Abstract
Ophthalmic bromfenac sodium sesquihydrate is a topically applied selective cyclooxygenase (COX)-2 inhibitor. It is similar to amfenac, except for a bromine atom at the C4 of the benzoyl ring position, which markedly affects its in vitro and in vivo potency, extends the duration of anti-inflammatory activity, and enhances its inhibitory effect on COX-2 absorption across the cornea and penetration into ocular tissues. The United States Food and Drug Administration approved bromfenac in 2005 for the treatment of postoperative inflammation and the reduction of ocular pain in patients who have undergone cataract surgery. Nonsteroidal anti-inflammatory drugs (NSAIDs), and among them bromfenac, could be even more effective than steroids at reestablishing the blood–aqueous barrier, as revealed by flare on slit-lamp examination and as quantitatively measured using ocular fluorophotometry. Similar to other NSAIDs, it has a role in inhibiting intraoperative miosis during cataract surgery. However, bromfenac also seems to be useful in other situations, such as refractive surgery, allergic conjunctivitis (not useful in dry eye), choroidal neovascularization, and even ocular oncology. No reports of systemic toxicity have been published and bromfenac has good topical tolerance with a low incidence of adverse effects.
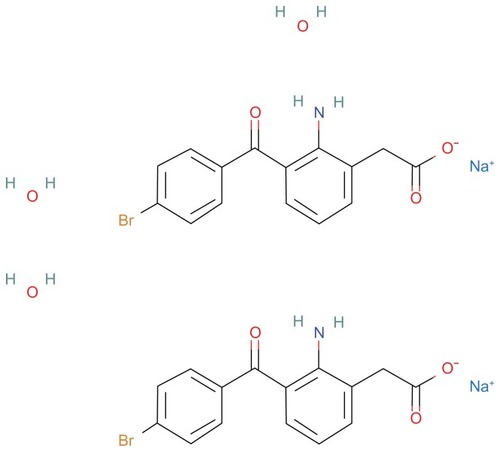
Bromfenac sodium sesquihydrate
Molecule
Bromfenac sodium sesquihydrate, also denominated as sodium 2-amino-3-(4-bromobenzoyl) phenylacetate sesquihydrate, has a molecular weight of 766.34 and a molecular formula of C30H28Br2 N2 Na2O9 (). The chemical structure is similar to amfenac, except for the addition of a bromine atom at the C4 of the benzoyl ring position. Containing a halogen in its structure enhances the molecule’s lipophilicity, thereby facilitating absorption across ocular tissues. The bromine atom of bromfenac has a marked effect on its in vitro and in vivo potency, extends the duration of anti-inflammatory activity, and enhances its inhibitory effect on cyclooxygenase (COX)-2 absorption across the cornea and penetration into ocular tissues.Citation1,Citation2
Mechanism of action
Bromfenac, a nonsteroidal anti-inflammatory drug (NSAID), has anti-inflammatory activity thought to be due to its ability to block prostaglandin synthesis by inhibiting COXs, an important group of enzymes active in the inflammatory process, which catalyzes the biosynthesis of eicosanoids from arachidonic acid to produce prostaglandins (PG) and thromboxanes.Citation1 Prostaglandins are mediators of certain kinds of intraocular inflammation. In studies of animal eyes, PG have been shown to disrupt the blood–aqueous humor barrier and increase vascular permeability, vasodilatation, intraocular pressure, and leukocytosis.Citation3,Citation4
Two cyclooxygenases (COX-1 and COX-2) encoded by two different genes have been cloned and characterized. Citation5,Citation6 Although COX-1 and COX-2 share a high level of homology (65%), the activity and expression of these enzymes are regulated differentially, and they can function independently within the same cell type.Citation7 COX-1 is important for homeostatic functions, such as maintaining the integrity of the gastrointestinal mucosa, mediating platelet function, and regulating renal blood flow. COX-2 is often induced at sites of inflammation. Animal models have shown that COX-2 is the primary mediator for ocular inflammation.Citation3 Therefore, inhibition of COX-2 is thought to be the most important therapeutic mechanism of ophthalmic NSAIDs.
NSAIDs have different relative potencies against COX-1 and COX-2. Bromfenac has been shown to be the most potent ophthalmic NSAID in inhibiting the COX-2 enzyme.Citation4 It was approximately 32 times more active against COX-2 than COX-1 in a rabbit model and 18 times more potent than ketorolac in inhibiting COX-2.Citation8
Ocular pharmacokinetics
The plasma concentration of bromfenac following ocular administration of bromfenac 0.09% ophthalmic solution in humans is unknown. Based on the maximum proposed dose of one drop in each eye (0.09 mg) and pharmacokinetic information from other routes of administration, the systemic concentration of bromfenac is estimated to be below the limit of quantification (50 ng/mL) at steady-state in humans.Citation9
Studies evaluating the pharmacokinetic profile of topical bromfenac 0.09% revealed a maximum concentration of 95.3 ng/g in the aqueous humor after instillation of a single dose in rabbits.Citation10 The time to maximum concentration in the aqueous humor in both studies was 2 hours.Citation10,Citation11 The half-life was 2.2 hours, but the drug remained detectable at 24 hours.Citation10,Citation11 In a separate multi-dose study, rabbits received one drop of bromfenac 0.09% three times daily for 14 days. The bromfenac concentration was 1103.0 ± 424.2 ng/g in the sclera; 78.1 ± 13.0 ng/g in the choroid; 32.4 ± 5.4 ng/g in the retina; 1.3 ± 0.2 ng/g in the vitreous; and 55.9 ± 9.2 ng/g in the aqueous humor.Citation10
Human studies have revealed that the drug concentration required to reduce human COX-2 activity to half maximal (IC50) was achieved after a single dose of bromfenac,Citation12 and the concentration of bromfenac in the aqueous humor of patients undergoing cataract surgery, after the instillation of one drop of bromfenac 12 hours prior to surgery, was in excess of the IC50 value for COX-2, but not the IC50 value for COX-1.Citation13
Ocular commercial presentations
Bromfenac sodium ophthalmic solution 0.1% was first approved in 2000 as Bronuck® (Senju Pharmaceutical Company, Ltd, Osaka, Japan) and is currently approved for the clinical indications of the treatment of postoperative inflammation, blepharitis, conjunctivitis, and scleritis.Citation14 The same formulation was approved in the United States in 2005 as XibromTM 0.09% (Ista Pharmaceuticals Inc, Irvine, CA) for the treatment of postoperative inflammation and the reduction of ocular pain in patients who have undergone cataract surgery. However, it has been unavailable since February 2011Citation9 because in October 2010, BromdayTM 0.09% (Ista Pharmaceuticals Inc) was approved by the Food and Drug Administration (FDA) as the first once-daily ophthalmic NSAID for the treatment of postoperative ocular inflammation and the reduction of ocular pain after cataract surgery.Citation15 A generic formulation in 2011 was approved in the United States by Coastal Pharms. In Europe, Yellox™ 0.09% (Croma Pharma, GmbH, Leobendorf, Austria) was approved in 2011 for the treatment of postoperative ocular inflammation following cataract extraction in adults.Citation16
Once-daily dose
Several studies have found that ophthalmic medications dosed less frequently tend to yield improved compliance and patient satisfaction.Citation17 The benefits of less frequent dosing also include less exposure to benzalkonium chloride and a lower potential for adverse effects.Citation18,Citation19
Based on previous studies, which showed detectable concentrations of bromfenac at 24 hours after a single dose,Citation10–Citation12 the possibility of bromfenac 0.09% dosed once daily was studied.
Overall, bromfenac ophthalmic solution 0.09% dosed once a day appeared to be as effective as when dosed twice a day in treating post-cataract surgery anterior chamber inflammation, although no direct comparisons were made and the designs of the clinical trials were different.Citation20,Citation21 However, there are no studies determining the efficacy of once-daily doses in the treatment of posterior segment inflammation.
Adverse effects of ophthalmic bromfenac
Systemic adverse effects
Oral bromfenac was initially approved for the treatment of short-term pain in 1997.Citation22 However, cases of fulminant hepatic failure were reported after prolonged use, and it was withdrawn from the market in 1998.Citation23,Citation24 Although the plasma concentration of bromfenac following ocular administration of bromfenac 0.09% ophthalmic solution in humans is unknown, it is supposed to be below the limit of quantification (50 ng/mL) and no reports of systemic toxicity have been published.Citation9
Shiffman et alCitation25 also confirmed the systemic safety. They showed normal liver function test values for more than 90% of subjects and no evidence of hepatic toxicity after 14 days of twice-daily dosing of topical bromfenac.Citation18
Although exacerbation of asthmatic symptoms has been reported with the use of topical NSAIDs, such as diclofenac or indomethacin, these events have not been described with the use of topical bromfenac.Citation26,Citation27
Ocular adverse effects
The rate of serious ocular adverse events, reported 6 years after the use of topical bromfenac in one study, was 0.0002%.Citation18 These serious ocular adverse events included five corneal ulcers, three corneal erosions, three corneal perforations, three corneal infiltrates, and two cases of corneal thinning.Citation18 The most commonly reported adverse effects in the Phase III clinical trials of topical bromfenac included iritis, an abnormal sensation in the eye, eye pain, eye pruritus, headache, eye irritation (burning/stinging), and conjunctival hyperemia.Citation18
The adverse effects of bromfenac eye drops in the ocular surface have been described as being particularly associated with the preservatives used in ophthalmic solutions, such as benzalkonium chloride. However, the experimental use of preservative-free bromfenac (commercially unavailable) also exhibited considerable toxicity in corneoconjunctival cell lines.Citation28 In contrast, in Phase III clinical trials of topical bromfenac, safety assessments for subjects treated with bromfenac were generally equivalent to or better than those for subjects treated with a vehicle.Citation18 Animal studies have implicated NSAIDs in delaying corneal wound healingCitation29 and decreasing the migration of corneal epithelium.Citation30 There are four reported cases of corneal melting and subsequent perforation in one of them after topical bromfenac use.Citation31,Citation32 In three of the reported cases of bromfenac-induced melting, the patients had severe ocular surface compromise. A role for metalloproteinase (MMP)-1 and MMP-8 has been proposed in NSAID-induced melting. An increase in MMP-1 and MMP-8 expression in eyes treated with diclofenac has been reported.Citation33 Proteases play an important role in corneal degradation and NSAID use may be implicated in their overexpression.
One study assessed the effects of topical bromfenac in human corneal endothelial cells, and revealed it was unlikely to cause endothelium damage at the concentration used under the usual conditions.Citation28
Bromfenac in cataract surgery
NSAIDs are one of the most commonly prescribed classes of medications worldwide. Aspirin and other chemically related compounds, the properties of which in oral presentations have been well known for many decades, have recently been prepared in topical ophthalmic formulations. Systemic indications are due to their analgesic, antipyretic, and anti-inflammatory properties.
In the United States, bromfenac ophthalmic solution 0.09% (XibromTM; Ista Pharmaceuticals Inc) was approved by the US Food and Drug Administration (FDA) in 2005 for the treatment of postoperative inflammation and the reduction of ocular pain in patients who have undergone cataract surgery.Citation9
Mydriasis maintenance during surgery
Topical NSAIDs are FDA-approved for the inhibition of intraoperative miosis during cataract surgery. Several clinical studies have demonstrated similar mydriatic properties for diclofenac 0.1%,Citation34,Citation35 ketorolac 0.4% and 0.5%,Citation36,Citation37 suprofen 1%,Citation38,Citation39 flurbiprofen 0.03%,Citation35 and bromfenac 0.09%.Citation40 However, the effectiveness of topical NSAIDs on pupil size in vitreoretinal surgery appears to vary from one study to another.Citation39
Reducing postoperative inflammation in cataract surgery
There is evidence that topical NSAIDs are capable of reducing postoperative inflammation after cataract surgeryCitation38,Citation39 when used correctly and cause no significant side effects or toxicity. Citation41–Citation44 However, there are only four FDA-approved drugs for this use: diclofenac, ketorolac, nepafenac, and bromfenac. Although studies comparing NSAIDs to corticosteroids have not revealed differences in the reduction of intraocular inflammation after cataract surgery,Citation42,Citation45–Citation47 NSAIDs appear to be more effective at reestablishing the blood–aqueous barrier, as revealed by flare on slit-lamp examination and as quantitatively measured using ocular fluorophotometry.Citation38,Citation39,Citation42,Citation48 This is true for both bromfenac presentations currently available in the USA.Citation20,Citation21,Citation40,Citation49,Citation50 Bromfenac has not been demonstrated to be superior to ketorolac in reducing the inflammation rate, but the incidence of corneal epitheliopathy was reported to be significantly higher.Citation18 Takamatsu et al and Ohara et al’s case series comparing bromfenac and diclofenac drops after cataract extraction did not show any differences in the inflammation rate on days 7 and 28, respectively.Citation51,Citation52
Masuda et al compared several concentrations of twice-daily 0.01%, 0.1%, and 0.2% bromfenac drops in 228 cataract cases. The 0.1% and 0.2% solutions showed superior anti-inflammatory activity in contrast to the 0.01% solution. There were no adverse events that were concentration-dependent; thus, 0.1% bromfenac was deemed optimal to minimize any potential for corneal toxicity.Citation53
Both once- and twice-daily dosing of bromfenac have been demonstrated to be beneficial for patients with a low rate of compliance or who need to minimize exposure to medications and excipients.Citation18,Citation20 However, efficacy data from clinical trials of bromfenac 0.09% with once-daily dosing cannot be directly compared to twice-daily dosing because the protocols for these clinical trials differ in terms of the predosing criteria and the Summed Ocular Inflammation Score (SOIS) end point scales used.Citation21 Both dosing presentations achieved the prespecified efficacy end points of their respective clinical trials and were thus effective in comparison to the placebo group.
Miyanaga et al compared topical bromfenac to a topical steroid or a combination in 72 patients following cataract surgery. Citation54 They did not find any significant difference in anterior chamber inflammation in this case series. There is no recent larger controlled trial comparing the efficacy of bromfenac alone versus a steroid or a combination in cataract surgery. There are no publications about using bromfenac in glaucoma, strabismus, or vitreoretinal surgeries to reduce inflammation.
Reducing ocular pain in cataract surgery
Among subjects who experienced ocular pain after surgery, the median time for resolution of pain was 2 days for those treated with bromfenac compared with 5 days for those treated with a vehicle. The proportion of subjects who were pain-free was statistically significantly greater for the bromfenac group than for the vehicle group over all visits. Citation55,Citation56 In January 2006, the FDA expanded the indication for bromfenac to include the reduction of ocular pain after cataract surgery on the basis of these results.
Cystoid macular edema
Cystoid macular edema (CME) remains the most common cause of vision loss after cataract surgery, with up to 58% of patients being reported to have some angiographic evidence of CME.Citation57 On the other hand, the rate of clinically evident CME was much lower at 4.7% in those treated with a placebo and 1.8% in those treated with steroids.Citation18 Currently, there is no FDA-approved treatment for the prevention or treatment of CME following cataract surgery. However, reviewing the world literature, Rossetti et al determined from a meta-analysis that the prevention and treatment of CME with NSAIDs is beneficial.Citation58
It remains unclear whether prophylactic treatment prevents the onset of chronic CME or in some way decreases its severity. Therefore, the long-term benefit of prophylactic treatment remains unproven, making this FDA-unapproved indication controversial.Citation59
Other ophthalmic uses of bromfenac
NSAIDs have increasingly been included in the therapeutic arsenal in ophthalmology. The treatment of intraocular inflammation and pain after cataract surgery and the treatment and prevention of cystoid macular edema are the main indications. However, NSAIDs have also been suggested to have some effectiveness in some pathology, but with a more limited action than other available agents. Their use in these cases can be considered additional therapy.
Allergic conjunctivitis
The pathogenesis of allergic conjunctivitis is complicated, but the mast cell and its chemical mediators are probably the most important components. These mediators comprise a wide range of molecules, including many different PG. Among them, PGE1 and PGE2 diminish the threshold of itching and become NSAIDs in potential therapy for allergic conjunctivitis. Topical ketorolac 0.5% is the only NSAID approved by the FDA for the relief of ocular itching in patients with seasonal allergic rhinoconjunctivitis.
In an experimental model, Hashimoto et al found that ketotifen was less effective in reducing PG than bromfenac, but it reduced plasma exudation significantly more. Moreover, ketotifen was much more effective in inhibiting the itch–scratch response than bromfenac.Citation60
Miyake-Kashima et al compared bromfenac to the mast-cell stabilizer, pemirolast potassium. Improvements in objective clinical findings were similar for both, and subjective symptoms after one week of twice-daily dosing did not show any improvement for either agent.Citation61
Uchio et al evaluated the efficacy and safety of bromfenac 0. 1% in the long-term management of vernal keratoconjunctivitis patients while mast cell stabilizers and topical steroids were continued. They found that the role of bromfenac in these patients might be to suppress the trigger-inducing recurrence of the disease by controlling the COX pathways. Once vernal keratoconjunctivitis recurred, bromfenac could not reduce the severity of the disease.Citation62
Ocular surface inflammatory diseases
Oral NSAIDs are used as first-line agents in scleritis and episcleritis with good results. However, topical presentations have not had the same results and are therefore infrequently used.Citation63 There is evidence that NSAIDs are more useful in the treatment of inflamed pinguecula and pterygium compared to topical steroids.Citation64
Dry eye
Inflammation was included in the new definition of dry eye given by the International Dry Eye Workshop.Citation65 Inflammatory mediators in the dry eye tear film are a potential target for molecules such as NSAIDs with lower side effects than topical corticosteroids. A recent study in a mouse model of dry eye raised the possibility that NSAIDs may help alleviate some of the signs and symptoms of ocular surface disease.Citation66 However, corneal melting and perforations have been reported with NSAIDs, even with bromfenac, which prevents routine use in dry eye patients for these potential adverse events, especially in cases with corneal involvement.Citation31,Citation67
Refractive surgery
Surface laser procedures remove the corneal epithelium in different ways (laser, manual debridement, diluted alcohol, epikeratome) prior to laser ablation of the stroma. Regardless of which technique is used, laser reshaping of the cornea damages the sensory nociceptive fibers. Patients can experience various degrees of pain during the epithelial regeneration process, and NSAIDs are effective in controlling it. Moreover, they have the additional benefit of reducing inflammation by inhibiting COX. In ophthalmology, the primary topical analgesia used to control pain is NSAIDs and this medication has shown to be effective in photorefractive keratectomy.Citation68,Citation69
Sher et al found no significant differences in discomfort and safety between topical bromfenac 0.09% and ketorolac 0.4%, approved by the FDA to control postoperative photorefractive keratectomy ablation pain.Citation69 However, Wang et al found there was less pain in eyes treated with bromfenac 0.09% than in those treated with ketorolac 0.5% following LASEK or epi-LASEK surgery. There were no statistically significant differences in uncorrected visual acuity between groups.Citation70
Diabetic retinopathy
Both animal and human studies have demonstrated elevated levels of PG in eyes with diabetic retinopathy.Citation71,Citation72 Therapeutic inhibition of COX-2 in the retina may now be achievable with both topical nepafenac 0.1% and bromfenac 0.09%. The first one has demonstrated the ability to inhibit diabetes-induced retinal microvascular disease in animal models.Citation73 Bromfenac may be as useful as nepafenac in animal models. However, there are no studies in humans and consequently, there is insufficient evidence to recommend the use of NSAIDs as prophylaxis or for primary treatment of diabetic retinopathy or even diabetic macular edema. Furthermore, other routes, including periocular and intravitreal, are currently being investigated to achieve greater therapeutic effects.Citation74,Citation75
Age-related macular degeneration
Age-related macular degeneration is the leading cause of blindness in developed countries.Citation76 While the etiology and pathogenesis of this disease are complex and remain poorly understood, there is evidence from human and animal studies that inflammatory and immunological events play a central role.Citation77
COX-2 is a promoter of angiogenesis and can be detected in human choroidal neovascular membranes.Citation78 Thus, the inhibition of COX-2 by NSAIDs reduces vascular endothelial growth factor production and directly inhibits choroidal neovascularization in both trauma-induced and ischemia-induced animal models.Citation74 In an experimental model, the use of topical bromfenac led to the translocation of Nrf2 and the induction of the antioxidant protein heme oxygenase (HO-1) in neovascularization lesions. The sizes of lesions were significantly smaller in the group of rats treated with bromfenac, indicating a potential therapeutic effect for intraocular angiogenic diseases.Citation79 These findings can justify the results that showed bromfenac worked as an adjunctive therapy to intravitreal ranibizumab in a prospective randomized controlled trial, showing a beneficial effect in the change of macular central thickness.Citation80 However, Zweifel et al found no benefit to the addition of topical bromfenac 0.09% twice daily over 2 months for patients receiving monthly intravitreal antivascular endothelial growth factor; in fact, there was limited response in terms of reducing persistent exudation.Citation81
At present, there is insufficient evidence to recommend NSAIDs for prophylaxis or treatment of age-related macular degeneration in human beings.
Ocular tumors
It is well known that systemic NSAIDs reduce the incidence of colon cancer by 40%–50%, and several epidemiological, clinical, and experimental studies have established NSAIDs as promising cancer chemopreventive agents.Citation82,Citation83 At the same time, COX-2 expression is increased in both uveal melanoma and retinoblastoma. Some COX-2 inhibitors have been demonstrated in experimental studies to inhibit the proliferation of human retinoblastoma cell lines, limit the progression of uveal melanoma, and increase the radiosensitivity of uveal melanoma.Citation84–Citation87 Moreover, COX-2 expression appears to be correlated with tumor malignancy.Citation88
Future studies about the therapeutic potential of NSAIDs in the treatment and prevention of ocular tumors are under way.
Method of literature search
In order to prepare this review, we conducted a Medline and PubMed search of the literature for the period between 2000 and 2011 using the following key words, as well as various combinations of them: bromfenac, Xibrom, ophthalmic NSAIDs. Reference lists from the selected articles were used to obtain further relevant articles not included in the electronic database.
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- SancilioLFNolanJCWagnerLEWardJWThe analgesic and anti-inflammatory activity and pharmacologic properties of bromfenacArzneimittelforschung19873755135193497637
- RuizJLopezMMilaJLozoyaELozanoJJPouplanaRQSAR and conformational analysis of the antiinflammatory agent amfenac and analoguesJ Comput Aided Mol Des1993721831988320556
- OkaTShearerTAzumaMInvolvement of cyclooxygenase-2 in rat models of conjunctivitisCurr Eye Res2004291273415370364
- Guex-CrosierYNon-steroidal anti-inflammatory drugs and ocular inflammationKlin Monbl Augenheilkd2001218530530811417322
- MoncadaSGryglewskiRBuntingSVaneJRAn enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregationNature19762635579663665802670
- O’BanionMKWinnVDYoungDAcDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenaseProc Natl Acad Sci U S A19928911488848921594589
- DavidgeSTProstaglandin H synthase and vascular functionCirc Res200189865066011597987
- WaterburyLDSillimanDJolasTComparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodiumCurr Med Res Opin20062261133114016846546
- Xibrom (bromfenac ophthalmic solution) 0.09% [package insert full prescribing information]Irvine, CAIsta Pharmaceuticals2008
- SiECBowmanLMHosseiniKPharmacokinetic comparisons of bromfenac in DuraSite and XibromJ Ocul Pharmacol Ther2011271616621332395
- BaklayanGAPattersonHMSongCKGowJAMcNamaraTR24-hour evaluation of the ocular distribution of (14)C-labeled bromfenac following topical instillation into the eyes of New Zealand White rabbitsJ Ocul Pharmacol Ther200824439239818665811
- MiyakeKOgawaTTajikaTGowJAMcNamaraTROcular pharmacokinetics of a single dose of bromfenac sodium ophthalmic solution 0.1% in human aqueous humorJ Ocul Pharmacol Ther200824657357819049295
- BucciFAJrWaterburyLDComparison of ketorolac 0.4% and bromfenac 0.09% at trough dosing: aqueous drug absorption and prostaglandin E2 levelsJ Cataract Refract Surg20083491509151218721711
- Bronuck [package insert full prescribing information]Osaka, JapanSenju Pharmaceutical Company, Ltd2003
- Bromday (bromfenac ophthalmic solution) 0.09% [package insert full prescribing information]Irvine CAIsta Pharmaceuticals2010
- Yellox (bromfenac sodium sesquihydrate) [package insert full prescribing information]Leobendorf, AustriaCroma Pharma GmbH2011
- OlthoffCMSchoutenJSvan de BorneBWWebersCANoncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based reviewOphthalmology2005112695396115885795
- DonnenfeldEDDonnenfeldAGlobal experience with Xibrom (bromfenac ophthalmic solution) 0.09%: the first twice-daily ophthalmic nonsteroidal anti-inflammatory drugInt Ophthalmol Clin2006464214017060789
- ChaSHLeeJSOumBSKimCDCorneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitroClin Experiment Ophthalmol200432218018415068436
- SilversteinSMCableMGSadriEOnce daily dosing of bromfenac ophthalmic solution 0.09% for postoperative ocular inflammation and painCurr Med Res Opin20112791693170321751945
- HendersonBAGaytonJLChandlerSPGowJAKlierSMMcNamaraTRSafety and efficacy of bromfenac ophthalmic solution (Bromday) dosed once daily for postoperative ocular inflammation and painOphthalmology2011118112120212721762992
- Bromfenac marketed for short-term pain reliefAm J Health Syst Pharm1997541921512152
- HunterEBJohnstonPETannerGPinsonCWAwadJABromfenac (Duract)-associated hepatic failure requiring liver transplantationAm J Gastroenterol19999482299230110445569
- FontanaRJMcCashlandTMBennerKGAcute liver failure associated with prolonged use of bromfenac leading to liver transplantation. The Acute Liver Failure Study GroupLiver Transpl Surg19995648048410545534
- ShiffmanMLDonnenfeldEDHollandEJGrilloneLRInvestigation of liver toxicity following topical treatment with Xibromt 001%, an NSAID for post-cataract surgery inflammationProceedings of the American Society of Cataract & Refractive Surgery (ASCRS) MeetingWashington DCApril 15–20, 2005
- SharirMExacerbation of asthma by topical diclofenacArch Ophthalmol199711522942959046277
- PolachekJShvartzmanPAcute bronchial asthma associated with the administration of ophthalmic indomethacinIsr J Med Sci19963211110711098960083
- AyakiMIwasawaASodaMYaguchiSKoideRCytotoxicity of five fluoroquinolone and two nonsteroidal anti-inflammatory benzalkonium chloride-free ophthalmic solutions in four corneoconjunctival cell linesClin Ophthalmol201041019102420922036
- HershPSRiceBABaerJCTopical nonsteroidal agents and corneal wound healingArch Ophthalmol199010845775832322160
- HashizumeNSaikaSOkadaYMiyamotoTShimizuKOhnishiYEffects of antiinflammatory drugs on migration of the rabbit corneal epitheliumJ Cataract Refract Surg20012791499150211566537
- IsawiHDhaliwalDKCorneal melting and perforation in Stevens Johnson syndrome following topical bromfenac useJ Cataract Refract Surg20073391644164617720085
- AsaiTNakagamiTMochizukiMHataNTsuchiyaTHottaYThree cases of corneal melting after instillation of a new nonsteroidal anti-inflammatory drugCornea200625222422716371788
- ReviglioVERanaTSLiQJAshrafMFDalyMKO’BrienTPEffects of topical nonsteroidal antiinflammatory drugs on the expression of matrix metalloproteinases in the corneaJ Cataract Refract Surg200329598999712781288
- RobertsCWComparison of diclofenac sodium and flurbiprofen for inhibition of surgically induced miosisJ Cataract Refract Surg199622Suppl 17807879279672
- ThallerVTKulshresthaMKBellKThe effect of pre-operative topical flurbiprofen or diclofenac on pupil dilatationEye (Lond)200014Pt 464264511040914
- DonnenfeldEDPerryHDWittpennJRSolomonRNattisAChouTPreoperative ketorolac tromethamine 0.4% in phacoemulsification outcomes: pharmacokinetic-response curveJ Cataract Refract Surg20063291474148216931258
- SolomonKDTurkaljJWWhitesideSBStewartJAAppleDJTopical 0.5% ketorolac vs 0.03% flurbiprofen for inhibition of miosis during cataract surgeryArch Ophthalmol19971159111911229298051
- FlachAJTopical nonsteroidal antiinflammatory drugs in ophthalmologyInt Ophthalmol Clin200242111112189605
- FlachAJCyclo-oxygenase inhibitors in ophthalmologySurv Ophthalmol19923642592841549810
- JonesJFrancisPOphthalmic utility of topical bromfenac, a twice-daily nonsteroidal anti-inflammatory agentExpert Opin Pharmacother200910142379238519735215
- FlachAJGrahamJKrugerLPStegmanRCTanenbaumLQuantitative assessment of postsurgical breakdown of the blood-aqueous barrier following administration of 0.5% ketorolac tromethamine solution. A double-masked, paired comparison with vehicle-placebo solution studyArch Ophthalmol198810633443473345153
- FlachAJKraffMCSandersDRTanenbaumLThe quantitative effect of 0.5% ketorolac tromethamine solution and 0.1% dexamethasone sodium phosphate solution on postsurgical blood-aqueous barrierArch Ophthalmol198810644804833355415
- FlachAJThe incidence, pathogenesis and treatment of cystoid macular edema following cataract surgeryTrans Am Ophthalmol Soc19989655763410360304
- Efficacy of diclofenac eyedrops in preventing postoperative inflammation and long-term cystoid macular edemaItalian Diclofenac Study GroupJ Cataract Refract Surg1997238118311899368162
- RobertsCWBrennanKMA comparison of topical diclofenac with prednisolone for postcataract inflammationArch Ophthalmol19951367257277786212
- SimoneJNPendeltonRAJenkinsJEComparison of the efficacy and safety of ketorolac tromethamine 0.5% and prednisolone acetate 1% after cataract surgeryJ Cataract Refract Surg199925569970410330648
- MissottenLRichardCTrinquandCTopical 0.1% indomethacin solution versus topical 0.1% dexamethasone solution in the prevention of inflammation after cataract surgery. The Study GroupOphthalmologica20012151435011125269
- MirshahiADjalilianARafieeFNamavariATopical administration of diclofenac (1%) in the prevention of miosis during vitrectomyRetina20082891215122019430389
- ChoHWolfKJWolfEJManagement of ocular inflammation and pain following cataract surgery: focus on bromfenac ophthalmic solutionClin Ophthalmol2009319921019668566
- BucciFAJrWaterburyLDProstaglandin e(2) inhibition of ketorolac 0.45%, bromfenac 0.09%, and nepafenac 0.1% in patients undergoing phacoemulsificationAdv Ther201128121089109522105509
- TakamatsuFShiroyamaNSaitoYIchikawaSEfficacy and adverse effects of bromfenac ophthalmic solution following cataract surgeryRinsho Ganka Jpn J Clin Ophthalmol200357712331237 Japanese
- OharaKOkuboAMiyamotoTMiyakuboHNezuNMatsudaAEffect of bromfenac sodium on postoperative inflammationJpn J Cataract Refract Surg2004182112 Japanese
- MasudaKFukadoYShimizuHEffect of bromfenac sodium ophthalmic solution on inflammation following intraocular lens implantationGanka Rinsho Iho (Jpn Rev Clin Ophthalmol)199791745750
- MiyanagaMMiyaiTNejimaRMaruyamaYMiyataKKatoSEffect of bromfenac ophthalmic solution on ocular inflammation following cataract surgeryActa Ophthalmol200987330030519183412
- SewardMSCDGrilloneLRTopical XibromTM 0.09%, significantly reduced ocular pain following cataract surgeryARVO Meeting AbstractsMay 1, 200647679
- DonnenfeldEDHEStewartRHTopical XibromTM 0.1% an investigational NSAID, significantly and rapidly decreased post- cataract surgery inflammation and reduced ocular painARVO Meeting AbstractsMay 1, 200546791
- AsanoSMiyakeKOtaIReducing angiographic cystoid macular edema and blood-aqueous barrier disruption after small-incision phacoemulsification and foldable intraocular lens implantation: multicenter prospective randomized comparison of topical diclofenac 0.1% and betamethasone 0.1%J Cataract Refract Surg2008341576318165082
- RossettiLChaudhuriJDickersinKMedical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta-analysisOphthalmology199810533974059499767
- KimSJFlachAJJampolLMNonsteroidal anti-inflammatory drugs in ophthalmologySurv Ophthalmol201055210813320159228
- HashimotoTIgarashiAHoshinaFEffects of nonsteroidal antiinflammatory drugs on experimental allergic conjunctivitis in Guinea pigsJ Ocul Pharmacol Ther200319656957714733714
- Miyake-KashimaMTakanoYTanakaMComparison of 0.1% bromfenac sodium and 0.1% pemirolast potassium for the treatment of allergic conjunctivitisJpn J Ophthalmol200448658759015592786
- UchioEItohYKadonosonoKTopical bromfenac sodium for long- term management of vernal keratoconjunctivitisOphthalmologica2007221315315817440276
- Sainz de la MazaMFosterCSJabburNSScleritis associated with systemic vasculitic diseasesOphthalmology199510246876927724185
- Frucht-PeryJSiganosCSSolomonAShvartzenbergTRichardCTrinquandCTopical indomethacin solution versus dexamethasone solution for treatment of inflamed pterygium and pinguecula: a prospective randomized clinical studyAm J Ophthalmol1999127214815210030555
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Work Shop (2007)Ocul Surf200752759217508116
- LekhanontKParkCYSmithJAEffects of topical anti- inflammatory agents in a botulinum toxin B-induced mouse model of keratoconjunctivitis siccaJ Ocul Pharmacol Ther2007231273417341147
- PrasherPAcute corneal melt associated with topical bromfenac useEye Contact Lens2011Epub Dec13
- DurrieDSKennardMGBoghossianAJEffects of nonsteroidal ophthalmic drops on epithelial healing and pain in patients undergoing bilateral photorefractive keratectomy (PRK)Adv Ther20072461278128518165210
- SherNAGolbenMRBondWTrattlerWBTauberSVoirinTGTopical bromfenac 0.09% vs ketorolac 0.4% for the control of pain, photophobia, and discomfort following PRKJ Refract Surg200925221422019241773
- WangXJWongSHGivergisRChynnEWEvaluation of analgesic efficacy of bromfenac sodium ophthalmic solution 0.09% versus ketorolac tromethamine ophthalmic solution 0.5% following LASEK or Epi-LASIKClin Ophthalmol201151451145722034570
- JohnsonEIDunlopMELarkinsRGIncreased vasodilatory prostaglandin production in the diabetic rat retinal vasculatureCurr Eye Res1999182798210223650
- NavehNBelkinMBen-ChaimOWeissmanCTreisterGProstanoids in the vitreous of diabetic and nondiabetic human eyes with retinal detachmentOphthalmic Res19902213112111530
- KernTSMillerCMDuYTopical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiologyDiabetes200756237337917259381
- AmriteACAyalasomayajulaSPCheruvuNPKompellaUBSingle periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakageInvest Ophthalmol Vis Sci20064731149116016505053
- KimSJAdamsNATomaHSSafety of intravitreal ketorolac and diclofenac: an electroretinographic and histopathologic studyRetina200828459560518398362
- BresslerNMAge-related macular degeneration is the leading cause of blindnessJAMA2004291151900190115108691
- PatelMChanCCImmunopathological aspects of age-related macular degenerationSemin Immunopathol20083029711018299834
- AyalasomayajulaSPKompellaUBCelecoxib, a selective cyclooxygenase- 2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat modelEur J Pharmacol2003458328328912504784
- YoshinagaNArimuraNOtsukaHNSAIDs inhibit neovascularization of choroid through HO-1-dependent pathwayLab Invest20119191277129021709668
- FlaxelCSchainMBHamonSCFrancisPJProspective randomized controlled trial of combination ranibizumab (lucentis) and bromfenac (xibrom) for neovascular age-related macular degeneration: a Pilot StudyRetina201232341742321862953
- ZweifelSAEngelbertMKhanSFreundKBRetrospective review of the efficacy of topical bromfenac (0.09%) as an adjunctive therapy for patients with neovascular age-related macular degenerationRetina200929101527153119898185
- GuadagniFFerroniPPalmirottaRDel MonteGFormicaVRoselliMNon-steroidal anti-inflammatory drugs in cancer prevention and therapyAnticancer Res2007275A3147316217970056
- RaoCVReddyBSNSAIDs and chemopreventionCurr Cancer Drug Targets200441294214965265
- Souza FilhoJPMartinsMCCorreaZMThe expression of cyclooxygenase 2 in retinoblastoma: primary enucleated eyes and enucleation after conservative treatmentAm J Ophthalmol2006142462563117011855
- de Souza FilhoJPCorreaZMMarshallJCThe effect of a selective cyclooxygenase-2 (COX-2) inhibitor on the proliferation rate of retinoblastoma cell linesEye (Lond)200620559860116123787
- FigueiredoACaissieALCallejoSAMcLeanIWGoldPBurnierMNJrCyclooxygenase-2 expression in uveal melanoma: novel classification of mixed-cell-type tumoursCan J Ophthalmol200338535235612956275
- MarshallJCFernandesBFDi CesareSThe use of a cyclooxygenase- 2 inhibitor (Nepafenac) in an ocular and metastatic animal model of uveal melanomaCarcinogenesis20072892053205817434930
- CryanLMParaoanLHiscottPExpression of COX-2 and prognostic outcome in uveal melanomaCurr Eye Res200833217718418293189