Abstract
Purpose
To evaluate the effects of D-sorbitol addition on changes in the extrusion force of ophthalmic viscosurgical devices (OVDs).
Methods
OVD formulations; the mixtures of 3% hyaluronic acid (HA) and 4% chondroitin sulfate (CS) containing 0%, 0.5%, or 1.0% D-sorbitol were prepared. Each prefilled syringe of OVD was stored at room temperature for 0, 15, 30, 60, or 120 mins after a small amount of viscoelastic agent was discharged from the needle. The extrusion force values (kgf) of these OVDs when reused after storage were measured with a texture analyzer. Moreover, 10 healthy adults (5 men and 5 women) used a pinch sensor to measure the extrusion force values for the HA/CS combination without D-sorbitol which was stored in the above manner, and used a 4-step scale to score the usability of OVD.
Results
For the HA/CS combination without D-sorbitol, the extrusion force value was increased from its initial value (storage duration, 0 min) as storage duration increased. However, for the HA/CS combination containing 0.5% or 1.0% D-sorbitol, this value remained almost unchanged over time. Likewise, the pinch sensor-determined extrusion force values of HA/CS combination without D-sorbitol increased, depending on storage duration.
Conclusion
The addition of D-sorbitol to viscoelastic agent may suppress the needle clogging that occurs with OVD storage, and may improve the usability of OVDs during surgery.
Introduction
There are many ophthalmic viscosurgical devices (OVDs) on the market including those formulated with hyaluronic acid (HA). OVDs are mainly divided into two types (cohesive and dispersive) based on their cohesion-dispersion index.Citation1,Citation2 Opegan-Hi, a 1% high-molecular-weight (>2000 kDa) HA formulation, is classified as a cohesive OVD and is effective in protecting the corneal endothelium from mechanical invasion during intraocular lens implantation and penetrating keratoplasty. Protection is afforded by maintaining space in the eye through high viscosity of viscoelastic agents.Citation3,Citation4 The cohesive type and the dispersive type have different properties. It is easy to remove cohesive types en bloc in phacoemulsification and aspiration, while dispersive types such as a combination of 3% HA and 4% chondroitin sulfate (CS) tend to adhere to tissues.Citation5–Citation7 Therefore, surgeons need to understand the different properties of OVDs to use them effectively in individual cases and particular surgical procedures.
Anterior segment surgeries in which cohesive 1% HA formulations are indicated include cataract surgery, intraocular lens implantation, and penetrating keratoplasty.Citation8 Routine cataract surgery takes approximately 15 mins, while triple surgery, in which cataract extraction, intraocular lens implantation, and keratoplasty are performed simultaneously, has been reported to take approximately 60 mins.Citation9 Moreover, when cataract surgery and vitreous surgery are performed simultaneously, it has been reported to take 120 mins or longer in several cases.Citation10,Citation11 Drying of the cornea during such a long surgery increases the risk for corneal epithelial damage. Therefore, to moisturize the corneal epithelium and prevent a reduction of visibility, viscoelastic agents are often placed on the cornea during ophthalmic surgery.Citation12,Citation13 Although the use of OVDs in such a surgical procedure is off-label, dispersive types such as the HA/CS combinations are mainly used because they spread easily and evenly over the corneal epithelial surface.Citation14
In the ophthalmic procedures mentioned above, the same OVD syringe is used more than once at certain intervals during the surgery because the moisturizing effect from viscoelastic agents is not permanent. To use the OVDs many times during surgery, they must be able to be consistently delivered. In such usage, we often experience difficulty in extruding viscoelastic agent when the OVD is used more than once. We presumed that this difficulty is due to the drying and hardening of the viscoelastic agent remaining in the lumen of the needle. Thus, we hypothesized that by keeping the viscoelastic agent from drying, consistent use of OVDs can be expected without compromising their usability during long surgeries. There have been no reports of drying-induced changes in the extrusion force of OVDs or of the methods to prevent these changes.
In the current study, D-sorbitol, which is known to have hygroscopic and moisturizing effects,Citation15–Citation18 was added to the combination of 3% HA and 4% CS, and examined whether it could be used to prevent needle clogging caused by the drying of the OVD formulation. The changes in the extrusion force depended on time of OVD needle tip storage and exposure to air at room temperature; these were measured with a texture analyzer and pinch sensor. Our experimental results showed that the addition of D-sorbitol to viscoelastic agent suppressed increase in the extrusion force of OVDs.
Materials And Methods
Questionnaire Survey On Needle Clogging During Operations
To investigate the occurrence of needle clogging of OVDs and the associated coping methods, we surveyed surgeons who consented to answer the questionnaires on the web. In obtaining informed consent, we explained that participation was on a voluntary basis and without penalty, and that the survey results were strictly managed with personal information de-identified while securing anonymity. In a web survey conducted in December 2018, there were 299 cataract surgeons in Japan who were asked the following 2 questions (surveyed by Ipsos K.K., Tokyo, Japan). Among the 299 surgeons, 127 surgeons who applied viscoelastic agent to the corneal surface during vitreous surgery or cataract surgery (83 surgeons working at hospitals with 100 beds or more and 44 surgeons working at hospitals with fewer than 100 beds) answered Q1.
Q1: During cataract surgery or vitreous surgery, when applying viscoelastic agent to the corneal surface, have you experienced needle clogging with storage of OVDs? The responders answered this question by choosing 1 of the following 4 options that described their experience: 1) I do not store OVDs but have experienced needle clogging. 2) I have experienced needle clogging due to storage. 3) I sometimes store OVDs but have not experienced needle clogging. 4) I have not experienced needle clogging because I do not store OVDs.
Q2: During cataract surgery or vitreous surgery, when applying viscoelastic agent to the corneal surface, how do you deal with needle clogging? The responders who chose 1 or 2 for Q1 answered Q2 by choosing 1 of the following 3 strategies: 1) Immersed the needle tip in physiological saline. 2) Immersed the needle tip in balanced salt solution (BSS). 3) Others.
Materials
OVDs used in the study are shown in . The homemade HA/CS-A was prepared by dissolving HA powder (derived from chicken combs, Seikagaku Corp., Tokyo, Japan) and CS powder (derived from shark cartilage, Seikagaku Corp.) in phosphate-buffered saline (PBS) at final concentrations of 3% and 4%, respectively. HA/CS-B and -C were prepared by adding D-sorbitol (Junsei Chemical Co., Ltd., Tokyo, Japan) to HA/CS-A at final concentrations of 0.5% and 1.0%, respectively. Prepared specimens were passed through 0.22-μm filters (Merck KGaA, Darmstadt, Germany). In order to exclude possible differences in the extrusion force resulting from differences in syringe structure, approximately 0.5 mL of each prepared specimen was put into the same type of plastic syringes as those used for the Shellgan®. Opegan® and Shellgan were obtained from Santen Pharmaceutical Co., Ltd. (Osaka, Japan). Viscoat® and Discovisc® were purchased from Alcon Inc. (Hünenberg, Switzerland).
Table 1 Investigational OVDs
Measurement Of The Extrusion Force Of OVDs With A Texture Analyzer
A total of 27-gauge ophthalmic needles (needle accompanying Shellgan) were attached to prefilled syringes of HA/CS-A, -B, and -C, and a small amount of viscoelastic agent was discharged. These prefilled syringes were stored in a room under ambient conditions (temperature of 23.1 ± 1.0°C and humidity of 44.3 ± 8.9%) for 15, 30, 60, and 120 mins. After storage, the extrusion force (kgf) of the OVDs was measured by with a texture analyzer (TA.XT plus, Stable Micro Systems, Surrey, UK, ) equipped with a probe made of aluminum (n = 3). The probe (50 mm in diameter) was moved downward at the extrusion speed of 90 mm/min. Extrusion speed was calculated by measuring the distance the piston traveled during extrusion of the Shellgan viscoelastic agent (in mm) and the time it took for extrusion (in min). The extrusion force was defined as the maximum force required to extrude viscoelastic agent from the syringe in 5 s. The initial extrusion force was the value measured at 0 min (no storage): the initial relative extrusion force was 100%. The relative extrusion force (%) over time was calculated according to the following formula: [The maximum extrusion force of the OVDs at each exposure time (kgf)]/[The maximum of extrusion force initially (kgf)] × 100. The HA/CS-A was also tested with and without immersing the needle tip in PBS after 60-mins storage (n = 5), and the rate of increase in extrusion force was calculated for both groups. The relative extrusion force (%) was calculated as follows: [The maximum extrusion force of OVDs exposed to the open air for 60 mins (kgf)]/[The maximum of extrusion force initially (kgf)] × 100.
Figure 1 Instruments that evaluate the extrusion force of OVDs.
Notes: (A) Texture analyzer and (B) pinch sensor.
Abbreviation: OVD, ophthalmic viscosurgical devices.
Before using the method described above to measure the extrusion force after 30-mins storage, a 27-gauge Opegan needle (TOP Co., Ltd., Tokyo, Japan) was attached to the Opegan and 27-gauge ophthalmic needles accompanying each OVD were attached to the Shellgan, Viscoat and Discovisc.
Measurement Of The Extrusion Force Applied By Healthy People When Extruding OVD (Extrusion Force-p)
A total of 10 healthy people from the Seikagaku Corp. (Tokyo, Japan) staff provided written informed consent to participate in the study after being briefed on the procedure. This study was conducted conforming to principles of the Declaration of Helsinki (2013). This study has been judged not to require ethical reviews because the study satisfied the following criteria: 1) No handling personal information. 2) No using samples derived from the human body. 3) No loading on the human body. 4) It is assumed that no psychological distress. In healthy people [5 women (34.6 ± 10.1 years) and 5 men (34.6 ± 5.0 years)], the extrusion force of OVD needed for discharging viscoelastic agent (extrusion force-p) was measured with a pinch sensor (SAKAI Medical Co., Ltd., Tokyo, Japan) ().
First, we measured pinch strength, which was defined as the force (kgf) exerted when the sensor is pinched with the thumb and index finger of the dominant hand as hard as possible. Next, HA/CS-A was stored in the same manner as described above in the method for 0 (initial), 30, 60, and 120 mins. As shown in , the plunger of a syringe after storage was pushed manually with the thumb until the viscoelastic agent was discharged, and the maximum value measured during the push was considered as the extrusion force-p value (kgf). In addition, to quantify the “feel” during extrusion, the subjects were asked to choose one of the following descriptions: 1) Felt no difference from the initial extrusion. 2) Felt more resistance compared to the initial extrusion, but the viscoelastic agent could be extruded without effort. 3) Felt considerably more resistance compared to the initial extrusion; it was difficult to discharge the viscoelastic agent but eventually the entire volume was released. 4) When pushed, the viscoelastic agent was not discharged at all.
Results
Results From The Questionnaire On The Occurrence Of Needle Clogging Of OVDs And The Associated Coping Methods
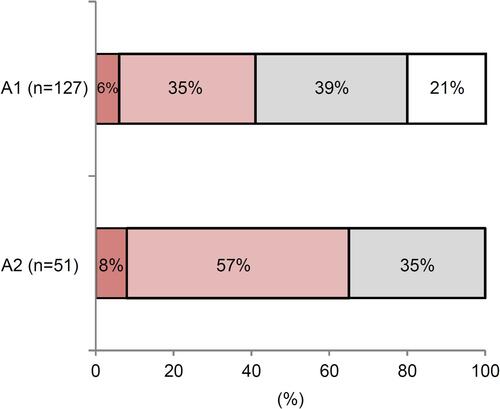
From a survey of 127 surgeons who applied viscoelastic agents on the cornea during cataract or vitreous surgery, 41% of surgeons (51 individuals) experienced needle clogging (, A1). When the needle tip was clogged and extrusion became difficult, 57% of the surgeons attempted to resolve the issue by immersing the needle tip in BSS, and 8% of the surgeons immersed it in saline (, A2). Most of the remaining 35% of surgeons chose “Others” and dealt with the problem “by pushing viscoelastic agent out with power” or “by replacing the needle.”
Figure 2 The survey on OVD needle clogging. A1: Answer to the Q1 “During cataract surgery or vitreous surgery, when applying viscoelastic agent to the corneal surface, have you experienced needle clogging with storage of OVDs?” 
 ; I have experienced needle clogging due to storage,
; I have experienced needle clogging due to storage,  ; I sometimes store OVDs but have not experienced needle clogging, □; I have not experienced needle clogging because I do not store OVDs. A2: Answer to the Q2 “During cataract surgery or vitreous surgery, when applying viscoelastic agent to the corneal surface, how do you deal with needle clogging?”
; I sometimes store OVDs but have not experienced needle clogging, □; I have not experienced needle clogging because I do not store OVDs. A2: Answer to the Q2 “During cataract surgery or vitreous surgery, when applying viscoelastic agent to the corneal surface, how do you deal with needle clogging?”  ; Immersed the needle tip in physiological saline,
; Immersed the needle tip in physiological saline,  ; Immersed the needle tip in BSS,
; Immersed the needle tip in BSS,  ; Others.
; Others.Abbreviations: OVD, ophthalmic viscosurgical devices; BSS, balanced salt solution.
Change In The Force Required To Extrude The 3% HA/4% CS Combination With Different D-sorbitol Concentrations Over Time Measured With A Texture Analyzer
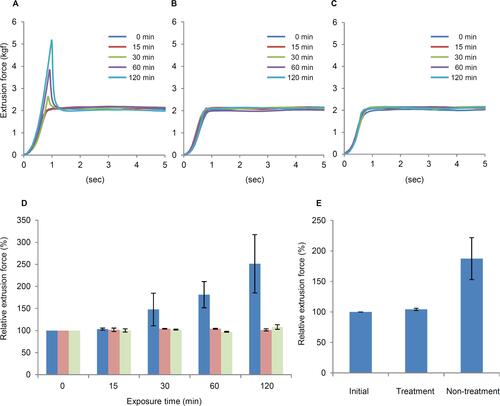
As shown for HA/CS-A, which contained no D-sorbitol, the extrusion force increased temporarily in the initial phase of extrusion, and the rate of increase in extrusion force depended on storage duration after the initial viscoelastic agent discharge ( and ). On the other hand, there was no increase in the extrusion force due to storage over time compared to initial values for HA/CS-B and -C containing 0.5% or 1.0% D-sorbitol ( and ) and no increase in extrusion force due to 60-mins storage following the initial viscoelastic agent discharge with needle tip immersed in PBS for HA/CS-A ().
Figure 3 Dependence of the extrusion force on the storage time of OVDs with different concentrations of D-sorbitol as measured by a texture analyzer. Effect of D-sorbitol addition on the extrusion force of OVD samples containing 3% HA and 4% CS with no D-sorbitol (A), 0.5% D-sorbitol (B), or 1.0% D-sorbitol (C). Effect of open-air exposure time on the extrusion force of OVDs (D): 
 ; 0.5% D-sorbitol, and
; 0.5% D-sorbitol, and  ; 1.0% D-sorbitol. The relative extrusion force (%) is presented as the mean ± standard deviation (n = 3). Effect of soaking the needle in PBS on the extrusion force of HA/CS-A exposed to the open air for 60 mins (E). Treatment: the needle tip was soaked in PBS after exposure to the open air for 60 mins. Non-treatment: the needle tip was not soaked in PBS. The relative extrusion force (%) is presented as the mean ± standard deviation (n = 5).
; 1.0% D-sorbitol. The relative extrusion force (%) is presented as the mean ± standard deviation (n = 3). Effect of soaking the needle in PBS on the extrusion force of HA/CS-A exposed to the open air for 60 mins (E). Treatment: the needle tip was soaked in PBS after exposure to the open air for 60 mins. Non-treatment: the needle tip was not soaked in PBS. The relative extrusion force (%) is presented as the mean ± standard deviation (n = 5).Abbreviations: OVD, ophthalmic viscosurgical devices; HA, hyaluronic acid; CS, chondroitin sulfate; kgf, kilogram-force; PBS, phosphate-buffered saline.
Change In The Force-p Required To Extrude The 3% HA/4% CS Combination Without D-sorbitol Over Time Measured With A Pinch Sensor
As with the texture analyzer measurements, pinch sensor measurements also demonstrated an increase in the extrusion force-p, irrespective of gender, for 3% HA/4% CS-A with time after an initial discharge of viscoelastic agent (). The correlation coefficient between the extrusion force values measured by a texture analyzer and the extrusion force-p values by a pinch sensor at 30 or more minutes after the start of storage was 0.84 for men and 0.87 for women, demonstrating the presence of correlation. In men, the extrusion force-p value was 1.8 kgf initially, increased to 3.4 kgf after 30-mins storage, and finally reached 5 kgf after 120-mins storage. The pinch strength was approximately 30% weaker in women than in men, and the extrusion force-p value was 1.5 kgf initially, increased to 2.5 kgf after 30-mins storage, and reached 3.4 kgf after 60-mins storage. At the same time as this examination, the usability of OVDs was scored at each extrusion time point (the score is described in the method). These devices clearly became more difficult to extrude after 60-mins in both men and women and the difficulty was particularly marked in women.
Table 2 Extrusion Forces-p Dependence On Exposure Time Of The HA/CS-A Measured By Pinch Sensor
Discussion
According to Shiba et al, the extrusion force values of OVDs varied even between products with an equivalent viscoelastic agent composed of HA such as Opegan-Hi and Healon.Citation19 They demonstrated that the syringe structure, which differs among products, influenced the extrusion force on syringe content.
When using an OVD that a surgeon is not accustomed to, even a slight difference in extrusion may affect the usability or operability during surgery. In addition, changes in product operability such as “Felt resistance to extrusion, and it became difficult to discharge the viscoelastic agent completely” and “When pushed, the viscoelastic agent did not exit the needle” during the surgery are expected to interrupt its smooth progress for many surgeons. Such events will occur with a certain probability when OVDs are reused after a while. Actually, approximately 40% of surgeons have experienced needle clogging during ophthalmic surgery (). However, no report has evaluated such properties of OVDs in detail or mentioned methods to prevent expected problems. Therefore, to establish methods that can be implemented to avoid changes in the usability of OVDs resulting from needle clogging, we focused on evaluation of the extrusion force of OVDs.
Dispersive OVDs are subdivided based on their zero-share viscosity as viscous (100–1000 Pa·s), medium viscosity (10–100 Pa·s), or very low viscosity (1–10 Pa·s). The dispersive OVDs used in this study are classified as follows: Discovisc; viscous dispersive, Viscoat; medium viscous dispersive, Opegan; very low viscous dispersive.Citation1 We have reported that the classification of OVDs based on their rheological properties overlapped with the classification based on the cohesion-dispersion index previously proposed by Arshinoff.Citation20 According to the rheological properties, we classified Shellgan as medium viscous dispersive. Thus, the prepared HA/CS combination described herein is the same formulation as the Shellgan medium viscous dispersive, which is the type most commonly used as a viscoelastic agent on the corneal surface during surgery.
The changes in extrusion force described above are presumed to be due to clogging by viscoelastic agent dried and hardened at the needle tip. We hypothesized that if the adhesion of dried viscoelastic agent to the needle tip can be prevented, the elevation of extrusion force will be suppressed. D-sorbitol, a known additive in pharmaceuticals and cosmetics, possesses hygroscopicity and moisture-retention capacity.Citation15–Citation18 The addition of D-sorbitol to viscoelastic agents is expected to improve the extrusion resistance of OVDs. In other words, we thought that adding D-sorbitol would suppress the elevation of extrusion force due to drying of the viscoelastic agents. Thus, we evaluated the effects of D-sorbitol addition to the HA/CS combination on changes in the extrusion force. As shown in , in OVDs containing no D-sorbitol, extrusion force increased temporarily after viscoelastic agent was discharged, even if the amount discharged was small. The rate of increase escalated as the interval lengthened. On the other hand, in OVDs containing D-sorbitol, no temporary increase in the extrusion force was observed. These results suggested that the addition of D-sorbitol to viscoelastic agent can suppress a temporary increase in the extrusion force of OVDs.
We presumed that sorbitol’s hygroscopic or moisturizing effect prevents the hardening of viscoelastic agent remaining at the needle tip. However, since HA and CS are also known to possess hygroscopicity and moisture-retention ability,Citation18 it may be difficult to conclude that these effects of D-sorbitol alone overcame the problem. Nishiyama et al showed that an HA solution applied to the skin decreases its flexibility and increases its hardness, while a mixed solution of HA and polyols such as glycerin markedly increases the flexibility of the skin compared to HA alone.Citation17 The results suggested that an HA solution forms a film over time and hardens, but added polyols in the HA solution function as a plasticizer for the HA solution, markedly facilitating its effect. It is presumed that as with glycerin, D-sorbitol, which is also a polyol, functioned as a plasticizer in solutions of HA/CS and prevented the needle tip clogging observed with HA/CS solutions lacking D-sorbitol.
The suppression of an extrusion force increase by immersing the needle tips in PBS after OVDs storage suggested that PBS had dissolved and removed the viscoelastic agent hardened at the needle tip (). In general, the hygroscopicity and moisture-retention property of organic matter are increased by high humidity and decreased by low humidity. These properties of organic matter are influenced by the conditions of its external environment such as humidity depending on the molecular weight of the organic matter.Citation17 Considering the contribution of the external environment to the hardening of viscoelastic agent at the needle tip, it can be said that a drier environment facilitates and higher humidity retards hardening, which means that needle tip clogging might occur even in OVDs containing D-sorbitol in certain operating room environments and under certain OVD storage conditions.
shows the results of our analysis of the extrusion force of various dispersive OVDs with different components that were stored for 30 mins after viscoelastic agent discharge. Although it varied among the products, the extrusion force was increased after 30-mins storage in all products except for Shellgan, which contains D-sorbitol. All dispersive OVDs use 27-gauge needles, but they differ slightly, for example, in the length of their needles. However, the extrusion force was increased irrespective of needle type, suggesting that needle structure is unlikely to influence extrusion force. As for the composition of viscoelastic agent, increase in the extrusion force after storage was observed not only in HA/CS combination but also in OVDs containing 1% HA formulations, suggesting that this event is not related to the composition of HA or CS. However, a cohesive type OVD containing a high molecular weight HA (>2000 kDa) but without D-sorbitol did not result in an increase in the extrusion force (data not shown). As described above, the addition of D-sorbitol is considered to be a factor suppressing the increase in the extrusion force after OVDs storage; however, the influence of other factors was also suggested.
Table 3 Extrusion Forces After Storing OVDs For 30 Mins
OVDs are used more than once at intervals during surgery. For example, vitreous surgery takes 120 mins or longer for some patients. During such operations, viscoelastic agent is applied more than once to the cornea to prevent the corneal epithelium from drying. Because of the length of this operative procedure, we evaluated extrusion force at a maximum OVDs storage duration of 120 mins after initial viscoelastic agent discharge. However, the texture analyzer only evaluates extrusion force mechanically, and from such an analysis, it is difficult to infer the usability. Thus, we used a pinch sensor to measure the manually exerted extrusion force-p and, in parallel, quantified the usability of OVDs. It was found that viscoelastic agent discharge from OVDs became difficult for men, or impossible for some men, when the pinch-sensor extrusion force-p value reached 5 kgf. Even when a subject was able to extrude viscoelastic agent with 5-kgf power, we observed that the subject’s hand was shaking and that the discharge was sudden and vigorous. For women, it was suggested the limit of extrusion force-p was approximately 4 kgf. That the pinch strength differed between men and women also indicated the presence of a gender difference in extrusion force. For some OVD products, the extrusion force value was over 4 kgf after 30-mins storage (), indicating that extrusion can become difficult in a short period of time, particularly for women. This is the first study to use the pinch sensor, which is normally used to evaluate upper limb function in rheumatic patients and elderly people,Citation21,Citation22 for measurement of the extrusion force on syringe plungers as described above. It was suggested that this instrument can be useful for quantifying the usability of not only OVDs but also other injectable drug delivery systems and medical devices.
As shown in , 65% of the surgeons dealt with it by immersing the needle tip in saline or BSS when the needle tip was clogged. By immersing the needle tip in an intraocular irrigating solution or by replacing the needle, the OVD may be used without problems; however, these procedures may be burdensome to surgeons. Arshinoff SA advocated that surgeons learn the rheological properties of each OVD sufficiently to use it.Citation1,Citation2 There are many OVDs. We think that there should be greater understanding of not only intraocular behavior expected from the rheological properties of OVDs but also the usability of OVD including extrusion force.
Conclusion
In this study, it was suggested that the addition of D-sorbitol to the viscoelastic agent can suppress needle clogging due to OVD storage. The extrusion force values of OVDs are presumed to be related to OVD usability and operability during surgery. It is important for surgeons to know these properties of OVDs thoroughly in order to select OVDs that suit their needs.
Abbreviations
BSS, balanced salt solution; CS, chondroitin sulfate; HA, hyaluronic acid; kgf, kilogram-force of extrusion force values; OVDs, ophthalmic viscosurgical devices, PBS, phosphate-buffered saline.
Author Contributions
All authors contributed to data analysis, drafting and revising the paper, gave approval for the final version to be published and agree to be accountable for all aspects of the work.
Acknowledgments
The questionnaire survey was supported by a collaboration between Santen Pharmaceutical Co., Ltd. (Japan) and Seikagaku Corp. The authors thank the staff of Seikagaku Corp. for their participation in this study using a pinch sensor. We also thank surgeons who answered the questionnaire. We thank Naoto Honda (Seikagaku Corp.) and Masakazu Kasahara (Seikagaku Corp.) for their technical assistance. The authors are grateful to Masataka Arihara (Seikagaku Corp.), Kiyoshi Suzuki (Seikagaku Corp.) and Jun Takeuchi (Seikagaku Corp.) for carefully reviewing the manuscript and helpful discussion. The authors also thank ASCA Corporation for language editing of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Arshinoff SA, Jafari M. New classification of ophthalmic viscosurgical devices – 2005. J Cataract Refract Surg. 2005;31(11):2167–2171. doi:10.1016/j.jcrs.2005.08.056
- Arshinoff SA. Dispersive-cohesive viscoelastic soft shell technique. J Cataract Refract Surg. 1999;25(2):167–173. doi:10.1016/S0886-3350(99)80121-7
- Hütz WW, Eckhardt HB, Kohnen T. Comparison of viscoelastic substances used in phacoemulsification. J Cataract Refract Surg. 1996;22(7):955–959. doi:10.1016/S0886-3350(96)80198-2
- Caporossi A, Baiocchi S, Storzi C, Frezzotti R. Healon GV versus Healon in demanding cataract surgery. J Cataract Refract Surg. 1995;21(6):710–713. doi:10.1016/S0886-3350(13)80572-X
- Bissen-Miyajima H. In vitro behavior of ophthalmic viscosurgical devices during phacoemulsification. J Cataract Refract Surg. 2006;32(6):1026–1031. doi:10.1016/j.jcrs.2006.02.039
- Assia EI, Apple DJ, Lim ES, Morgan RC, Tsai JC. Removal of viscoelastic materials after experimental cataract surgery in vitro. J Cataract Refract Surg. 1992;18(1):3–6. doi:10.1016/S0886-3350(13)80376-8
- Miyata K, Maruoka S, Nakahara M, et al. Corneal endothelial cell protection during phacoemulsification: low-versus high-molecular-weight sodium hyaluronate. J Cataract Refract Surg. 2002;28(9):1557–1560. doi:10.1016/S0886-3350(02)01540-7
- Healon [Package Insert]. Santa Ana, CA: Abbott Medical Optics Inc; 2018.
- Yang S, Wang B, Zhang Y, et al. Evaluation of an interlaced triple procedure: penetrating keratoplasty, extracapsular cataract extraction, and nonopen-sky intraocular lens implantation. Medicine (Baltimore). 2017;96(35):e7656. doi:10.1097/MD.0000000000007656
- Hasebe H, Takada R, Terashima H, et al. Factors of wound sutures at 3 ports in 25-Gauge pars plana vitrectomy. Jpn J Ophthalmic Surg. 2013;26(3):458–460.
- Sato T, Emi K, Bando H, Ikeda T. [Retrospective comparison of 25-gauge vitrecctomy with 20-gauge vitrectomy in the repair of retinal detachment complicated with proliferative vitreoretinopathy]. Nihon Ganka Gakkai Zasshi. 2012;116(2):100–107.
- Kwon SH, Shin JP, Kim IT, Park DH. Comparative study of corneal wetting agents during 25-gauge microincision vitrectomy surgery under a noncontact wide-angle viewing system. Ophthalmic Surg Lasers Imaging Retina. 2013;44(4):360–365. doi:10.3928/23258160-20130715-07
- Garcia-Valenzuela E, Abdelsalam A, Eliott D, et al. Reduced need for corneal epithelial debridement during vitreo-retinal surgery using two different viscous surface lubricants. Am J Ophthalmol. 2003;136(6):1062–1066. doi:10.1016/S0002-9394(03)00634-2
- Karaca C, Ozkose M, Unlu M, Sevim DG, Mirza E. Comparison of corneal wetting properties of different dispersive ophthalmic viscosurgical devices: an optical coherence tomography study. Retina. 2018;38(11):2137–2142. doi:10.1097/IAE.0000000000001854
- Peng CG, Chow AHL, Chan CK. Hygroscopic study of glucose, citric acid, and sorbitol using an electrodynamic balance: comparison with UNIFAC predictions. Aerosol Sci Technol. 2001;35(3):753–758. doi:10.1080/02786820152546798
- Chen WL, Guo DW, Shen YY, Guo SR, Ruan KP. Effects of highly hygroscopic excipients on the hydrolysis of simvastatin in tablet at high relative humidity. Indian J Pharm Sci. 2012;74(6):527–534. doi:10.4103/0250-474X.110587
- Nishiyama S, Komatsu M. Humectant. J Jpn Soc Colour Mater. 1993;66(6):371–379. doi:10.4011/shikizai1937.66.371
- Tonooka N. Water-holding property of sodium hyaluronate. Hifu. 1985;27(2):296–302. doi:10.11340/skinresearch1959.27.296
- Shiba T, Tsuneoka H. Prefilled syringes and usability of ophthalmic viscosurgical devices. Clin Ophthalmol. 2014;8:1697–1702. doi:10.2147/OPTH.S67689
- Watanabe I, Hoshi H, Sato M, Suzuki K. Rheological and adhesive properties to identify cohesive and dispersive ophthalmic viscosurgical devices. Chem Pharm Bull (Tokyo). 2019;67(3):277–283. doi:10.1248/cpb.c18-00890
- Helliwell P, Howe A, Wright V. Functional assessment of the hand: reproducibility, acceptability, and utility of a new system for measuring strength. Ann Rheum Dis. 1987;46(3):203–208. doi:10.1136/ard.46.3.203
- Lam NW, Goh HT, Kamaruzzaman SB, Chin AV, Poi PJ, Tan MP. Normative data for hand grip strength and key pinch strength, stratified by age and gender for a multiethnic Asian population. Singapore Med J. 2016;57(10):578–584. doi:10.11622/smedj.2015164
