?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To compare choroidal thickness (CT) between patients with systemic lupus erythematosus (SLE) without ophthalmologic manifestations and a control group. To study the effects in CT of disease duration, activity index, medication and systemic comorbidities.
Methods
Cross-sectional study where spectral-domain optical coherence tomography with enhanced depth imaging was used to measure CT in 13 locations, subfoveally and at 500-µm intervals along a horizontal and a vertical section from the fovea. Linear regression models were used.
Results
Sixty-eight SLE patients and fifty healthy controls were enrolled. CT multivariable analysis revealed lower values in SLE patients (12.93–26.73 µm thinner) in all locations, except the inferior quadrants (6.48–10.44 µm thicker); however, none of these results reached statistical significance. Contrary to the control group, the normal topographic variation in CT between macular quadrants and from the center to the periphery was not observed in the SLE group. Multivariable analysis in the SLE group alone revealed a significant negative association with anticoagulants (50.10–56.09 µm thinner) and lupus nephritis (40.79–58.63 µm thinner). Contrary to controls, the CT of SLE patients did not respond to changes in mean arterial pressure.
Conclusion
CT in SLE appears to be thinner, particularly in the subset of patients with nephritis and taking anticoagulants, suggesting more advanced systemic vascular disease. Choroidal responses to hemodynamic changes may also be altered in SLE.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, autoimmune, connective tissue disease that can involve multiple organ systems. Similar to other autoimmune disorders, it can affect the vascular system in multiple territories either directly by inducing vasculitis and an increased atherosclerotic or thrombotic burden or indirectly by interfering with normal vasoregulatory mechanisms.Citation1–Citation4 Up to one-third of SLE patients present ocular manifestations, which may precede extraocular systemic disease.Citation5 In the eye, SLE can affect almost any structure, including the vascular supply of the retina, the choroid and the optic nerve head.Citation6 Lupus choroidopathy is rare and can present with serous retinal detachment, retinal pigment epithelium detachment, retinal pigment epitheliopathy, choroidal ischemia or effusion.Citation5 When present, lupus choroidopathy is usually a marker of high disease activity and often associated with the central nervous system and renal disease.Citation7 On the other hand, subtle and subclinical changes in choroidal circulation have been described in patients with lupus nephritis.Citation8 These facts raise the hypothesis that choroidal vascular bed may be affected early in the course of the disease, possibly reflecting existing microvasculopathy in other organ systems.
Spectral domain optical coherence tomography (SD-OCT) with enhanced depth imaging (EDI) software is a noninvasive imaging modality that provides high-resolution three-dimensional images of the retina and choroid.Citation9 In recent years, this technique enabled us to characterize the morphology and thickness of the choroid in several ocular and systemic pathologies.Citation10–Citation12 However, only a very limited number of publications have evaluated the choroid in SLE patients using EDI SD-OCT.Citation13–Citation16 These studies had heterogeneous samples and presented contradictory results.
In this study, we compared choroidal thickness (CT) using SD-OCT between SLE patients without ophthalmologic manifestations and a control group in whom systemic autoimmune diseases were excluded. We also studied the relationship between CT and, among others, disease duration, disease activity score, hydroxychloroquine intake and cumulative dosage, systemic medications and systemic comorbidities frequently present in SLE patients, namely, lupus nephritis, neuropsychiatric SLE (NP-SLE), Sjogren’s syndrome and anti-phospholipid syndrome.
Materials and methods
Subject groups
This was a cross-sectional study nested in a prospective cohort study that is still ongoing. The study was conducted at the Ophthalmology Department and at the Autoimmune Disease Unit of the Central Lisbon Hospital and University Center. The study occurred between July 2017 and August 2018. Consecutive SLE patients sent by the Autoimmune Disease Unit for ophthalmological screening were observed for inclusion/exclusion criteria. Patients fulfilled the 1997 revised American College of Rheumatology (ACR) criteria for the diagnosis of SLECitation17 and were aged between 18 and 80 years old. Only those without signs of optic neuropathy, retinopathy or choroidopathy were included in the study.
The control group, in whom rheumatologic diseases were excluded, was recruited from the General Ophthalmology Department.
The exclusion criteria were a refractive error >5 diopters or/and axial length >25 mm in the studied eye, keratic astigmatism >3 diopters, diabetes mellitus, pregnancy, signs or previous history of optic neuropathy, retinopathy or choroidopathy (namely, lupus-related, age-related macular degeneration, vascular occlusion, macular dystrophy, hydroxychloroquine retinopathy, glaucoma, ocular hypertension or neurodegenerative diseases, such as Alzheimer’s or Parkinson’s disease), ocular tumor, previous episodes of intraocular inflammation, history of intraocular or refractive surgery and significant media opacities that precluded fundus imaging.
The Institutional Ethics Committee approved the study, and written informed consent was obtained from all participants. The tenets of the Declaration of Helsinki were respected.
Study procedures
All patients underwent a prescreening visit where demographic, background medical history, full ophthalmological examination with visual acuity, anterior segment examination, Goldmann applanation tonometry, dilated fundus examination and optic biometry (using Lenstar LS 900®, Haag-Streit AG, Koeniz, Switzerland) were recorded. After this visit, patients were assigned to a specific study visit where SD-OCT was performed. Blood pressure (BP) was also measured before SD-OCT. SLE patients currently or previously treated with hydroxychloroquine also underwent fundus autofluorescence imaging and 10–2 macular automated threshold visual field testing to exclude retinal toxicity according to the American Academy of Ophthalmology recommendations.Citation18 An evaluation by an autoimmune disease specialist was also performed in all SLE patients, including a complete physical examination and laboratory tests required to access disease activity state and systemic involvement. Disease activity was scored using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).Citation19 One eye per patient was randomly selected for the study.
Visual acuity
Best corrected distance visual acuity (BCVA) was evaluated for each eye using Snellen charts. The value was then converted to the logarithm of the minimum angle of resolution (LogMAR).
Intraocular pressure
Before pupillary dilation, intraocular pressure was measured using Goldmann applanation tonometry, and a mean of 3 measurements was recorded.
Mean arterial pressure
The BP was measured using an automatic sphygmomanometer in the left arm in the seated position. Systolic (SBP) and diastolic (DBP) blood pressures were recorded, and the mean arterial pressure (MAP) was calculated according to the following formula:
Imaging
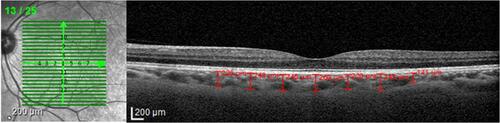
All patients were examined with SD-OCT (Spectralis® Heidelberg Engineering, Heidelberg, Germany). Scans were performed in the EDI mode using a previously described method.Citation9 Twenty-five sections, each comprising 100 averaged scans, were obtained in a 20×20⁰ (5.8 mm×5.8 mm) square centered on the fovea. All OCT examinations were performed by an ophthalmologist (J.T.F.) and were assessed by another ophthalmologist (A.D.S.); both were masked to the patients’ diagnosis. All scans were performed between 2 PM and 4 PM. CT was measured perpendicularly from the outer border of the hyperreflective line corresponding to the retinal pigment epithelium (RPE) to the inner scleral border. These measurements were taken at the subfoveal choroid and at 500-µm intervals from the fovea to 1500 µm temporal, 1500 µm nasal, 1500 µm superior and 1500 µm inferior (13 locations) ().
Statistical evaluation
First, an exploratory analysis was performed for all variables. Categorical variables were presented as frequencies (percentages), and continuous variables were presented as the mean (standard deviation [SD]) or median and interquartile range (25th percentile–75th percentile). Nonparametric Chi-Square test and Mann-Whitney test were used. To study progressive CT decrease from the center to the periphery, Friedman test was applied. Linear regression models were used to identify the variables that explain the variability of CT in the SLE and control groups. The variables gender, age, BMI, IOP, axial length, spherical equivalent, MAP, BCVA, and pharmacological variables were considered for these analyses. A second study for the SLE group alone was performed also using linear regression models and scatterplots with locally weighted scatterplot smoothers. Disease duration, disease activity score, hydroxychloroquine intake and cumulative dosage, systemic medications, and other comorbidities frequently present in these patients were added to this analysis.
Those variables attaining a p-value <0.25 in the univariable analyses were selected as candidates for the multivariable models. Normality assumption of the residuals was verified using Shapiro-Wilk test. Given the multiple testing inherent to this study, Bonferroni corrections were applied. A level of significance α=0.05 was considered. Data were analyzed using the Statistical Package for the Social Science for Windows (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
Patient demographics and clinical characteristics
A total of 68 eyes of 68 SLE patients (58 women and 10 men) and 50 eyes of 50 healthy controls (43 women and 7 men) were enrolled in this study. The median duration of SLE diagnosis was 11.0 (6.25–19.00) years, and 26 patients (38.8%) had active disease (SLEDAI >3) at the time of the study visit. Among SLE patients, 19 (27.9%) had NP-SLE, including 16 with the central type and 3 with the peripheral type; 18 (26.5%) had biopsy-proven lupus nephritis; 21 (30.9%) had anti-phospholipid syndrome; and 5 patients (7.4%) had Sjogren’s syndrome. Demographic and clinical characterization of the two groups is summarized in . Pharmacological history of patients and controls, except for hydroxychloroquine (HCQ), is presented in .
Table 1 Demographic and clinical characteristics of the patients by group
Table 2 Pharmacological history of the patients by group
Enhanced depth imaging optical coherence tomography
Comparison of CT between groups in all 13 study locations is depicted in . CT is generally higher in the control group except in the inferior quadrants, particularly at 1000 and 1500 µm inferior to the fovea. However, these differences did not reach statistical significance. The overall distribution of CT in the four quadrants showed different patterns between groups. The choroid in the superior quadrants is thicker than the inferior choroid in the control group. In the SLE group, this relationship is reversed. Regarding the temporal-nasal relationship, both groups presented a thicker choroid in the temporal quadrants. In addition, a normal progressive decrease in CT from the center to the periphery was observed in the horizontal meridian for both groups (p<0.05). However, regarding the vertical meridian, this progressive decrease was not observed in the SLE group (p>0.05).
Table 3 Choroidal thickness (µm) at 13 locations by group
In multivariable regression models, after correcting for gender, age, BMI, MAP, BCVA, IOP, spherical equivalent, axial length and medication, CT in SLE patients was lower in all locations, except for the inferior quadrants (1000 µm and 1500 µm inferior to the fovea) where it was higher (). However, evidence of a difference was exclusively found in the temporal 1500-µm location (regression coefficient estimate: −26.73; 95% confidence interval: −53.40 to −0.05; p=0.050); however, after applying Bonferroni adjustment for multiple testing, the result was not statistically significant. Therefore, there is only a tendency for a lower CT in SLE patients (−1.35 to −26.73 µm) in subfoveal, superior, nasal and temporal quadrants and at 500 µm inferior to the fovea and a tendency for higher CT in the 1000 µm and 1500 µm inferior to the fovea (6.48 to 10.44 µm). Independently from the group, age and axial length were negatively associated with CT (there was a mean decrease in CT between 20.63 and 35.62 µm for each 10 additional years and a mean decrease of 23.90 to 37.85 µm for each additional 1 mm in axial length) in all locations. CT was negatively associated with MAP at 1000 µm nasal, 500 µm and 1000 µm inferior, 500 µm and 1000 µm temporal to the fovea (there was a mean decrease of 1.26 to 1.48 µm for each increase of one mmHg). Finally, CT was negatively associated with BMI at 500 µm, 1000 µm, 1500 µm superior and 1500 µm temporal to the fovea (with a mean decrease of 4.21 to 4.96 µm in the CT for each increase of one kg/m2 in BMI).
Table 4 Results of choroidal thickness multivariable regression models
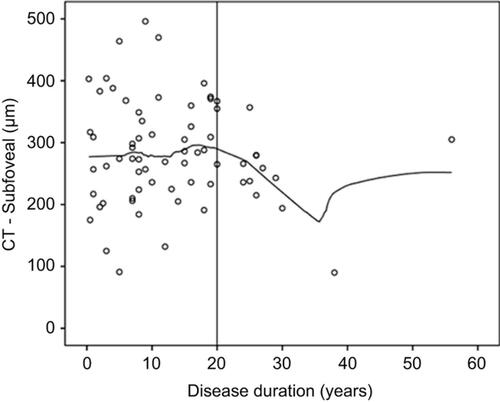
A multivariable regression analysis was also performed for the SLE group alone (). Age and axial length maintained a negative association with CT for all locations. Anticoagulants presented a negative association with CT in the subfoveal and 500 µm inferior to the fovea locations (CT was 50.10 and 56.09 µm thinner, respectively, in patients taking anticoagulants). Sjogren’s syndrome was associated with a thicker choroid in the 1000 µm superior location (CT was 73.07 µm thicker). Corticotherapy showed a negative association with CT at 1500 µm temporal from the fovea (with a 2.43 µm decrease in CT for each one mg increase of prednisone equivalent). Diuretics also presented a negative association with CT at 1000 µm superior to the fovea (in patients taking diuretics CT was 85.44 µm thinner). Finally, lupus nephritis revealed a negative association with CT at 500 µm, 1000 µm and 1500 µm nasal, 500 µm, 1000 µm superior and 1500 µm inferior to the fovea (with a mean decrease in CT of 40.79 to 58.63 µm in patients with lupus nephritis). However, apart from axial length and age, after applying Bonferroni corrections, statistical significance was only observed for lupus nephritis in the 500 µm nasal location. CT was not statistically associated with disease duration, MAP, SLEDAI, NP-SLE, anti-phospholipid syndrome or HCQ treatment duration or dosage. However, it is possible to document a constant pattern of CT distribution according to disease duration in SLE patients. In all 13 locations, CT remains stable through the first 20 years of disease and then starts to decrease slightly with time ().
Table 5 Results of choroidal thickness multivariable regression models for SLE group
Figure 2 Association between choroidal thickness (CT) and disease duration in the systemic lupus erythematosus group.
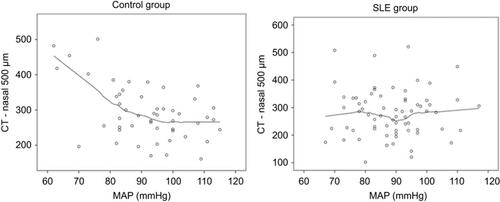
Regarding MAP, there is a different CT response between groups. In the control group, there is a reduction of CT with increasing MAP in all locations, showing stabilization for higher MAP-values (greater than 100 mmHg), while CT does not change throughout the whole range of MAP in SLE (eg, see for location 500 µm nasal).
Discussion
The choroid is the tissue with the highest blood flow per unit of weight and plays a key role in the nutrition and homeostasis of the outer layers of the retina.Citation20 On the other hand, it may be a target and reflect the microvasculature damage of systemic vascular pathologies. For example, systemic arterial hypertension has been associated with choroidal thinning,Citation21 while diabetes mellitus induces choroidal thickening in an early phase before the development of diabetic retinopathy.Citation22 Coronary heart disease is also associated with a decrease in CT independently of diabetes mellitus or systemic hypertension.Citation23
In this study, we compared the CT of SLE patients without ophthalmological manifestations with a healthy control group. We observed an overall decrease in the CT of SLE patients in the central subfoveal choroid and the nasal, temporal and superior quadrants. In the inferior quadrants, SLE patients presented a thicker choroid. However, these differences did not reach statistical significance. Moreover, the normal pattern of CT was observed in the control group with a thicker superior quadrant than the inferior and a thicker temporal quadrant than the nasal.Citation24,Citation25 In the SLE group, the temporal-nasal relationship was preserved, but the superior-inferior relationship was reversed. In addition, normal progressive decrease of CT from the center to the peripheryCitation25 was preserved in the horizontal meridian but lost in the vertical meridian in the SLE group, suggesting a flatter and less reactive choroid.
Previous studies on the CT of SLE presented conflicting results. Altinkaynak et al reported a series of SLE with a statistically significant decrease in CT.Citation13 However, this study only included patients in the “inactive” state and only measured the choroid in 3 locations: subfoveally, 1500 µm nasally and 1500 µm temporally from the foveal center. Given the irregularity of the chorioscleral border, measuring CT in more locations significantly increases the consistency of the results. Additionally, systemic factors, such as medication, the presence of lupus nephritis, NP-SLE, anti-phospholipid syndrome or other systemic comorbidities, were not included in the analysis. Ferreira et al published a series of SLE patients who had thicker choroids than healthy controls.Citation14 However, this was a retrospective study performed in patients who performed SD-OCT in the context of a HCQ screening program. Therefore, disease activity status, blood pressure at the time of the examination and SLE-related systemic comorbidities were not included in the analysis. In addition, CT was only measured in the horizontal foveal meridian. Agin et al in a study on juvenile SLE and Braga et al in a study on adult SLE patients described an increase in CT compared to healthy controls. However, none of these studies included multivariable analysis, and the effects of ocular and systemic variables known to influence CT, namely, axial length, spherical equivalent, IOP, blood pressure, BMI or systemic medication, were not taken into consideration in their analysis.Citation15,Citation16
In our study, we included 68 eyes of 68 SLE patients, and only one eye per patient was randomly selected for the study. All patients underwent a complete ophthalmologic evaluation previous to the exam as well as an appointment with an autoimmune disease specialist to assess disease activity and systemic comorbidities. In the multivariable analysis, after correcting for gender, age, BMI, MAP, BCVA, IOP, spherical equivalent, axial length and medication, CT comparison between SLE and controls showed that CT was lower in the SLE group for all locations, except in the inferior 1000 and 1500 µm locations. CT was also negatively associated with age and axial length independently from the group, which is consistent with the literature.Citation26–Citation29 In some locations, there was a negative association between MAP and CT, which is consistent with previous studies.Citation30,Citation31 A higher BMI was also associated with a reduction in CT in some of the locations, and this relation was previously reported.Citation32,Citation33
In the multivariable regression analysis for the SLE group alone, in addition to age and axial length, anticoagulants presented a negative association with CT in some locations. This association may represent a subset of SLE patients in whom the ischemic and atrophic process of the choroidal vasculature is more advanced. The subgroup of SLE patients with Sjogren’s syndrome presented a thicker choroid in the 1000 µm superior location. This finding was not described previously; however, it should be interpreted with caution since only five patients in our sample had the diagnosis of secondary Sjogren’s syndrome. Unlike Altinkaynak et al, we did not find a significant relation between choroidal thickness and disease duration.Citation13 However, when we analyze the scatter plot for disease duration, there seems to be a tendency for CT reduction after 20 years of disease. Finally, biopsy-proven lupus nephritis revealed a significant negative association with CT in some of the locations. Subtle changes in choroidal circulation in SLE patients with nephropathy and no other signs of ophthalmic involvement was previously demonstrated with indocyanine green angiography.Citation8,Citation34 However, to our knowledge, this is the first study to demonstrate a reduction of CT with SD-OCT in patients with lupus nephritis. This interesting finding is of utmost importance as it may reflect the burden of systemic microvascular damage, particularly at the renal vasculature. The relation between the duration or cumulative dosage of HCQ and CT has provided inconsistent results in previous publications.Citation14,Citation35 In our study, we did not observe a significant association between HCQ treatment duration or cumulative dosage and CT.
Histopathology studies of the choroid in SLE patients have demonstrated mononuclear inflammatory cells within the choroid, reflecting choroidal vasculitis as well as immunoglobulin and complement deposition in the choroidal vasculature.Citation6,Citation36 Consequently, choroidal blood supply is compromised, leading to choroidal thinning. Chronic ischemia induces long-term atrophy of choroidal stromal, which also leads to choroidal thinning.Citation37 These physiopathologic events may justify the decrease in CT of SLE patients and the loss of the normal topographic CT distribution observed in our study. This decrease in CT was more obvious in patients taking anticoagulants and patients with biopsy-proven lupus nephritis. In these subsets of patients, the prothrombotic and inflammatory state of repeated flares and long-term disease results in a prolonged insult to choroidal vasculature that ultimately leads to choroidal atrophy. As a matter of fact, patients with lupus nephritis present a significantly increased risk of carotid atherosclerotic plaques, myocardial infarction and cardiovascular disease mortality than nonnephritis SLE patients and healthy controls.Citation38,Citation39
The results of this study also point to a defective vascular autoregulation in the choroid of SLE patients. Choroidal blood flow (BF) is a function of perfusion pressure (PP) and vascular radius (r): BF = PP/r. PP subsequently depends on arterial blood pressure and IOP.Citation40 The main resistance to choroidal BF is located in choroidal arterioles. As stated before, choroidal BF is higher than that noted in most tissues with estimates ranging from 500 to 2000 ml/min/100 g tissue.Citation41,Citation42 Several studies suggest the existence of autoregulation in choroidal BF, which offsets fluctuations in blood pressure and IOP.Citation43–Citation45 Some of the proposed vasoregulatory mechanisms include nitric oxide, endothelins, prostaglandins and the autonomic nervous system.Citation46–Citation50 In our study, CT decreased with increasing MAP in healthy controls. On the other hand, CT in SLE patients remained unchanged throughout the entire range of MAP in the 13 studied locations. This behavior is probably related to defective mechanisms of blood flow regulation in response to changes in ocular perfusion pressure. In fact, autonomic dysfunction has been largely demonstrated in SLE patients, even in patients without manifest peripheral neuropathy, and there seems to be no relationship with disease duration, disease activity or disease damage.Citation51 Moreover, a high rate of endothelial dysfunction and vascular stiffness has been reported in patients with early SLE, even without cardiovascular risk factors and disease.Citation52
Our study has some limitations. First, CT measurements were manually obtained. Nevertheless, this manual technique has proven to have high intraobserver and interobserver reproducibility.Citation53 Second, hydration status, which may affect the CT, was not taken into account. To minimize this issue, we managed to decrease any circadian variability by performing all measurements at the same time of the day and in the same environment.
Conclusion
In summary, this study using SD-OCT to evaluate the choroid in SLE has the largest sample in the literature and was the first to evaluate the effect of systemic comorbidities in the CT of SLE patients. We documented a generalized thinning of the choroid in SLE patients, except in the inferior quadrants. This pattern is associated with a loss of normal CT topographic distribution in these patients. Moreover, a significant decrease in CT was observed in SLE patients taking anticoagulants and those with lupus nephritis. A different response of CT to variations in MAP was also observed in SLE patients compared to healthy controls. These results probably reflect existing atrophy of choroidal tissue as well as defective vascular autoregulatory mechanisms. These findings may contribute to a better understanding of the pathogenesis of SLE choroidopathy and its associations with systemic vasculopathy. Further studies, with a longitudinal design and Doppler blood flow analysis may contribute to a better understanding of our findings and the alterations occurring in the choroid as the disease progresses.
Acknowledgments
The content of this manuscript was accepted for oral presentation at the 19th EURETINA Congress in Paris in September 2019. A grant for this study was given by José de Mello Saúde – Hospital CUF Descobertas. The authors have no commercial associations.
Disclosure
The authors report no conflicts of interest in this work.
References
- Bugała K, Mazurek A, Gryga K, et al. Influence of autoimmunity and inflammation on endothelial function and thrombosis in systemic lupus erythematosus patients. Clin Rheumatol. 2018. doi:10.1007/s10067-018-4104-4
- Taraborelli M, Sciatti E, Bonadei I, et al. Endothelial dysfunction in early systemic lupus erythematosus patients and controls without previous cardiovascular events. Arthritis Care Res (Hoboken). 2017. doi:10.1002/acr.23495
- Alam MM, Das P, Ghosh P, et al. Cardiovascular autonomic neuropathy in systemic lupus erythematosus. Indian J Physiol Pharmacol. 2015;59(2):155–161.
- Liu Y, Kaplan MJ. Cardiovascular disease in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018. doi:10.1097/BOR.0000000000000528
- Silpa-Archa S, Lee JJ, Foster CS. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. 2016;100(1):135–141. doi:10.1136/bjophthalmol-2015-306629
- Dias-Santos A, Proença RP, Tavares Ferreira J, et al. The role of ophthalmic imaging in central nervous system degeneration in systemic lupus erythematosus. Autoimmun Rev. 2018:617–624. doi:10.1016/j.autrev.2018.01.011
- Palejwala NV, Walia HS, Yeh S. Ocular manifestations of systemic lupus erythematosus: a review of the literature. Autoimmune Dis. 2012;1:1. doi:10.1155/2012/290898
- Baglio V, Gharbiya M, Balacco-Gabrieli C, et al. Choroidopathy in patients with systemic lupus erythematosus with or without nephropathy. J Nephrol. 2011;24(4):522–529. doi:10.5301/JN.2011.6244
- Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi:10.1016/j.ajo.2008.05.032
- Wood A, Binns A, Margrain T, et al. Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol. 2011. doi:10.1016/j.ajo.2011.05.021
- Tan KA, Gupta P, Agarwal A, et al. State of science: choroidal thickness and systemic health. Surv Ophthalmol. 2016. doi:10.1016/j.survophthal.2016.02.007
- Dias-Santos A, Ferreira J, Abegão Pinto L, et al. Choroidal thickness in nonarteritic anterior ischaemic optic neuropathy: a study with optical coherence tomography. Neuro-Ophthalmology. 2014;38:4. doi:10.3109/01658107.2014.926943
- Altinkaynak H, Duru N, Uysal BS, et al. Choroidal thickness in patients with systemic lupus erythematosus analyzed by spectral-domain optical coherence tomography. Ocul Immunol Inflamm. 2015:1–7. doi:10.3109/09273948.2015.1006790
- Ferreira CS, Beato J, Falcão MS, Brandão E, Falcão-Reis FCÂ. Choroidal thickness in multisystemic autoimmune diseases without ophthalmologic manifestations. Retina. 2017;37(3):529–535. doi:10.1097/IAE.0000000000001193
- Braga J, Rothwell R, Oliveira M, et al. Choroid thickness profile in patients with lupus nephritis. Lupus. 2019. doi:10.1177/0961203319828525
- Ağın A, Kadayıfçılar S, Sönmez HE, et al. Evaluation of choroidal thickness, choroidal vascularity index and peripapillary retinal nerve fiber layer in patients with juvenile systemic lupus erythematosus. Lupus. 2019. doi:10.1177/0961203318814196
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997. doi:10.1002/art.1780400928
- Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF, Lum F. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123(6):1386–1394. doi:10.1016/j.ophtha.2016.01.058
- Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the sledai. A disease activity index for lupus patients. Arthritis Rheum. 1992. doi:10.1002/art.1780350606
- Alm ABA. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. doi:10.1016/0014-4835(73)90185-1
- Akay F, Gundogan FC, Yolcu U, Toyran S, Uzun S. Choroidal thickness in systemic arterial hypertension. Eur J Ophthalmol. 2015. doi:10.5301/ejo.5000675
- Tavares Ferreira J, Vicente A, Proença R, et al. Choroidal thickness in diabetic patients without diabetic retinopathy. Retina. 2018. doi:10.1097/IAE.0000000000001582
- Ahmad M, Kaszubski PA, Cobbs L, Reynolds H, Smith RT. Choroidal thickness in patients with coronary artery disease. PLoS One. 2017. doi:10.1371/journal.pone.0175691
- Esmaeelpour M, Považay B, Hermann B, et al. Three-dimensional 1060-nm OCT: choroidal thickness maps in normal subjects and improved posterior segment visualization in cataract patients. Investig Ophthalmol Vis Sci. 2010. doi:10.1167/iovs.10-5196
- Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects RID F-2586-2011. Invest Ophthalmol Vis Sci. 2010. doi:10.1167/iovs.09-4383
- Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2016. doi:10.1016/j.ajo.2008.12.008
- Ouyang Y, Heussen FM, Mokwa N, et al. Spatial distribution of posterior pole choroidal thickness by spectral domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2011. doi:10.1167/iovs.11-8046
- Li XQ, Larsen M, Munch IC. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Investig Ophthalmol Vis Sci. 2011. doi:10.1167/iovs.11-8108
- Barteselli G, Chhablani J, El-Emam S, et al. Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology. 2012. doi:10.1016/j.ophtha.2012.06.065
- Alm A. The effect of sympathetic stimulation on blood flow through the uvea, retina and optic nerve in monkeys (Macaca irus). Exp Eye Res. 1977. doi:10.1016/0014-4835(77)90241-X
- Alm A, Bill A. The effect of stimulation of the cervical sympathetic chain on retinal oxygen tension and on uveal, retinal and cerebral blood flow in cats. Acta Physiol Scand. 1973. doi:10.1111/j.1748-1716.1973.tb05436.x
- Öner Rİ, Karadağ AS. Evaluation of choroidal perfusion changes in obese patients: ocular effects of insulin resistance. Arq Bras Oftalmol. 2018;81(6):461–465.
- Yilmaz I, Ozkaya A, Kocamaz M, et al. Correlation of choroidal thickness and body mass index. Retina. 2015. doi:10.1097/IAE.0000000000000582
- Gharbiya M, Pecci G, Baglio V, Gargiulo A, Allievi F, Balacco-Gabrieli C. Indocyanine green angiographic findings for patients with systemic lupus erythematosus nephropathy. Retina. 2006;26(2):159–164. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16467671.
- Ahn SJ, Ryu SJ, Joung JY, Lee BR. Choroidal thinning associated with hydroxychloroquine retinopathy. Am J Ophthalmol. 2017. doi:10.1016/j.ajo.2017.08.022
- Nag TC, Wadhwa S. Histopathological changes in the eyes in systemic lupus erythematosus: an electron microscope and immunohistochemical study. Histol Histopathol. 2005. doi:10.14670/HH-20.373
- Nag TC, Wadhwa S. Vascular changes of the retina and choroid in systemic lupus erythematosus: pathology and pathogenesis. Curr Neurovasc Res. 2006. doi:10.2174/156720206776875821
- Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatol. 2017. doi:10.1093/rheumatology/kew475
- Gustafsson JT, Herlitz Lindberg M, Gunnarsson I, et al. Excess atherosclerosis in systemic lupus erythematosus, - A matter of renal involvement: case control study of 281 SLE patients and 281 individually matched population controls. PLoS One. 2017. doi:10.1371/journal.pone.0174572
- Riva CE, Alm A, Pournaras C. Ocular Circulation. In: Levin LA, Nilsson SFE, Ver HJ, Wu SM, Kaufman PL, Alm A, editors. Adler’s Physiology of the Eye. 11th ed. Edingburg: Saunders/Elsevier; 2011:243–273.
- Yu DY, Alder VA, Cringle SJ, Brown MJ. Choroidal blood flow measured in the dog eye in vivo and in vitro by local hydrogen clearance polarography: validation of a technique and response to raised intraocular pressure. Exp Eye Res. 1988. doi:10.1016/S0014-4835(88)80021-6
- Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975. doi:10.1152/physrev.1975.55.3.383
- Polska E, Simader C, Weigert G, et al. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Ophthalmol Vis Sci. 2007;48:3768–3774. doi:10.1167/iovs.07-0307
- Riva CE, Titze P, Hero M, Petrig BL. Effect of acute decreases of perfusion pressure on choroidal blood flow in humans. Investig Ophthalmol Vis Sci. 1997;38(9):1752–1760.
- Riva CE, Titze P, Hero M, Movaffaghy A, Petrig BL. Choroidal blood flow during isometric exercises. Investig Ophthalmol Vis Sci. 1997;38(11):2338–2343.
- Simader C, Lung S, Weigert G, et al. Role of NO in the control of choroidal blood flow during a decrease in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2009. doi:10.1167/iovs.07-1614
- Schmetterer L, Polak K. Role of nitric oxide in the control of ocular blood flow. Prog Retin Eye Res. 2001. doi:10.1016/S1350-9462(01)00014-3
- Kiel JW. Endothelin modulation of choroidal blood flow in the rabbit. Exp Eye Res. 2000. doi:10.1006/exer.2000.0911
- Chemtob S, Beharry K, Rex J, Chatterjee T, Varma DR, Aranda JV. Ibuprofen enhances retinal and choroidal blood flow autoregulation in newborn piglets. Investig Ophthalmol Vis Sci. 1991;32(6):1799–1807.
- Steinle JJ, Krizsan-Agbas D, Smith PG. Regional regulation of choroidal blood flow by autonomic innervation in the rat. Am J Physiol Regul Integr Comp Physiol. 2000. doi:10.1152/ajpregu.2000.279.1.R202
- Shalimar HR, Deepak KK, Bhatia M, Aggarwal P, Pandey RM. Autonomic dysfunction in systemic lupus erythematosus. Rheumatol Int. 2006. doi:10.1007/s00296-005-0093-0
- Taraborelli M, Sciatti E, Bonadei I, et al. Endothelial dysfunction in early systemic lupus erythematosus patients and controls without previous cardiovascular events. Arthritis Care Res. 2018. doi:10.1002/acr.23495
- Shao L, Xu L, Chen CX, et al. Reproducibility of subfoveal choroidal thickness measurements with enhanced depth imaging by spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2013. doi:10.1167/iovs.12-10351