Abstract
Background:
The purpose of this study was to evaluate the efficacy and safety of a new instrument to optimize the size, shape, and centration of anterior capsulorhexis.
Methods:
The study was divided into two parts. The first part was done in 10 goats’ eyes. A specially designed capsulorhexis marker was used. The lower edge of the instrument was stained by gentian violet and then applied to the anterior capsule to produce a circular mark of 5.5 mm. In five eyes, the gentian violet was applied to the marker using a corneal marking pen; in the remaining five eyes, the gentian violet was applied using a corneal marking pad. In the second part, the toxicity of gentian violet was tested as follows: ten albino rabbits received a gentian violet injection into the anterior chamber in one eye (experimental group) and an equal volume of balanced salt solution in the fellow eye (control group). Five rabbits were sacrificed one day after surgery and the remaining five rabbits after one week.
Results:
In the first part, there was no difficulty in the introduction or removal of the instrument from all eyes. In the first two eyes, the circular mark was diffuse due to sliding of the marker on the surface of the anterior capsule. In the remaining eight eyes, there was a well-centered, rounded mark which was adequately stained. In the second part, there was no histopathological evidence of corneal toxicity in either group. There was loss of ganglion cells from the neurosensory retina one day after surgery in one eye from the experimental group. At one week, there was no evidence of retinal toxicity in any of the rabbits.
Conclusion:
This capsulorhexis marker can guide the surgeon to a better centration and proper sizing of anterior capsulorhexis using gentian violet staining. More refinement of the instrument is needed to be able to use it in human eyes.
Keywords:
Introduction
With the introduction of phacoemulsification as standard cataract surgery, anterior capsulorhexis has become an essential step. Anterior capsulorhexis can be performed using a 26-gauge needle or capsulorhexis forceps.Citation1,Citation2 However, when performed free-hand, it is not always easy to create a perfectly sized anterior capsulorhexis that is also perfectly centered relative to the pupil or the limbus. Although the anterior capsulorhexis obtained is usually continuous, it may still be noncurvilinear, varying from kidney-shaped to heart-shaped, and in some cases it may be discontinuous.Citation3 Moreover, the introduction of multifocal intraocular lenses has increased the need for better surgical performance and precision.
Many investigators have attempted to design new tools for better control of the size and shape of the capsulorhexis, such as diathermy rhexisCitation4 and vitrectorhexis.Citation5 Certain styles of capsule forceps have also been designed with laser marks to help surgeons produce various capsulotomy diameters. However, these forceps are limited in their ability to provide a diameter reference in the horizontal meridian parallel to the phacoemulsification incision.Citation6
The aim of this study was to evaluate the efficacy and safety of a new instrument to optimize the size, shape, and centration of anterior capsulorhexis during phacoemulsification by making a circular mark on the anterior capsule using gentian violet stain.
Materials and methods
This study was divided into two parts. The first part was performed in a fully equipped wet laboratory (Alcon Laboratories, Cairo, Egypt) where goats’ eyes were used. The second part was done at Cairo University Medical School animal house facility using albino rabbits. We adhered to the tenets of the National Institutes of Health statement on the use of animals in research.
Use of capsulorhexis marker to stain the anterior capsule
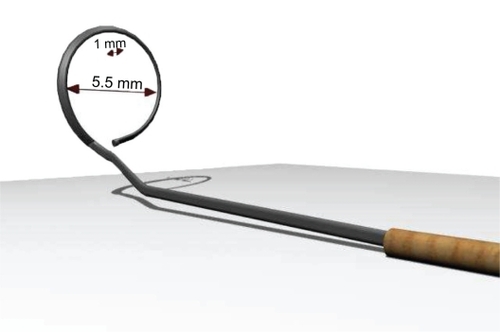
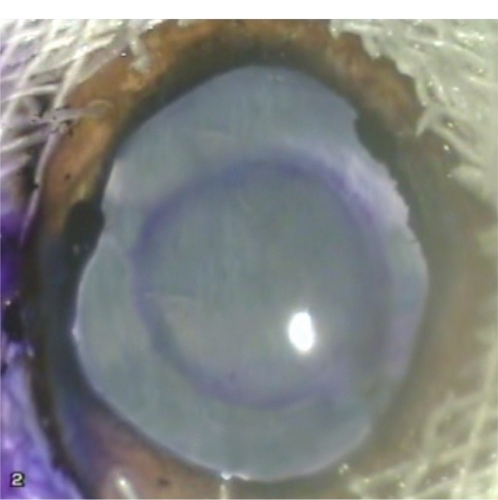
A stainless steel capsulorhexis marker was specially designed for this study. It was formed of a handle attached to a ring with an inner diameter of 5.5 mm and a height of 1 mm (). Ten goats’ eyes were prepared for the procedure. A 3 mm corneal tunnel was performed in each eye followed by injection of viscoelastic material into the anterior chamber. The wound was then widened to 6 mm to allow easy introduction of the instrument. In five eyes, the lower edge of the ring was stained by gentian violet using a surgical marking pen (Viscot Medical, East Hanover, NJ) whereas in the other five eyes, the lower edge of the ring was stained by gentian violet using a surgical marker pad (Vismark®, Viscot Medical). The capsulorhexis marker was introduced into the anterior chamber and the ring was centered within the papillary area. The lower edge of the ring was applied onto the anterior capsule for a few seconds without pressure to avoid zonular stress. The marker was then removed from the eye and assessment of the mark was done as regards centration and degree of staining of the anterior capsule ().
Assessment of intraocular toxicity
Because we were using a preparation of gentian violet designed for extraocular use (on the corneal surface), we needed to assess the intraocular toxicity in an animal model. Accordingly, 10 albino rabbits weighing 2–4 kg received a gentian violet injection into the anterior chamber in one eye (experimental group), and an equal volume of balanced salt solution in the fellow eye (control group).
Surgical technique
The rabbits were anesthetized with an intramuscular injection of xylazine 5–8 mg/kg and ketamine hydrochloride 35–44 mg/kg. The pupils were dilated with tropicamide and phenylephrine 10% eye drops. In the experimental group, the lower edge of the ring was stained by gentian violet using the surgical marking pen. The ring was then left in the well of a sterile contact lens case containing balanced salt solution 0.1 mL for two minutes. The stained balanced salt solution was then injected into the anterior chamber using a 27-gauge needle. In the control group, balanced salt solution 0.1 mL was injected into the anterior chamber using a 27-gauge needle. All animals were kept in the animal house and were handled according to the Association for Research in Vision and Ophthalmology statement on use of animals in ophthalmic and vision research. Five rabbits were sacrificed one day after surgery and the remaining five after one week. The eye balls were enucleated and immediately fixed in Bouin’s solution, dehydrated in ascending grades of alcohol (70%, 90%, and 100%), cleared in xylol, and embedded in paraffin. The embedded specimens were then sectioned (5–7 μm thickness), stained with hematoxylin and eosin, and examined by light microscopy.
Results
Use of capsulorhexis marker to stain anterior capsule
There was no difficulty in the introduction or removal of the instrument from any of the eyes. There was no difference in the degree of capsular staining using either the marker pen or the marker pad. In the first two eyes, the mark was not clear due to sliding of the ring on the surface of the anterior capsule; this resulted in spread of the stain, leading to a diffuse mark. In all other cases, care was taken not to touch the anterior capsule during introduction of the instrument into the anterior chamber. Once in place, the ring was pressed down on the anterior capsule without undue sliding. This resulted in a well-centered rounded mark which was adequately stained, precise, and clear enough to guide the surgeon to perform a 5.5 mm capsulorhexis.
Assessment of intraocular toxicity
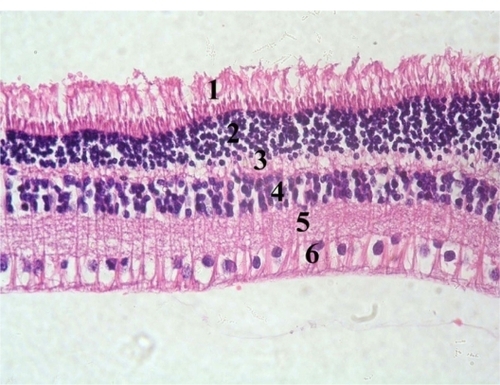
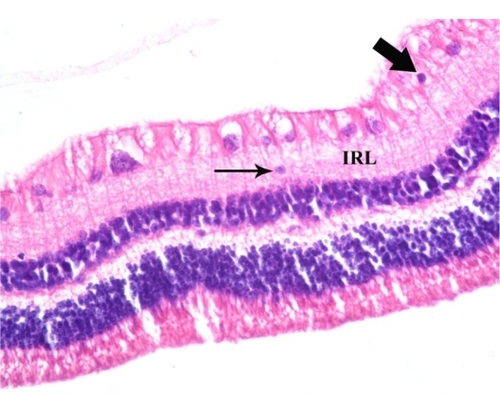
Histopathologically, none of the eyes in the study or control group showed any sign of corneal toxicity at one day and one week after surgery ( and ). Light microscopy examination of multiple sections from each eye showed normal iris tissue, ciliary body epithelium, and lens epithelium. The retina was normal in all eyes of the control group at one day and one week after the surgery (). In the experimental group, there was no evidence of retinal toxicity at one day and one week after surgery with the exception of one eye that showed evidence of loss of ganglion cells from the neurosensory retina one day after surgery ().
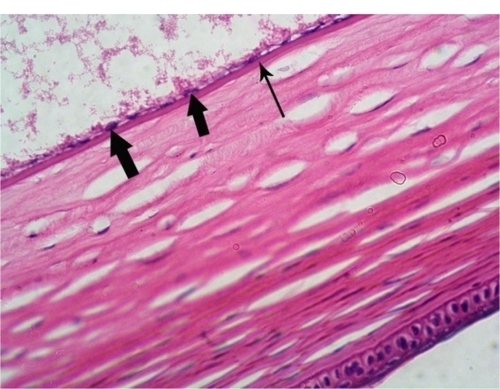
Figure 3 Photomicrograph of a control rabbit cornea showing flat endothelial cells with flat nuclei (thick arrow) resting on Descemet’s membrane (thin arrow). Hematoxylin and eosin staining, 400×.
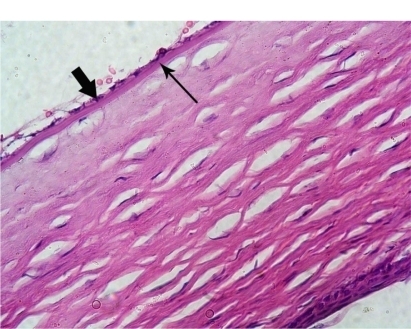
Figure 4 Photomicrograph of an experimental rabbit’s cornea showing normally appearing flat endothelial cells (thick arrow) and intact Descemet’s membrane (thin arrow). Hematoxylin and eosin staining, 400×.
Discussion
A well constructed capsulorhexis is the foundation of complication-free phacoemulsification and intraocular lens surgery. Ophthalmic surgical dyes have become valuable tools, and are now widely used in both anterior and posterior segment indications. In this study, we present a preliminary prototype of a capsulorhexis marker which is first stained by gentian violet and then applied directly onto the anterior capsule in order to make a rounded mark to guide the surgeon to a better centering and proper sizing of the rhexis. Moreover, it may help phacoemulsification beginners and shorten their learning curve for this crucial step in modern cataract surgery.
Today’s demanding refractive results require a well-centered, perfectly circular capsulorhexis that slightly overlaps the intraocular lens optic. This construction is important for achieving the so-called capsular “shrink-wrap” effect during the postoperative period. A 360° overlapping capsular edge creates a capsular bend, which acts as a barrier against proliferating lens epithelial cells and thus significantly delays the onset of posterior capsular opacification.Citation7–Citation9 This overlap also sets the anteroposterior positioning of the intraocular lens, which prevents capsular fibrosis from shifting the lens optic forward and creating an unwanted late refractive shift. Moreover, with a perfectly circular capsulorhexis, any contraction of the anterior capsule (ie, phimosis) will be symmetrical, so late in-the-bag decentering of the optic is prevented.Citation10 Also, if the capsulorhexis is too small, it can result in glare, halos, and night driving problems with a diffractive multifocal intraocular lens. Capsular phimosis is more pronounced with small capsulotomies.Citation11,Citation12 A small capsular opening makes surgery more difficult and places greater stress on the anterior capsule during nuclear division or quadrant removal. Furthermore, a smaller capsular opening can obscure peripheral retinal visibility and treatment. A capsulorhexis with a 5.5 mm diameter is usually ideal.
Many investigators have tried to improve the predictability of size, shape, and centration of capsulorhexis. WallaceCitation6 performed a study to make a central corneal mark with a 6.0 mm optical zone marker, previously used for radial keratotomy (with the center of the optical zone as the central visual axis), as the patient fixates on the light of the microscope. He performed the capsulorhexis in the usual way with the intention of leaving the borders of the capsulotomy inside the capsulotomy diameter mark. However, this technique has some disadvantages. First, because corneal curvatures are not uniform between patients, a different mark size would be required for each patient to achieve the correct capsulorhexis size. Moreover, use of this marker incorrectly implies that corneal curvature remains constant after the incisions are made.Citation3 Finally, the corneal plane and the anterior capsule plane must be parallel with the focal plane of the operating microscope to avoid parallax errors, and the surgeon may confuse the corneal mark with the anterior capsule rim at the time of intraocular lens implantation.Citation13
Tassignon et alCitation3 designed a 0.25 mm ring-shaped caliper with an internal diameter of 5.0 mm or 6.0 mm. After its insertion, the ring is gently pushed on top of the anterior capsule with additional Healon GV. A small opening is made in the center of the capsule. The surgeon then carefully follows the internal border of the ring caliper. However, this technique is not precise because the ring is not fixed on the anterior surface of the capsule, and thus any movement of the ring will affect the size, shape, and centration of the capsulorhexis.
A promising technological solution involves overlaying a reference ring on the surgeon’s view through the microscope. Carl Zeiss Meditec Inc developed an interface module that adjusts the size of the ring appropriately with microscope magnification while keeping it centered within the limbus using real-time eye tracking.Citation14 Moreover, several companies are developing femtosecond technologies to perform the capsulorhexis.
To our knowledge, this is the first published article in the literature describing an instrument that makes a circular mark on the anterior capsule using a stain. Gentian violet (hexamethyl rosaniline chloride) is a triphenylmethane (rosaniline) dye. The pure compound is known as crystal violet. It was chosen for this study because it is cheap, easily available, and intensely and homogeneously stains the anterior capsule.Citation15
Different techniques have been proposed for staining of the anterior capsule by different dyes. Many surgeons continue to inject capsular dye beneath an air bubble, as originally described by Horiguchi et alCitation16 and Melles et al.Citation17 Laureano and CoroneoCitation18 suggested simply omitting the air bubble. Kayikicioglu et alCitation19 have used a viscoelastic-mixing technique with 0.4% trypan blue. In this study, we applied the dye directly onto the anterior surface of the capsule by the lower edge of the capsulorhexis marker and this helped in decreasing the amount of the dye inserted into the anterior chamber and also in avoiding inadvertent application of the dye towards the corneal endothelium.
Many studies have investigated the ocular toxicity of gentian violet. Previously, gentian violet 0.1% with methylene blue 1% was used for staining.Citation17 This combination is no longer used because the endothelial toxicity of the dyes causes corneal edema. Ünlü et alCitation15 examined the effects of gentian violet 0.001% and gentian violet 0.01% without methylene blue and found that each concentration of gentian violet alone was effective in staining the anterior capsules of white cataracts and found that both concentrations had no toxic effects on intraocular tissues. Gamal Eldin et alCitation20 reported that gentian violet 0.05%–2% stained the anterior capsule of the rabbit eye effectively, but that only the lowest concentration (0.05%) showed excellent preservation of the cornea during one week of observation. In the second part of our study, we studied the toxicity of gentian violet derived from the marker pen. This pen is generally used to make an external mark on the outer surface of the cornea during keratoplasty and keratorefractive procedures. No toxic changes were evident in the cornea, iris tissue, ciliary body epithelium, or in the lens epithelium. Only one eye had partial ganglion cell loss from the retina one day after surgery. This may represent a preparation artifact rather than a true toxic effect.
In this study, we used a simple preliminary prototype that needs a large corneal incision (6 mm) to be introduced inside the eye. We considered this as the first step in our research and we are looking forward to changing the material of the marker in order to form a memory ring that is able to be introduced and removed from the eye through a small incision and become ergonomically feasible. However, it can be used as such in cases of combined cataract extraction with penetrating keratoplasty because there is enough room to introduce the instrument after trephination of the recipient cornea. An important issue to be addressed is the proper way to centralize the marker on the surface of the anterior capsule in relation to the visual axis.
Conclusion
This capsulorehxis marker is an instrument designed to optimize the size, shape, and centering of the capsulorhexis during phacoemulsification by impressing a circular pattern on the anterior capsule to guide the surgeon in creating a 5.5–6.0 mm diameter capsulorhexis. More refinement of the instrument is needed to be able to use it in human eyes.
Acknowledgements
We express our deep gratitude to Dr Dina Helmy for her help and support in preparation and interpretation of the histopathologic specimens in the second part of the study. We also express our appreciation to Alcon Egypt for allowing use of its wet laboratory facility in the first part of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- ZaminiMBurattoLSavinGCapsulorhexisBurattoLWernerLZaniniMAppleDPhacoemulsification: Principles and TechniquesThorafore, NJSlack Incorporated2003
- NeuhanTFCapsulorhexisSteinertRFFineHGimbelHVCataract SurgeryNew York, NYElsevier Science2004
- TassignonMJRozemaJGobinLRing-shaped caliper for better anterior capsulorhexis sizing and centrationJ Refract Surg20063212531255
- ShoheiFMasayukiMKiyoshiSMorphological changes in cataract anterior capsule in diathermy capsulorhexis using different types of viscoelastic agentsJournal of the Eye200118795799
- HazirolanDOAltiparmakUEAslanBSDumanSVitrectorhexis versus forceps capsulorhexis for anterior and posterior capsulotomy in congenital cataract surgeryJ Pediatr Ophthalmol Strabismus20094610410719343972
- WallaceRCapsulotomy diameter markJ Refract Surg20032918661868
- AppleDJPengQVisessookNEradication of posterior capsule opacification: documentation of a marked decrease in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortemOphthalmology200110850551811237905
- AykanUBilgeAHKaradayiKThe effect of capsulorhexis size on development of posterior capsule opacification: small (4.5 to 5.0 mm) versus large (6.0 to 7.0 mm)Eur J Ophthalmol20031354154512948312
- HollickEJSpaltonDJMeacockWRThe effect of capsulorhexis size on posterior capsular opacification: one-year results of a randomized prospective trialAm J Ophthalmol199912827127910511019
- AltmannGENichaminLDLaneSSPeposeJSOptical performance of 3 intraocular lens designs in the presence of decentrationJ Cataract Refract Surg20053157458515811748
- SugimotoYTakayanagiKTsuzukiSPostoperative changes over time in size of anterior capsulorhexis in phacoemulsification/aspirationJpn J Ophthalmol1998424954989886742
- JooCKShinJAKimJHCapsular opening contraction after continuous curvilinear capsulorhexis and intraocular lens implantationJ Cataract Refract Surg1996225855908784631
- KellenRCapsulotomy diameter markJ Refract Surg2004301020312032
- DickHGPena-AcevesAMannsMKrummenauerFNew technology for sizing the continuous curvilinear capsulorhexis: prospective trialJ Cataract Refract Surg2008341136114418571082
- ÜnlüKAsküngerASökerSGentian violet solution for staining the anterior capsuleJ Cataract Refract Surg2000261228123211008053
- HoriguchiMMiyakeKOhtaIItoYStaining of the lens capsule for circular continuous capsulorhexis in eyes with white cataractArch Ophthalmol19981165355379565058
- MellesGde WaardPPameyerJBeekhuisWTrypan blue capsule staining to visualize the capsulorhexis in cataract surgeryJ Cataract Refract Surg199925799888070
- LaureanoJSCoroneoMTCrystalline lens capsule staining with trypan blueJ Cataract Refract Surg2004302046204915474812
- KayikiciogluOErakgunTGulerCTrypan blue mixed with sodium hyaluronate for capsulorhexisJ Cataract Refract Surg20032797011496911
- Gamal EldinSAEl MehelmyEMEl ShazliEMMostafaYMSExperimental staining of the anterior lens capsule in albino rabbitsJ Cataract Refract Surg1999251289129410476517