising amplitudes, the better the vision gains. This difference also shows that a patient’s VA has a concordance with the signal amplitude or response density of mfERG, not the implicit time. However, the implicit time indicates an early functional improvement after the treatment. Gerth et alCitation8 reported that the implicit times were found to be more sensitive in detecting abnormal retinal responses compared with the amplitude or response density. Thus, in the recovering retinal area a decrease in the implicit time may occur earlier than an increase in the amplitude.
After treatment, the OCT revealed a decrease in macular thickness by a strong significance (P < 0.003) while the mean VA had a lesser degree of significant improvement at the same time (P < 0.034). Our study shows similar results as in the bevacizumab and ranibizumab treatments from the previous reports.Citation2–Citation4 Therefore, anti-VEGF therapy can structurally reduce the macular edema, but may not fully recover the neuroretinal function. The next question is whether or not we can accelerate the improvement of vision. A method of vision restoration therapy (VRT)Citation9 may be interesting because the early improvement area is mostly confined to the central and mid zones of the macula.
The limitation of this study is a lack of OCT data in some patients during an unfortunate period of machine malfunction. We not only recommend a future study with a longer follow-up period and a larger population, but also encourage the possibility of a careful visual rehabilitation trial of the watershed area by vision restoration therapy in the patients undergoing intravitreal ranibizumab treatments.
Conclusion
The mean implicit time of the central zone improved significantly at 3 months after treatment, whereas the mean amplitude had no significant change. There was a correlation between baseline VA and the amplitude of mfERG, but not the implicit time. The OCT also showed a decrease in macular thickness along with an improvement of VA after treatment. Continuous treatment with intravitreal ranibizumab monotherapy is required to evaluate the long-term results.
Acknowledgements
This study was supported by the Faculty of Medicine, Prince of Songkla University, Hat Yai, Songkhla, Thailand.
Disclosure
The authors have no commercial association or any conflict of interest in the drugs or machines mentioned in the study.
References
- LükeMJanuschowskiKLükeJThe effects of ranibizumab (Lucentis) on retinal function in isolated perfused vertebrate retinaBr J Ophthalmol200993101396140019628500
- MaturiRKBleauLAWilsonDLElectrophysiologic findings after intravitreal bevacizumab (Avastin) treatmentRetina200626327027416508425
- MoschosMMBrouzasDApostolopoulosMKoutsandreaCLoukianouEMoschosMIntravitreal use of bevacizumab (Avastin) for choroidal neovascularization due to ARMD: a preliminary multifocal-ERG and OCT studyDoc Ophthalmol20071141374417216267
- FeiglBGreavesABrownBFunctional outcomes after multiple treatments with ranibizumab in neovascular age-related macular degeneration beyond visual acuityClin Ophthalmol20071216717519668506
- SutterEETranDThe field topography of ERG components in man-I. The photopic luminance responseVision Res19923234334461604830
- KaiserPKBrownDMZhangKRanibizumab for predominantly classic neovascular age-related macular degeneration: Subgroup analysis of first-year ANCHOR resultsAm J Ophthalmol2007144685085717949673
- MarmorMFHoodDCKeatingDKondoMSeeligerMWMiyakeYInternational Society for Clinical Electrophysiology of VisionGuidelines for basic multifocal electroretinography (mfERG)Doc Ophthalmol2003106210511512678274
- GerthCHauserDDelahuntPBMorseLSWernerJSAssessment of multifocal electroretinogram abnormalities and their relation to morphologic characteristics in patients with large drusenArch Ophthalmol2003121101404141414557176
- JungCSBruceBNewmanNJBiousseVVisual function in anterior ischemic optic neuropathy: Effect of vision restoration therapy (VRT) – A pilot studyJ Neurol Sci20082681–214514918207164