Abstract
Background:
It has been previously reported that oral administration of sodium pyruvate inhibits oxidative stress and cataract formation in diabetic animals. With a view to exploring the clinical usefulness of these findings, this study examined its preventive effect when administered topically as an eye drop.
Methods:
Diabetes was induced by intraperitoneal injections of streptozotocin. At the onset of diabetes, an eye drop preparation containing 2.5% sodium pyruvate was administered six times a day at 90-minute intervals. Treatment was continued for 6 weeks. Cataract formation was monitored ophthalmoscopically after mydriasis with 1% tropicamide eye drops. Subsequently, the treated and untreated diabetic animals and the age-matched normal controls were euthanized, their eyes enucleated, and the lenses isolated for biochemical assessment of protein glycation and glutathione levels.
Results:
Treatment with pyruvate eye drops was found to be significantly effective in inhibiting protein glycation. Glutathione levels were also better maintained. In addition, ophthalmoscopic examination revealed that the incidence of cataract in the pyruvate-treated group was only 12% as compared with the untreated diabetics in whom the incidence was 73%. Cataracts at this stage were largely equatorial.
Conclusion:
The results demonstrate that topical application of pyruvate can potentially be useful in attenuating or preventing cataract formation induced by diabetes and other conditions of oxidative stress.
Introduction
Cataract is one of the most common causes of vision impairment and blindness with significant adverse effects on the quality of life of the individual.Citation1 Even though surgical treatment is successful in restoring vision, the incidence of this disease is so high that surgery alone has not been able to eradicate cataract blindness, mainly because of replacement of old cases with new ones as well as the increase in longevity. Therefore, it is highly desirable to explore the possibility of preventing or treating cataracts pharmacologically.
Several previous studies strongly suggest that oxidative stress consequent to excessive generation of oxygen free radicals is one of the significant factors involved in the pathogenesis of this aging disease.Citation2–Citation5 This is attributable to decreased activity of endogenous antioxidant defense enzymes, viz., catalase, superoxide dismutase, and glutathione peroxidase, as well as accumulation of tryptophan degradation products, such as kynurenine. The latter can induce oxidative stress by acting as a photosensitizer and consequently generate excessive reactive oxygen species during photopic vision.Citation6,Citation7 The consequent increase in reactive oxygen species leads to oxidative modifications of cellular constituents, such as the enzymatic and nonenzymatic proteins, lipids, nucleic acids, and the pyridine nucleotides, with deleterious effects. Reactive oxygen species generation is further enhanced in certain diseases such as diabetes, wherein the cataract is known to start at an earlier age and progresses relatively faster than in nondiabetics.Citation8–Citation10 Enhancement of the process in diabetes has been attributed to increased generation of reactive oxygen species by metal-catalyzed auto-oxidation of glucose present at higher concentrations. In addition to increased reactive oxygen species generation, the process is accompanied by generation of highly reactive dicarbonylsCitation11–Citation13 which modify protein structures and functions by glycation, leading eventually to formation of advanced glycation end-products and the high molecular weight protein aggregates characteristic of cataracts. Glycated proteins can by themselves generate oxyradical species, thereby perpetuating the oxidative stress even further. The reactive oxygen species generated are known to be highly effective in causing −SH oxidation of several enzymes involved in the maintenance of tissue physiology, such as glyceraldehyde-3-phosphate dehydrogenase, a key glycolytic enzyme. Metabolic inhibition in diabetes is reflected also by a decreased respiratory quotient, ie, CO2/O2, first demonstrated by Richardson and Levine,Citation14 showing decreased utilization of molecular oxygen through normal metabolic pathways and its consequent diversion to auto-oxidation reactions known to proceed monovalently. In addition to its adverse effects on metabolic enzymes, reactive oxygen species inhibit membrane transport activity by inactivating Na+-K+ ATPase,Citation15,Citation16 the enzyme responsible for maintaining the lens in a deturgesced state by regulating active transport of cations. Hence the deleterious effects of reactive oxygen species are varied in nature. Therefore, it is expected that the use of reactive oxygen species scavengers would be beneficial in preventing oxidative stress-induced damage to the lens and cataract formation.
Previous studies, including those from our laboratory, have shown that nutritional antioxidants, such as ascorbate and vitamin E, are effective in preventing cataract formation in animal models of oxidative stress as well as diabetes.Citation17,Citation18 However, the clinical usefulness of these compounds becomes limited due to their tendency to become pro-oxidant following their reaction with reactive oxygen species.Citation19 Therefore, we believe that the effectiveness of such scavengers could be enhanced if they are able to provide bioenergetic support to the tissue simultaneously by facilitating ATP generation. In this regard, we have previously shown that pyruvate is highly effective in preventing oxidative stress to the lens in vitro,Citation16 as well as in preventing actual cataract formation in vivo when administered orally to rats or mice with experimentally-induced diabetes.Citation20,Citation21 The latter is the preferred model for such studies due to its low aldose reductase activity in the lens, similar to that in the human lens.Citation22 The ability of pyruvate in preventing cataract formation is also reflected biochemically in terms of the maintenance of levels of glutathione and inhibition of protein glycation in the lenses of diabetic mice. Its beneficial effect was further apparent in terms of prevention of diabetes-induced apoptotic changes in the lens.Citation23 Encouraged by these findings, we undertook the present investigation to examine the possibility of inhibiting diabetic cataract formation by topical treatment with pyruvate eye drops, a pharmacologically more acceptable and often more effective way of treating eye diseases.
Materials and methods
CD-1 mice (body weight 25 g) obtained from Charles River Laboratories (Wilmington, MA) were used in this research. The procedures used for animal handling and euthanasia followed the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Research and were approved by the Institutional Animal Care and Use Committee. Diabetes was induced by intraperitoneal injection of streptozotocin 40 mg/kg body weight for five consecutive days. Onset of hyperglycemia was ascertained one week following streptozotocin by determining the levels of blood glucose with a glucometer using tail blood. Animals with blood glucose of 350–400 mg/dL were included in the study. The diabetic animals were divided into two groups whereby one group was left untreated (control diabetics) and the second group was treated with 2.5% sodium pyruvate eye drops six times a day, the treatment being started immediately following the establishment of diabetes and continued until 6 weeks. The eye drops were freshly prepared daily by dissolving 2.5 g sodium pyruvate in a solution containing 0.3% hydroxypropyl methyl cellulose (3500–5600 cps, pH about 7) as a wetting agent. Development of cataract was monitored by ophthalmoscopy following mydriasis with 1% tropicamide eye drops. After six weeks of treatment, the untreated and treated diabetic animals as well as the age-matched normal controls were euthanized, their eyes enucleated, and the lenses isolated for biochemical analyses.
Penetration of sodium pyruvate in aqueous humor
That the pyruvate administered topically does penetrate through the cornea was ascertained by determining its level in the aqueous humor following instillation in normal mice before the start of the actual studies. This was done as follows. After induction of anesthesia by intraperitoneal ketamine:xylazine (80 mg/kg body weight ketamine, 10 mg/kg body weight xylazine), about 25 μL of the above eye drop preparation was administered in the cul-de-sac of the mouse eye. At 30 minutes after administering the eye drops, the eye was rinsed with normal saline and aqueous humor aspirated using a 25 G 5/8 disposable needle attached to an insulin syringe and analyzed for its pyruvate content. The basal level of pyruvate in the aqueous humor was determined in the anesthetized animals that did not receive the eye drops. Pyruvate concentration was determined enzymatically by mixing the sample with nicotinamide adenine dinucleotide and lactate dehydrogenase reagent and monitoring spectro-photometrically the decrease in absorption (at 340 nm) due to reduction of pyruvate to lactate, as reported previously.Citation24
Biochemical analyses of the lenses obtained from normal, untreated, and pyruvate-treated diabetic groups were done as follows: An aqueous extract of the tissue was prepared by homogenization in 0.5 mL distilled H2O and centrifugation at 14,000 rpm. The supernatant was used for determining glutathione and glycated protein content.
Determination of lens glutathione content
Glutathione was determined in an aliquot of the above supernatant using Ellman’s reaction.Citation25 After precipitating the proteins present therein by addition of trichloroacetic acid to a final concentration of 5%, the sample was centrifuged. About 100 μL of the supernatant so obtained was reacted with 100 μL of Ellman’s reagent after neutralization with 300 μL of 0.6 M Na2HPO4. The yellow color developed due to the formation of thionitrobenzoate was then read at 412 nm.
Measurement of protein glycation
Protein glycation was measured as follows.Citation20,Citation21 The content of water-soluble proteins in the aqueous supernatant prepared as described earlier was determined by Bradford’s method using BioRad protein reagent. An aliquot of the supernatant containing 100 μg of the proteins was then loaded on a boronate affinity chromatographic column (Sigma kit 442-B; Sigma-Aldrich, St Louis, MO) which binds glycated proteins containing cis-diols of the sugar bound to the protein. After 10 minutes of reaction on the column, the unglycated proteins were eluted with phosphate buffer pH 9 (Sigma 442-3). The glycated protein was then eluted with 50 mM sorbitol in 0.4 N NaOH. Total proteins were determined in the two fractions by Bradford’s method, and the percentage of glycated protein was calculated.
Results
Previous studies from our laboratory have demonstrated the effectiveness of pyruvate in preventing cataract formation in diabetic animals. The preventive effect was evaluated biochemically as well as morphologically. Pyruvate was administered systemically by mixing it in the diet. However, given that topical treatment is the preferred mode of treatment for ocular diseases, it was considered desirable to determine the efficacy of topical instillation of pyruvate. Therefore, initial experiments were done to examine if adequate levels of the compound in the aqueous humor are indeed attained following such treatment. The level of pyruvate in the aqueous increases from a basal level of about 0.2 mM to about 1.2 mM at 30 minutes following instillation of 2.5% sodium pyruvate eye drops in the normal mouse eye. It decreases to the basal level by 60 minutes. Instillation of such an eye drop preparation in the mouse eye six times daily was found to be highly effective in inhibiting the onset of cataract formation. The earliest sign of cataract in diabetic mice consisted of the appearance of a peripheral ring of cloudiness in the lens, visible ophthalmoscopically at about 5–6 weeks of diabetes. However, such opacity was difficult to discern by standard slit lamp examination due to the convexity of the lens and peripheral location of the opacity. As shown in , 73% of the eyes in the untreated diabetic group had such cataracts. Interestingly, in the diabetic animals receiving pyruvate eye drops, only 12.5% of the eyes had developed early cataractous changes.
Table 1 Effect of pyruvate eye drops on the development of cataract in diabetic mice was assessed by ophthalmoscopy after mydriasis. As shown, the number of cataracts in the untreated diabetic group was significantly higher as compared with the pyruvate-treated group. Duration of diabetes was 6 weeks
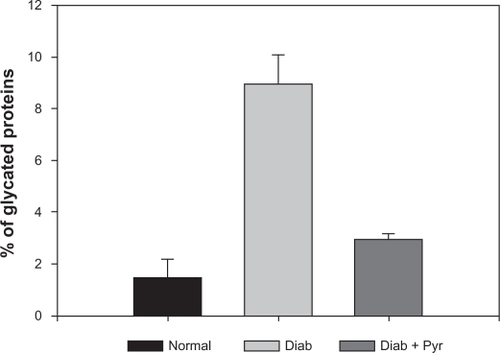
Inhibition of cataract formation by topical pyruvate treatment was due to inhibition of oxidative stress, as indicated by measurements of glutathione levels. As shown in , the level of glutathione, the primary antioxidant reserve of the tissue, decreased significantly (by about 50%) in the diabetic mouse lens to about 1.2 μmol/g from a normal value of approximately 2.4 μmol/g. Pyruvate administration prevented this decrease, the levels in this case being approximately 2.3 μmol/g, which is close to the normal. In addition to the direct effect of reactive oxygen species on glutathione, an additional mechanism by which glucose induces lens opacity is structural modification of the lens proteins by nonenzymatic glycation and subsequent formation of advanced glycation end-products and high molecular weight aggregates with decreased solubility. As shown in , the level of glycated proteins in the normal lens was about 1.5%, which increased to nearly 9% in the diabetic lens. Treatment with pyruvate eye drops substantially prevented such an increase, the level in this case being only about 3%.
Figure 1 Levels of glutathione in the diabetic mouse lens. Glutathione was determined in the acid extract of the tissue as described in the text to estimate the effect of topical pyruvate.
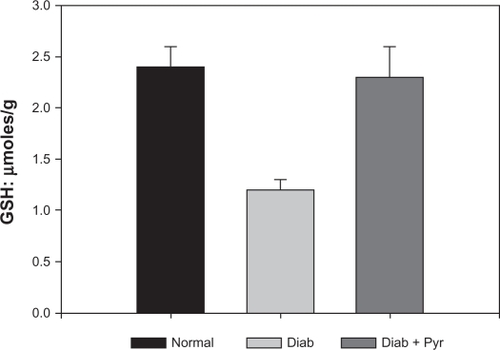
Figure 2 Effect of pyruvate eye drops on the extent of protein glycation in the diabetic mouse lens: Glycated proteins were determined in the aqueous lens extract by affinity chromatography using a boronate column.
Discussion
Diabetes is one of the significant risk factors for development of cataract. This is apparent in several studies showing that the incidence of cataracts in the diabetic population is higher than in nondiabetics. In addition, the onset of cataract occurs at an earlier age and progresses at a faster pace in diabetics. One of the important factors involved in the pathogenesis of cataracts is induction of oxidative stress consequent to excessive intraocular generation of reactive oxygen species triggered by the high levels of glucose in the extracellular as well as intracellular fluids. The latter is especially true in relatively insulin-insensitive tissues such as the lens. We have previously shown that such stress induced in the lens by diabetes is preventable by oral administration of pyruvate, a potent oxyradical scavenger and a metabolic agonist. The anticataractogenic effect of systemic treatment with pyruvate encouraged us to undertake further studies examining its potential effectiveness against cataract development by topical administration, which is the preferred route for such treatment clinically. It is expected that a higher level of the compound can be achieved in the aqueous humor when it is administered by this route. Indeed adequate levels of pyruvate were attained in the aqueous humor (about 1.2 mM) following its topical instillation. We have recently shown that it also penetrates the human cornea to a significant extent when administered topically.Citation26 The levels so attained in the aqueous humor were found to be significantly effective in inhibiting oxidative stress at the lens, apparent by the maintenance of glutathione levels. This effect is attributable primarily to the ability of pyruvate to scavenge reactive oxygen species. The reaction of pyruvate with all such species has been shown to be thermodynamically highly favorable, including its reaction with the hydroxyl radical.Citation27,Citation28
Pyruvate treatment was also effective in inhibiting protein glycation. It has previously been shown to exert this effect through competitive inhibition of glycation by glucose. The carbonyl group of pyruvate competes with the glucose carbonyl for reaction with the −NH2 protein group for the formation of the initial Schiff’s adduct. Because the carbonyl group of glucose and its derivatives remains masked in the hemiacetal configuration, it is less freely available for reacting with the −NH2 protein versus the pyruvate carbonyl which is exposed to react. In addition, due to the absence of vicinyl hydroxyl groups in the Schiff’s base formed between −NH2 protein and pyruvate, the complex does not undergo Amadori rearrangement and hence formation of advanced glycation end-products is prevented.
The protective effect of pyruvate eye drops against biochemical damage to the lens in diabetic animals was hence similar to that observed with its oral administration. We believe that the effectiveness of topical pyruvate can be further enhanced by using its ester, ethyl pyruvate, shown recently by us to attain significantly higher concentrations than its sodium salt in the aqueous humor and lens.Citation24 Further studies examining its effectiveness in preventing cataract formation in diabetic animals even until later stages of the disease are in progress.
Acknowledgements
The authors are grateful for the financial support of the National Eye Institute, National Institutes of Health.
Disclosure
The authors report no conflicts of interest in this work.
References
- ResnikoffSPascoliniDEtya’aleDGlobal data on visual impairment in the year 2002Bull World Health Organ20048284485115640920
- VarmaSDSrivastavaVKRichardsRDPhotoperoxidation in lens and cataract formation: preventive role of superoxide dismutase, catalase and vitamin COphthalmic Res1982141671757099536
- SpectorAGarnerWHHydrogen peroxide and human cataractExp Eye Res1981336736817318962
- VarmaSDEtsTKRichardsRDProtection against superoxide radicals in rat lensOphthalmic Res19779421431
- BhuyanDKBhuyanKCRegulation of hydrogen peroxide in eye humors. Effect of 3-amino-1H-1,2,4-triazole on catalase and glutathione peroxidase of rabbit eyeBiochim Biophys Acta1977497641651889879
- Van HeyningenRPhoto-oxidation of lens proteins by sun light in the presence of derivatives of kynurenine isolated from human lensExp Eye Res1973171371474759530
- DillonJWangRHAthertsoSPhoto-chemical and photo-physical studies on human lens constituentsPhotochem Photobiol199028498542089434
- HardingJJEgertonMvan HeyningenRHardingRSDiabetes, glaucoma, sex, and cataract: analysis of combined data from two case control studiesBr J Ophthalmol199377268435392
- KahnHALeibowitzHMGanleyJPThe Framingham eye study. Association of ophthalmic pathology with single variables previously measured in the Framingham heart studyAm J Epidemiol19771063341141882
- RoweNMitchellPCummingRGWansJJDiabetes, fasting blood glucose and age-related cataract: the Blue Mountains Eye StudyOphthalmic Epidemiol2000710311410934461
- WolffSPCrabbeMJCThornalleyPJThe autooxidation of glyceraldehydes and other simple monosaccharidesExperientia198440244246
- StevensMJRouzerCAMonnierVMCeramiADiabetic cataract formation and potential role of glycosylation of lens proteinsProc Natl Acad Sci U S A19787529182922275862
- BaynesJWRole of oxidative stress in development of complications in diabetesDiabetes1991404054122010041
- RichardsonHBLevineSZClinical calorimetry. Exercise and the respiratory quotient in diabetesJ Biol Chem 39;66161183
- KobatashiSRoyDSpectorASodium/potassium ATPase in normal and cataractous human lensesCurr Eye Res198223273346299650
- VarmaSDMorrisSMPeroxide damage to the eye lens in vitro: prevention by pyruvateFree Radic Res Comm19884283290
- DevamanoharanPLSHeneinMMorrisSMRamachandranSRichardsRDVarmaSDPrevention of selenite cataract by vitamin CExp Eye Res1991525635682065724
- RossWMCreightonMOPStewart-DeHaanPJSanwalMHirstMTrevithickJRModelling cortical cataractogenesis. 3. In vivo effects of vitamin E on cataractogenesis in diabetic ratsCan J Ophthalmol19821761667104839
- NagarajRHMonnierVMProtein modification by the degradation products of ascorbate: formation of a novel pyrrole from the Maillard reaction of L-threose with proteinsBiochim Biophys Acta1995125375847492603
- ZhaoWDevamanoharanPSHeneinMAliAHVarmaSDDiabetes induced biochemical changes in rat lens: attenuation of cataractogenesis by pyruvateDiabetes Obes Metab2000216517411220552
- HegdeKRVarmaSDPrevention of cataract by pyruvate in experimentally diabetic miceMol Cell Biochem200526911512015786723
- VarmaSDKinoshitaJHThe absence of cataracts in mice with congenital hyperglycemiaExp Eye Res1974195775824442467
- HegdeKRVarmaSDMorphogenetic and apoptotic changes in diabetic cataract: prevention by pyruvateMol Cell Biochem200426223323715532728
- HegdeKRKovtunSVarmaSDIntraocular penetration of pyruvate following its topical administration in miceMol Cell Biochem2010338879020012886
- EllmanGLTissue sulphydryl groupsArch Biochem Biophys195982707713650640
- ChandraPHegdeKRVarmaSDPossibility of topical antioxidant treatment of cataracts: corneal penetration of pyruvate in humansOphthalmologica200922313613819088495
- ErvensBGligorovskiSHerrmannHTemperature dependent rate constants for hydroxyl radical reactions with organic compounds in aqueous solutionsPhys Chem Chem Phys2003518111824
- MilloukiAMuYJOn atmospheric degradation of pyruvic acid in the gas phaseJ Photchem Photobiol A Chem2003157295300