Abstract
Purpose:
The evaluation of anatomic and visual outcomes in macular hole cases treated with internal limiting membrane (ILM) peeling, brilliant blue (BB), and 23-gauge pars plana vitrectomy (PPV).
Materials and methods:
Fifty eyes of 48 patients who presented between July 2007 and December 2009 with the diagnosis of stage 2, 3, or 4 macular holes according to Gass Classification who had undergone PPV and ILM peeling were included in this study. Pre- and postoperative macular examinations were assessed with spectral-domain optical coherence tomography. 23 G sutureless PPV and ILM peeling with BB was performed on all patients.
Results:
The mean age of patients was 63.34 ± 9.6 years. Stage 2 macular hole was determined in 17 eyes (34%), stage 3 in 24 eyes (48%), and stage 4 in 9 eyes (18%). The mean follow-up time was 13.6 ± 1.09 months. Anatomic closure was detected in 46/50 eyes (92%), whereas, in four cases, macular hole persisted and a second operation was not required due to subretinal fluid drainage. At follow-up after 2 months, persistant macular hole was detected in one case and it was closed with reoperation. At 12 months, an increase in visual acuity in 41 eyes was observed, while it remained at the same level in six eyes. In three eyes visual acuity decreased. There was a postoperative statistically significant increase in visual acuity in stage 2 and 3 cases (P < 0.05), however, no increase in visual acuity in stage 4 cases was observed.
Conclusion:
PPV and ILM peeling in stage 2, 3, and 4 macular hole cases provide successful anatomic outcomes, however, in delayed cases, due to photoreceptor loss, it has no effect on functional recovery. BB, used for clarity of ILM, may be beneficial due to its low retinal toxicity.
Introduction
Tangential tractions on the vitreomacular interface are usually responsible for the development of idiopathic macular holes. The prevalence is 3.3/1000 and it shows a strong female predominance.Citation1 In the past, it was known as a rare, untreatable disorder leading to central loss of vision. In 1991, Kelly and WendelCitation2 reported that idiopathic macular holes could be closed by vitrectomy and gas tamponade. With a better understanding of the pathogenesis of macular hole development and innovations in macular surgery, the rate of anatomical and functional success increased. One of the important developments in the surgical approach is peeling of the internal limiting membrane (ILM). Some authors have argued that ILM peeling has beneficial effects on the surgical outcome,Citation3,Citation4 whereas others have stated that this approach does not affect surgical success,Citation5,Citation6 and may lead to complications by increasing the surgery duration.Citation7,Citation8
The ILM is formed by Müller cell extensions and functions as a kind of basal membrane between retina and vitreus, acting as a surface for glial cell proliferation. Vitreoretinal interface changes due to cellular proliferation lead to ILM distortion and eventually to the development of epiretinal membrane (ERM), macular hole, and recurring macular edema.Citation9 With the removal of the ILM, successful results may be obtained in surgical treatment of such vitreoretinal diseases. The most difficult aspect of surgery is due to the tight attachment of the ILM to the retina and its extreme thinness and transparent nature. To increase the visibility of the ILM, several vital dyes have been used recently. Indocyanine green (ICG) and trypan blue have been used by many researchers in peeling of the ERM and ILM. There are different opinions on the amount and concentration of dye required, and exposure duration in the vitreous space. In many studies, because of intraretinal accumulation of ICG and its toxic effects on retinal pigment epithelium, visual field defects after surgery and possible optical nerve atrophy have been considered.Citation8,Citation10,Citation11 Recently, brilliant blue (BB) has been used in preclinical studies as it has minimal toxic effect and provides effective membrane staining.Citation9,Citation12 In this study, the anatomical and visual outcomes of pars plana vitrectomy (PPV) and ILM peeling with BB in the treatment of idiopathic macular holes has been examined.
Methods and materials
Fifty eyes of 48 patients who had 23 G PPV and ILM peeling as a treatment for macular holes between July 2007 and December 2009 were retrospectively evaluated in the authors’ clinic. All patients underwent measurement of best-corrected visual acuity and intraocular pressure, anterior segment and fundus examinations before surgery. The possible merits and risks of the treatment were explained to the patients, and informed consent was obtained from all patients in accordance with the Helsinki Declaration. Inclusion criteria were: diagnosis using noncontact-lens biomicroscopy and optical coherence tomography (OCT) of stages 2, 3, and 4 idiopathic macular hole according to the Gass classification. Exclusion criteria were: trauma history; previous macular surgery, rhegmatogenous retinal detachment together with macular hole; myopia higher than 10D; macular hole for more than 2 years; and previous retinal vessel disease. Macular examination was performed before surgery and 1, 3, and 6 months after surgery using spectral OCT (Optovue Inc, Fremont, CA).
All patients had a 23 G transconjunctival three port pars plana vitrectomy. In four eyes having both macular hole and ERM, both ILM and ERM peeling were performed. To visualize the ILM, 0.3 mL BB was used in all patients. At the end of the surgery, 16% C3F8 gas was injected into 28 eyes (56%) and 18% C2F6 gas into 22 eyes (44%).
Follow-up time points included 1-day, 1-week, 1-month, 3-month, and 6-month postsurgical evaluations and thereafter visits at 3-month intervals. Best-corrected visual acuity, intraocular pressure, and complications were recorded for each visit. Anatomical and functional outcomes of macular hole patients who underwent 23 G PPV and ILM peeling with BB were evaluated. Cases in different macular hole stages were compared in terms of anatomical and functional results.
Surgical technique
All operations were performed under local anesthesia. Following the introduction of a 23 G infusion cannula at the inferotemporal site, other 23 G trochars were inserted at the superotemporal and superonasal quadrants. Imaging systems used were the Volk MiniQuad XL (Volk Optical Ltd, Mentor, OH) during vitrectomy and Volk Central Retina (Volk Optical Ltd) during ILM peeling. After core vitrectomy, triamcinolone acetonide (Kenacort-A; Bristol Myers Squibb, New York, NY) was used to remove the posterior hyaloid. The posterior hyaloid was removed from the optic nerve head via high vacuum. Brilliant blue 0.3 mL (Fluoron Gmbh, Ludwigsfeld, Germany) was injected to the posterior pole via a 23 G dualbore cannula. After 60 seconds, peeling of the ILM was performed using a 23 G diamond-dusted membrane scraper (Synergetics Inc, Fort Collins, CO) and 23 G Eckardt end-gripping forceps (DORC, Zuidland, The Netherlands) for an area of at least 3 disc diameters. After liquid–air exchange, air–gas exchange was performed (16% C3F8 or 18% C2F6). None of the patients required sutures. Patients were asked to maintain a facedown position for 1 week.
Results
The patient group consisted of 33 (66%) female and 17 (34%) male subjects (mean age ± SD; 63.34 ± 9.6). Of these cases, 31 were phakic (62%) and 19 were pseudophakic (38%). Preoperative OCT examination revealed that, according to Gass classification, 17 eyes (34%) had stage 2 macular holes, 24 eyes (48%) had stage 3, and 9 eyes (18%) had stage 4. Four eyes with stage 2 macular holes also had an ERM. The mean follow-up period after surgery was 13.6 ± 1.09 months. Demographic characteristics of patients are given in .
Table 1 Patient (n = 50) characteristics
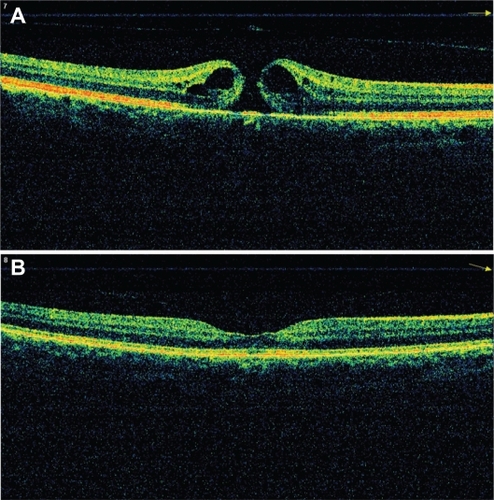
Anatomic success was assessed by the closure of the hole on OCT imaging and the withdrawal of subretinal fluid. The hole was closed in 46 (92%) of the 50 eyes after surgery but not closed in 4 (8%) eyes. When postoperative OCT controls revealed that subretinal fluid had disappeared, there was no need for a second surgery. Hole persistence was observed in only one patient 2 months after the surgery and it was closed by reoperation. shows pre- and postoperative third-month spectral OCT imaging of a patient with a stage 4 macular hole. Anatomic success was not different among the three groups.
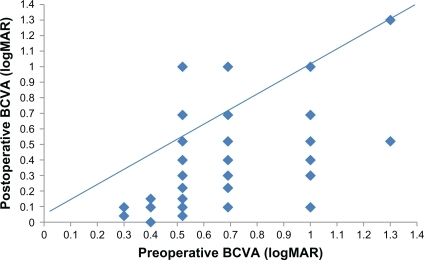
The mean best-corrected visual acuity of all patients before surgery was 0.71 ± 0.25 logMAR. Postoperative mean best-corrected visual acuity (and comparison with preoperative values) was obtained as: 0.48 ± 0.26 at 3 months (P < 0.05), 0.52 ± 0.27 at 6 months (P < 0.05), 0.42 ± 0.30 at 9 months (P < 0.05), and 0.41 ± 0.31 at 12 months (P < 0.05). When assessed together, the final visits (at 12 months) demonstrated that visual acuity had increased in 41 eyes (82%), whereas, in 6 eyes (12%), visual acuity remained unchanged. Because of recurrent macular holes, three cases showed a decrease in visual acuity. Two of these three cases had stage 4 macular holes, and one had a stage 3 macular hole. Best-corrected visual acuity results of patients before and after surgery are given in and . When evaluated according to the stage of macular hole, in stage 2 patients, all measurements of visual acuity after surgery showed that it was significantly higher when compared with preoperative measurements (P < 0.05). Increase in visual acuity between 6 and 12 months after surgery was statistically significant due to cataract surgery (P < 0.05).
Table 2 Visual acuity (logMAR)
In stage 3 patients, preoperative visual acuity compared with postoperative 3-month scores showed an increase, but this was not statistically significant (P = 0.056). During the postoperative period, visual acuity reduced between months 3 and 6 due to cataract development. In patients who underwent phacoemulsification after 6 months, visual acuity increased again. Therefore, comparisons of postoperative 9–12-month values showed significant increases (P < 0.05).
In contrast, in stage 4 patients, visual acuity scores of all time points after surgery failed to reach statistically significant levels with respect to preoperative values. Visual acuity changes of these three groups are shown comparatively in . An increase during the first month was not expected because of the gas endotamponade. Therefore, the first month visual acuity measurements were excluded from analysis. In 22 phakic patients, visual acuity increased after cataract surgery at 6 months.
Figure 2 Best-corrected visual acuity (BCVA) (logMAR) measurements of patients with stage 2, 3 and 4 macular hole after vitrectomy and brilliant blue–assisted internal limiting membrane peeling. Stages 2 and 3 patients showed significant improvements, whereas there was no significant change in stage 4 patients.
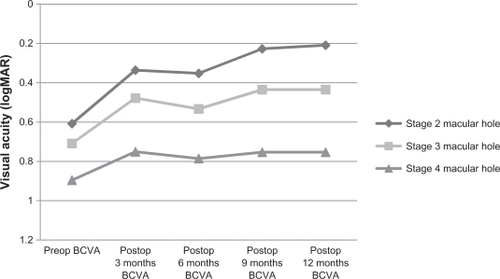
Figure 3 A representative patient with stage 4 macular hole. Preoperative (A) and postoperative 3 month (B) optical coherence tomography images.
The mean preoperative visual acuity of stage 2 macular hole patients was higher than that of stage 3 or 4 patients. Consequently, the most prominent increase in visual acuity was observed in the stage 2 group after surgery. There was no significant difference between stage 2 and 3 macular hole patients in terms of anatomical and visual outcomes, but the functional successes of these two groups were significantly higher than those of stage 4 patients. Iatrogenic retinal tears developed in four patients during surgery and were successfully treated intraoperatively. In one patient, a nasal retinal tear developed near the optic nerve while removing the posterior hyaloid. One of the phakic cases had preoperative lens opacity and vitrectomy and phacoemulsification was performed together with intracapsular lens implantation. Postoperative controls revealed hypotonia in six patients, which regressed in 3 days.
Discussion
Since the first report of Kelly and WendelCitation2 on the treatment of macular holes by pars plana vitrectomy and gas tamponade, anatomical and functional success rates have increased with developments in vitreoretinal surgery. Previous studies reported success rates over 90% after one session of surgeryCitation13,Citation14 and anatomical success rates as high as 100% in stage 2 or the initial phase of stage 3 macular holes.Citation15
In histopathological studies,Citation16,Citation17 various surgical approaches have been proposed based on the idea that collagen-containing myofibroblasts and actin-containing cells in the structure of ILM and ERM may cause contraction and lead to hole formation or widening of an existing hole. Many studiesCitation18–Citation21 have reported that ILM peeling increases anatomical and functional success in the treatment of macular hole, and during the late phases it reduces reformation of the hole. Kwok et alCitation18 compared anatomical closure of stage 3 or 4 holes in 40 patients with or without ILM peeling. Success rate was 89% in the ILM peeling group whereas it was 59% in the group without ILM peeling. The differences in anatomical closure success may be due to stage differences of macular holes, inclusion of traumatic cases, high myopic eyes, and recurrent cases.
Although there is no consensus on the effects of ILM peeling on anatomical and functional success, publications stating that ILM peeling increases hole closure are in the majority.Citation22,Citation23 There are several opinions regarding which patients require ILM peeling. Previous studies have stated that ILM peeling should be considered especially in patients with a hole wider than 400 microns and that is chronic (6 months or longer), traumatic, or recurring.Citation21–Citation25 ILM peeling was reported to increase anatomical success and prevent reopening of the hole by decreasing ERM development.Citation25,Citation26 Besides reports that suggest that ILM peeling increases anatomical success but not functional success,Citation27 there are also reports that suggest functional outcomes are better in ILM peeling groups.Citation18
In this study, ILM peeling was applied to all patients who underwent macular hole surgery. Anatomic closure was obtained in 46 (92%) of the 50 eyes, but the hole was not closed in four patients. Visual outcomes were significantly better in stage 2 or 3 patients than in stage 4 patients (P < 0.05). The most prominent postoperative visual acuity improvement was obtained in stage 2 patients. The authors consider that ILM peeling improved anatomical and functional success rates in stages 2 and 3 patients whereas it had no effect on visual outcomes in stage 4 patients due to photoreceptor loss.
Cataract development is the most prevalent complication of macular hole surgery in phakic eyes. In this study group, cataract development was observed in 23 (74%) of 31 patients due to an endotamponade application. Besides publications that report “early cataract surgery following macular hole surgery may increase the risk of re-opening of the hole,”Citation28 there are also studies that report no such risk.Citation29,Citation30 Therefore, in patients developing cataract, phacoemulsification surgery was performed at least 6 months after macular hole surgery to prevent a recurrence.
As the thin and semitransparent structure of the ILM complicates visualization during surgery, in patients with ILM peeling, there may be small asymptomatic paracentral scotomas, irregularity in nerve fiber layers, and retinal microhemorrhages due to iatrogenic retinal trauma.Citation31–Citation36 Chromovitrectomy has been developed as a method to improve ILM visibility, shorten the duration of surgery, and reduce iatrogenic retinal trauma.Citation36 Many dyes, such as indocyanine green (ICG), infracyanine green (IfCG), trypan blue (TB), BB, and triamcinolone acetonide (TA), are used to dye the ILM. Since ICG was first introduced for ILM peeling in macular hole surgery, several potential side effects have been noted.Citation37,Citation38 Visual field defects, retinal pigment epithelium, and ganglion cell defects are among the most reported side effects.Citation26,Citation32,Citation33 Dose-dependent biochemical damage to the retinal pigment epithelium and ganglion cells, photo-oxidative cell damage due to the phototoxic properties of ICG, and retinal pigment epithelium damage due to hypoosmotic solution are considered responsible for ICG-mediated ocular toxicity.Citation39–Citation41 Because of the potential toxic side effects of ICG in chromovitrectomy, other vital dyes with minimal toxicity have been tried.Citation32,Citation33,Citation38,Citation42 IfCG, unlike ICG, does not contain iodine, and it is widely thought that its toxicity to the retinal pigment epithelium is less than that of ICG.Citation31 Triamcinolone acetonide was reported as both toxicCitation43–Citation45 and nontoxicCitation46 to retinal pigment epithelium in preclinical studies. In the authors’ opinion, there is an insufficient number of randomized clinical trials to make a conclusion. However, in some studies of TA-assisted ILM peeling, it was found similar to ICG.Citation43,Citation47 Another vital dye, TB, is recommended for ERM staining because of its high affinity for proliferation-dense intraocular tissues.Citation48 Several preclinical studies with high-concentration TB reported toxic effects on tissue culture of retinal pigment epithelium.Citation49,Citation50 However, many other researchers claim that if it is used in lower concentrations, there will be no toxic effects.Citation51,Citation52
In this study, BB, a relatively new type of vital dye, was used to stain the ILM. It selectively dyes ILM and has no reported potential side effects or toxicity. The authors believe that it is the most effective alternative to ICG. Enaida et alCitation9 studied ICG on rats and found that low-dose ICG administration resulted in no retinal cell damage but high dose ICG led to morphological damage of retinal cells. In a rat study by Hisatomi et al and Enaida et al,Citation53,Citation54 low dose intravitreal BB administration caused no effects on retinal cells, but in higher doses electron microscopy revealed cyst formation in the inner layers of the retina. However, there was no apoptotic cell death. The authors of this present study think that biological adaptation to BB is better than to ICG. The use of BB is easier than ICG and TB because of its granular structure and easy dissolution in intraocular irrigation. It can be sterilized by a syringe filter. Additional surgical procedures such as fluid–air exchange are required before TB administration. Dissolving ICG is more difficult because of its structure. When compared with BB, higher concentrations of ICG are required to dye the ILM.Citation12 In addition, BB is not a fluorescent dye, so its phototoxicity is lower than ICG. The authors of this study have observed intraretinal accumulation in postoperative fundus fluorescein angiography of patients for whom ICG was used. Therefore, there may be long-term toxic effects.
Conclusion
ILM peeling in macular hole surgery provides beneficial effects on anatomical outcome, independent of disease stage. In terms of visual outcome, the highest success is obtained with stage 2 and 3 cases. It is not useful for stage 4 patients. Using BB for ILM peeling may provide beneficial effects with respect to retinal toxicity.
Disclosure
The authors report no conflicts of interest in this work.
References
- SchurmansAVan CalsterJStalmansPMacular hole surgery with inner limiting membrane peeling, endodrainage, and heavy silicone oil tamponadeAm J Ophthalmol2009147349550019019339
- KellyNEWendelRTVitreous surgery for idiopathic macular holes. Results of a pilot studyArch Ophthalmol199110956546592025167
- KwokAKLaiTYMan-ChanWWooDCIndocyanine green assisted retinal internal limiting membrane removal in stage 3 or 4 macular hole surgeryBr J Ophthalmol200387717412488266
- EckardtCEckardtUGroosSLucianoLRealeERemoval of the internal limiting membrane in macular holes. Clinical and morphological findingsOphthalmologe1997948545551 German9376691
- SmiddyWEFeuerWCordahiGInternal limiting membrane peeling in macular hole surgeryOphthalmology200110881471147611470703
- BensonWECruikshanksKCFongDSSurgical management of macular holes: a report by the American Academy of OphthalmologyOphthalmology200110871328133511425696
- KaracorluMKaracorluSOzdemirHIatrogenic punctate chorioretinopathy after internal limiting membrane peelingAm J Ophthalmol2003135217818212566021
- RodriguesEBMeyerCHFarahMEKrollPIntravitreal staining of the internal limiting membrane using indocyanine green in the treatment of macular holesOphthalmologica2005219525126216123549
- EnaidaHSakamotoTHisatomiTGotoYIshibashiTMorphological and functional damage of the retina caused by intravitreal indocyanine green in rat eyesGraefes Arch Clin Exp Ophthalmol2002240320921311935278
- HaritoglouCGandorferAGassCASchaumbergerMUlbigMWKampikAIndocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: a clinicopathologic correlationAm J Ophthalmol2002134683684112470751
- MaiaMHallerJAPieramiciDJRPE abnormalities after ILM peeling guided by ICG stainingRetina200424115716015076960
- EnaidaHHisatomiTHataYBrilliant blue G selectively stains the internal limiting membraneRetina200626663163616829804
- BensonWECruickshanksKCFongDSSurgical management of macular holes: a report by the American Academy of OphthalmologyOphthalmology200110871328133511425696
- ScottIUMoraczewskiALSmiddyWEFlynnHWFeuerWJLong term anatomic and visual acuity outcomes after initial anatomic success with macular hole surgeryAm J Ophthalmol2003135563364012719070
- MargherioRRMargherioARWilliamsGAChowDRBanachMJEffect of perifoveal tissue dissection in the management of acute idiopathic full-thickness macular holesArch Ophthalmol2000118449549810766135
- YoshidaMKishiSPathogenesis of macular hole recurrence and its prevention by internal limiting membrane peelingRetina200727216917317290198
- YoohHSBrooksHLJrCaponeAJrL’HernaultNLGrossniklausHEUltrastructural features of tissue removed during idiopathic macular hole surgeryAm J Ophthalmol1996122167758659600
- KwokAKLaiTYYuenKSTamBSWongVWMacular hole surgery with or without indocyanine green stained internal limiting membrane peelingClin Experiment Ophthalmol200331647047514641152
- FoulquierSGlacet-BernardASterkersMSoubraneGCoscasGStudy of internal limiting membrane peeling in stage-3 and -4 idiopathic macular hole surgery. FrenchJ Fr Ophthalmol2002251010261031
- SheidowTGBlinderKJHolekampNOutcome results in macular hole surgery: an evaluation of internal limiting membrane peeling with and without indocyanine greenOphthalmolgy2003110916971701
- BrooksHLJrMacular hole surgery with and without internal limiting membrane peelingOphthalmology2000107101939194811013203
- KuhnFPoint: To peel or not to peel, that is the questionOphthalmology2002109191111772571
- HassanTWilliamsGACounterpoint: To peel or not to peel: is that the question?Ophthalmology20021091111211772572
- MargherioARMacular hole surgery in 2000Curr Opin Ophthalmol200011318619010977225
- KumagaiKFurukawaMOginoNVitreous surgery with and without internal limiting membrane peeling for macular hole repairRetina200424572172715492625
- HaritoglouCGassCASchaumbergerMEhrtOGandorferAKampikAMacular changes after peeling of the internal limiting membrane in macular hole surgeryAm J Ophthalmol2001132336336811530049
- Al-AbdullaNAThompsonJTSjaardaRNResults of macular hole surgery with and without epiretinal dissection or internal limiting membrane removalOphthalmology2004111114214914711726
- BhatragorPKaiserPKSmithSDMeislerDMLewisHSearsJEReopening of previously closed macular holes after cataract extractionAm J Ophthalmol2007144225225917543876
- PassemandMYakoubiYMuselierALong-term outcome of idiopathic macular hole surgeryAm J Ophthalmol2010149112012619846059
- HagerAEhrichSWiegandWRate of reopening of macular holes following cataract operationOphthalmologe2007104538839217406812
- FarahMEMaiaMRodriguesEDyes in ocular surgery: principles for use in chromovitrectomyAm J Ophthalmol2009148333234019477708
- HaritoglouCEhertOGassCAKristinNKampikAParacentral scotoma: a new finding after vitrectomy for idiopathic macular holeBr J Ophthalmol200185223123311159494
- TsuikiEFujikawaAMiyamuraNYamadaKMishimaKKitaokaTVisual field defects after macular hole surgery with indocyanine green-assisted internal limiting membrane peelingAm J Ophthalmol2007143470470517386287
- ItoYTerasakiHTakahashiAYamakoshiTKondoMNakamuraMDissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular holesOphthalmology200511281415142016061095
- ChristensenUCKroyerKThomadsenJJorgensenTMla CourMSanderBNormative data of outer photoreceptor layer thickness obtained by software image enhancing based on Stratus optical coherence tomography imagesBr J Ophthalmol200892680080518523085
- Da MataAPBurkSERiemannCDIndocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repairOphthalmology200110871187119211425673
- Stanescu-SegallDJacksonTLVital staining with indocyanine green: a review of the clinical and experimental studies relating to safetyEye (Lond)200923350451818670454
- FerenczMSomfaiGMFarkasAFunctional assessment of the possible toxicity of indocyanine green dye in macular hole surgeryAm J Ophthalmol2006142576577017056360
- IriyamaAUchidaSYanagiYEffects of indocyanine green on retinal ganglion cellsInvest Ophthalmol Vis Sci200445394394714985315
- YipHKLaiTYSoKFKwokAKRetinal ganglion cell toxicity caused by photosensitizing effects of intravitreal indocyanine green with illumination in rat eyesBr J Ophthalmol20069019910216361677
- JacksonTLHillenkampJKnightBCSafety testing of indocyanine green and trypan blue using retinal pigment epithelium and glial cell culturesInvest Ophthalmol Vis Sci20044582778278515277504
- Stanescu-SegallDJacksonTLVital staining with indocyanine green: a review of the clinical and experimental studies relating to safetyEye (Lond)200923350451818670454
- NomotoHShiragaFYamajiHMacular hole surgery with triamcinolone acetonide-assisted internal limiting membrane peeling: one year resultsRetina200828342743218327134
- YuSYDamicoFMViolaFD’AmicoDJYoungLHRetinal toxicity of intravitreal triamcinolone acetonide: a morphological studyRetina200626553153616770259
- Ruiz-MorenoJMMonteroJABayonARuedaJVidalMRetinal toxicity of intravitreal triamcinolone acetonide at high doses in the rabbitExp Eye Res200784234234817141760
- MorrisonVLKohHJChengLBesshoKDavidsonMCFreemanWRIntravitreal toxicity of the kenalog vehicle (benzyl alcohol) in rabbitsRetina200626333934416508436
- KaracorluMOzdemirHArf KaracorluSDoes intravitreal triamcinolone acetonide assisted peeling of the internal limiting membrane affect the outcome of macular hole surgery?Graefes Arch Clin Exp Ophthalmol2005243875475715744526
- RodriguesEBMaiaMMeyerCHPenhaFMDibEFarahMEVital dyes for chromovitrectomyCurr Opin Ophthalmol200718317918717435423
- KwokAKYeungCKLaiTYChanKPPangCPEffects of trypan blue on cell viability and gene expression in human retinal pigment epithelial cellsBr J Ophthalmol200488121590159415548818
- RezaiKAFarrokh-SiarLGasynaEMErnestJTTrypan blue induces apoptosis in human retinal pigment epithelial cellsAm J Ophthalmol2004138349249515364242
- HaritoglouCEiblKSchaumbergerMFunctional outcome after trypan blue-assisted vitrectomy for macular pucker: a prospective, randomized, comparative trialAm J Ophthalmol200413811515234276
- BeutelJDahmenGZieglerAInternal limiting membrane peeling with indocyanine green or trypan blue in macular hole surgery: a randomized trialArch Ophthalmol2007125332633217353402
- HisatomiTEnaidaHMatsumotoHStaining ability and biocompatibility of brilliant blue G: preclinical study of brilliant blue G as an adjunct for capsular stainingArch Ophthalmol2006124451451916606877
- EnaidaHHisatomiTGotoYPreclinical investigation of internal limiting membrane peeling and staining using intravitreal brilliant blue GRetina200626662363016829803