Abstract
Purpose
To determine astigmatic changes of intrastromal limbal-relaxing incisions (LRIs) performed during femtosecond laser-assisted cataract surgery (FLACS).
Design
Retrospective case series.
Patients and Methods
Patients undergoing FLACS with adjunctive astigmatism management with intrastromal LRIs were included. All eyes had preoperative corneal cylinder (Kcyl) ≥0.20 D on ocular biometry. An intrastromal LRI nomogram of single, non-paired LRIs placed at the 9 mm optical zone was used. Keratometry was measured preoperatively, and postoperatively at 1 week, 1 month, and 3 months (POM3). Alpins astigmatism analysis was used to calculate target-induced astigmatism (TIA, equivalent to preoperative Kcyl), surgically induced astigmatism (SIA), difference vectors (DV), and correction indices (CI). Secondary analysis included multivariable binary logistic regression to determine clinical factors associated with corrections >125% (CI > 1.25).
Results
A total of 154 eyes (125 patients) were studied. Mean preoperative Kcyl was 0.87±0.42 D (SD), which did not significantly differ from POM3 Kcyl (0.87±0.51 D, p=0.470). Only the against-the-rule (ATR) subgroup demonstrated a small but significant reduction in Kcyl from preoperative (0.96±0.51D) to POM3 (0.89±0.55D, p=0.032). Sixteen eyes (10.4%) had Kcyl ≤0.5 D preoperatively, compared to 46 eyes (29.9%) at POM3 (p<0.0001). Mean SIA was 0.80±0.52 D. Mean DV was 0.85±0.47. Mean CI was 0.79. Fifty-one eyes (33%) had astigmatism correction >125%. On multivariable regression analysis, ATR astigmatism class (p=0.026) and lower arc lengths (30º) (p=0.005) were associated with correction >125%. Lower preoperative corneal astigmatism was inversely correlated with CI (p<0.001).
Conclusion
Although intrastromal LRIs can be conveniently performed during FLACS and appear safe, only patients with ATR astigmatism demonstrated a significant reduction in corneal astigmatism 3-months postoperatively under the current nomogram. Areas for future refinements to the nomogram were identified.
Introduction
Corneal astigmatism management is emerging as an integral component of present-day cataract surgery, as residual corneal astigmatism can lead to dissatisfying uncorrected distance visual acuity and spectacle dependence postoperatively.Citation1,Citation2 Corneal astigmatism is common in patients undergoing cataract surgery, with 36–45% having ≥1.0 diopter (D) of preoperative corneal astigmatism.Citation3,Citation4 Current modalities of astigmatism management during cataract surgery include toric intraocular lens implantation, post-cataract excimer laser refractive surgery, and incisional-based interventions.
Astigmatic keratotomies (AKs) are a form of incisional-based astigmatism management. Limbal relaxation incisions (LRIs) are a subset of AKs placed more peripherally than the traditional 7mm optical zone AKs.Citation5 In addition to assisting in cataract extraction, femtosecond laser technology can produce LRIs, which may improve precision and uniformity of incisions.Citation6 Femtosecond laser LRIs can be placed intrastromally, theoretically reducing the risk of infection and minimizing postoperative pain.
Few studies have investigated the efficacy of intrastromal AKs using femtosecond laser platforms.Citation7,Citation8 Specifically, the effect of unpaired intrastromal LRIs has never been investigated. Therefore, the purpose of this study was to determine astigmatic changes following single, non-paired, intrastromal LRIs performed during femtosecond laser-assisted cataract surgery (FLACs) through vector analysis.
Patients and Methods
A retrospective case series was performed on patients presenting to a single ophthalmologic institute for elective FLACS with adjunctive femtosecond laser LRIs for astigmatism management. Written informed consent was obtained for all patients. This study was approved by the Research Ethics Board of William Osler Health System (Brampton, Ontario, Canada) and followed the tenets of the Declaration of Helsinki.
Patients, Preoperative Measurements, and Follow-Up
Patients were included if they were >18 years of age presenting with a visually significant cataract and corneal cylinder (Kcyl) ≥0.20D. Eyes were excluded if they had prior corneal surgery, prior intraocular surgery, corneal endothelial disease, irregular astigmatism, myopia exceeding −18.00D spherical equivalent (SE), or hyperopia exceeding +7.00D SE.
Preoperative surgical planning included slit-lamp biomicroscopy, dilated fundoscopy, optical biometry, and corneal keratometry. Preoperative keratometry and ocular biometry were performed using IOLMaster700 (Carl Zeiss, Jena, Germany) and Pentacam total corneal refractive power (Oculus, Wetzlar, Germany). Postoperative keratometry was performed using Pentacam total corneal refractive power. Postoperative manifest refraction data were collected. All participants were scheduled postoperative follow-up at 1 week (POW1), 1 month (POM1), and 3 months (POM3).
Surgical Technique
All FLACS and intrastromal LRIs were performed by four surgeons between June 1 and September 30, 2018, using the Catalys femtosecond laser platform (Johnson & Johnson, Santa Ana, CA, USA), software version 3.0, under a standardized protocol for all eyes.
LRI arcuate length and position were determined by a standardized nomogram for all surgeons (). The nomogram was based on the surgeon group’s previous clinical experience as a starting point. With-the-rule (WTR) astigmatism was defined as steep Kcyl meridian at 45°–135°. Against-the-rule (ATR) astigmatism was defined as steep Kcyl meridian at 0°-44° or 136°-180°. LRIs were single, non-paired, and intrastromal. LRIs were created at the steep axis superiorly or nasally for WTR or ATR astigmatism, respectively. To ensure the LRI was placed at the required corneal meridian, patients were positioned under the laser system with proper orientation using the integrated real-time optical coherence tomography (OCT) system. All LRIs were placed orthogonally to the anterior corneal surface at the 9.0mm diameter optical zone. Incisions were non-penetrating, extending 60% stromal depth and leaving 20% margins anteriorly and posteriorly, as measured with the integrated-OCT. Other standardized LRI parameters included pulse energy of 5.0µJ, horizontal and vertical spot spacing of 5µm and 10µm, respectively, anterior line density of 10, anterior line distance of 30µm, and central line density of 4.
Table 1 Nomogram for Femtosecond Laser-Assisted Intrastromal LRIs
The programmed anterior capsulotomy size was 5.0mm, with an incisional depth of 600µm, horizontal spot spacing of 4µm, vertical spot spacing of 9µm, and pulse energy of 4.0µJ. Crystalline lens fragmentation was not standardized, but based on each surgeon’s own preference. Standardized corneal wounds were made using the femtosecond laser platform with the following parameters: one main temporal clear corneal incision measuring 2.5mm wide by 1.5mm long, and one side port incision measuring 0.8mm wide by 1.2mm long were created. The main incision was placed at 200° for Right eyes and 20° for Left eyes, whereas the side port incision was standardized at 135° for Right eyes and 315° Left eyes. Both incisions were created in tri-planar fashions and placed with limbus offsets of 0.3mm. Main incisions had anterior plane depths of 30% at side-cut angles of 100° and posterior plane depths of 70% at side-cut angles of 45°. Side port incision anterior plane depth and side-cut angle were 30% and 60°, respectively, whereas the posterior plane depth and side-cut angle were 70% and 45°, respectively. Cataract extraction was completed using a standardized phacoemulsification procedure with the Johnson & Johnson WhiteStar Pro system.
Astigmatism Analysis
Patients’ postoperative Kcyl were compared to their preoperative measurements. Astigmatism analyses were performed using Alpins method.Citation9–Citation12 Target Kcyl was defined as an ideal outcome of 0.00D postoperatively. The 4 main outcomes for Alpins analyses were target-induced astigmatism (TIA), surgically induced astigmatism (SIA), difference vector (DV), and correction indices (CI). TIA was the intended magnitude and axis of astigmatic correction, where the magnitude was equivalent to preoperative Kcyl.Citation10 SIA was the actual magnitude and axis of astigmatism created during surgery.Citation10 DV was the astigmatism remaining at the end of the procedure. For DVs, as the summated vector mean approaches the arithmetic mean, the greater overall trend and likelihood of systematic treatment error.Citation10 CI was defined as SIA/TIA, where values >1 or <1 represented overcorrection or undercorrection, respectively.Citation10
Additional descriptive parameters included were magnitude of error (ME), coefficient of adjustment (CoA), index of success (IoS), angle of error (AoE), flattening effect (FE), torque, and flattening index. ME was the arithmetic difference between SIA and TIA magnitudes, where ME was positive for overcorrections and negative for undercorrections.Citation10 CoA, defined as TIA/SIA, represented the nomogram modification required to achieve ideal correction.Citation10 IoS, defined as DV/TIA, and was a relative measure of success with an ideal value of 0.Citation10 AoE was the axis angle difference between the SIA and TIA, where values<0 represented clockwise error and values>0 represented counter-clockwise error with respect to the TIA axis.Citation10 FE was the magnitude of astigmatism reduction achieved at the intended meridian.Citation10,Citation13 Torque was the amount of astigmatic change induced by the SIA resulting from misalignment.Citation10,Citation13 Flattening index was the FE divided by the TIA, and represented the effective proportion of flattening achieved at the intended meridian.Citation10,13
Secondary astigmatism analyses were performed to elucidate factors that predicted significant overcorrection in the study population. Patients were grouped as being either corrected>125% (defined as CI>1.25), or not being significantly overcorrected (defined as CI≤1.25). Variables examined included patient demographics and baseline characteristics, LRI arc length, and astigmatism classification.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD). Categorical data were presented as ratios, numbers, or percentages. Wilcoxon signed-rank tests were used for paired comparisons. Fisher’s exact tests were used for categorical contingency analyses. When comparing Kcyl, IOLMaster was chosen as the primary preoperative Kcyl device as this was the instrument used for surgical planning. Comparisons between preoperative and postoperative Pentacam Kcyl were also performed to determine the validity of the primary Kcyl comparisons. Pearson correlation was used to evaluate the relationship between SIA and TIA, and between CI and preoperative Kcyl. Multivariable analysis by binary logistic regression was used for the overcorrection analysis, whereby variables achieving significance of p<0.1 on univariable analysis or determined clinically significant by expert consensus were added to the model as covariates in order to adjust for potential confounding variables.Citation14 Statistical parameters were determined a priori. Statistical analyses were performed using SPSS (SPSS Statistics, version 25, IBM Corp.). Alpins method was performed using ASSORT Vectrak Software (Cheltenham, Victoria, Australia). Alpins single-angle plots were generated using AstigMATIC Software.Citation15 Statistical significance was defined as P<0.05.
Results
Patient Demographics and Follow-Up
A total of 154 eyes of 125 patients were included. Demographics and baseline characteristics are presented in . Sixty-seven eyes had WTR astigmatism. Eighty-seven eyes had ATR astigmatism. Ninety eyes, 48 eyes, 3 eyes, and 13 eyes received 30º-, 40º-, 50º-, and 60º-arc LRIs, respectively. Seven patients (7 eyes) missed their POW1 visit. Seven patients (8 eyes) missed their POM1 visit. All missed visits were due to non-adherence to follow-up schedule. No patients missed their POM3 follow-up. IOLMaster and Pentacam measurements did not significantly differ when comparing preoperative Kcyl (p=0.803) and steep axis (p=0.253) (). There were no cases of LRI perforation, inadvertent placement within the visual axis, wound dehiscence, inflammation, or infection.
Table 2 Patient Demographics and Baseline Characteristics (N = 154 Eyes)
Preoperative and Postoperative Astigmatism
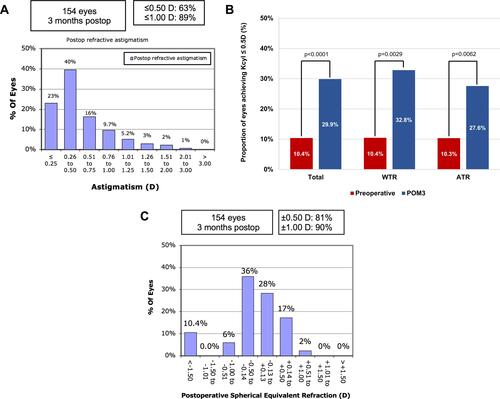
describes Kcyl measurements preoperatively, POW1, POM1, and POM3 in the total study population, WTR subgroup, and ATR subgroup. Preoperative and POM3 Kcyl did not significantly differ in the total study population (p=0.470) and WTR subgroup (p=0.170). In the ATR group, POM3 Kcyl was significantly lower than preoperative Kcyl (p=0.032). POM3 refractive cylinder data are shown in . POM3 refractive cylinder was significantly lower than preoperative Kcyl for the total study population, WTR subgroup, and ATR subgroup (each p<0.0001). illustrates a non-cumulative histogram of POM3 refractive astigmatism. shows the proportion of eyes achieving Kcyl≤0.5D at preoperative and POM3 visits. illustrates the spherical equivalent (SE) refraction at POM3, where 81% of the patients achieved a SE of ±0.50D. Fourteen (10.4% of total) patients were targeted to a sphere of −2.00 D, which accounts for the patients with SE <-1.50D as seen in . Similar to the IOLM results, preoperative Pentacam Kcyl demonstrated no significant difference to POM3 Kcyl in the total study population (p=0.759) and WTR subgroup (p=0.072) (); whereas POM3 Kcyl was significantly lower than preoperative Pentacam Kcyl in the ATR subgroup (p=0.047) ().
Table 3 Preoperative and Postoperative Astigmatism; Mean ± SD and Range
Figure 1 (A) Non-cumulative histogram of the magnitude of postoperative refractive astigmatism. (B) Proportion of eyes achieving corneal cylinder ≤0.5D preoperatively and 3-months postoperative in the total study population (n=154 eyes), with-the-rule (WTR) astigmatism class (n=67 eyes), and against-the-rule (ATR) astigmatism class (n=87 eyes). Kcyl, corneal cylinder. (C) Non-cumulative histogram of spherical equivalent refraction at postoperative month 3.
Alpins Vector Analysis
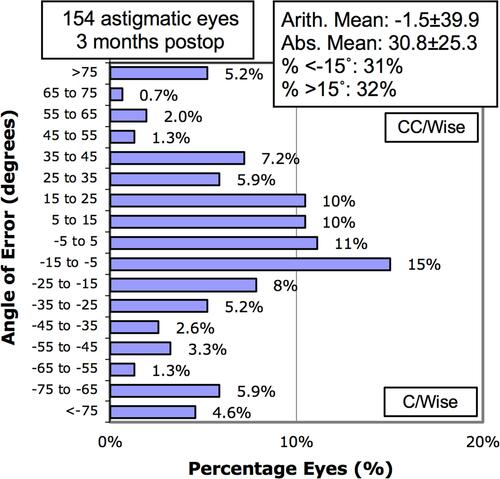
shows vector analysis outcomes for the total study population, WTR and ATR subgroups. shows a CI scatter plot of SIA versus TIA. shows the total study population TIA (), SIA (), and DV () single-angle plots, which include both arithmetic and summated vector means. By dividing the DV summated vector mean (0.25D) by the arithmetic DV mean (0.85D), the DV-plot demonstrates that 29% of the total treatment error is attributable to systematic treatment error ().Citation10 A histogram of keratometric angle of error is presented in .
Table 4 Alpins Vector Analysis Parameters; Mean ± SD, Rangea
Figure 2 Scatter plot for individual correction index values. Dashed lines represent ±0.5D limits.
Abbreviations: ATR, against-the-rule astigmatism class; SIA, surgically induced astigmatism; TIA, target-induced astigmatism; WTR, with-the-rule astigmatism class.
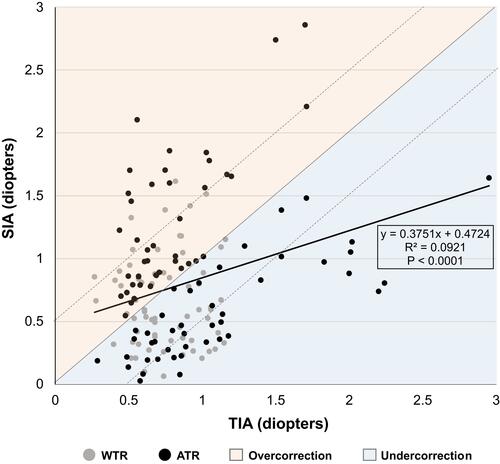
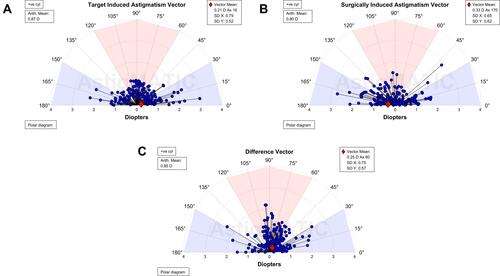
Figure 3 Alpins single-angle plots for the total study population (n=154 eyes). Target-induced astigmatism (TIA, A), surgically induced astigmatism (SIA, B), and difference vector (DV, C) arithmetic and vector means are shown. Images generated by AstigMATIC Software.Citation15
Overcorrection Analysis
In this study, 51/154 eyes were corrected>125%. Univariable and multivariable logistic regression analyses were performed for astigmatism correction>125% (defined as CI>1.25), shown in . In univariable analysis, correction>125% was associated with lower arc lengths (30º) (p=0.035). Central corneal thickness (CCT) trended towards association but did not achieve statistical significance (p=0.084) (). Age, gender, eye laterality, axial length, anterior chamber depth, central corneal thickness, predicted spherical equivalent, and astigmatism classification were not significantly different between groups ().
Table 5 Overcorrection Analysis (N = 154 Eyes)
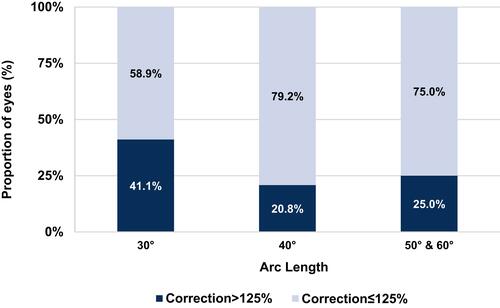
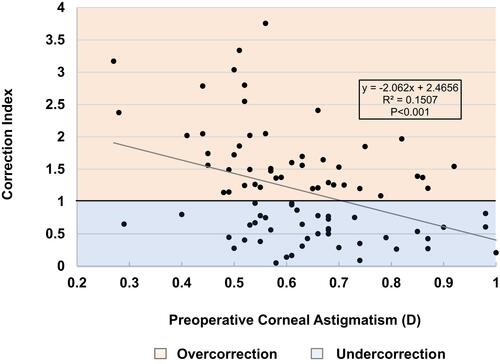
In multivariable analysis, lower LRI arc lengths and astigmatism class were independent predictors of correction>125% (p=0.005 and p=0.026, respectively). Specifically, odds of correction>125% were 2.465 times greater (95% CI, 1.114 to 5.457) in patients with ATR than WTR. Age and CCT were not associated with overcorrection (). Rates of overcorrection at each arc length are shown in . In patients who received 30º-arc LRIs, lower preoperative corneal astigmatism was inversely correlated with CI (p<0.001) (). Overall overcorrection was seen when preoperative Kcyl<0.7D and undercorrection when Kcyl>0.7D ().
Discussion
Femtosecond laser LRIs for astigmatism management can be easily performed during FLACS with limited additional cost, and may yield potential benefits over their manual counterparts with improved reproducibility and precision.Citation16 Although several nomograms for manual LRIs exist,Citation17–Citation19 these nomograms may require adjustments when being applied to femtosecond laser devices. This is the first study to report astigmatism analyses of single, non-paired, non-penetrating intrastromal LRIs performed using a femtosecond laser platform.
The current study’s nomogram demonstrated mixed findings of success. Only the ATR astigmatism class demonstrated a significant reduction in Kcyl postoperatively. This may be explained by the standardized temporal main corneal incisions for all patients. These main corneal wounds could have had an additive astigmatic effect with the LRIs when treating ATR astigmatism – thereby potentially acting similarly to paired LRIs. The nomogram still yielded some success in the total population, however, as 29.9% and 63.3% of eyes achieved ≤0.5D at POM3 in Kcyl and refractive astigmatism, respectively ( and ). The nomogram undercorrected on aggregate analysis (total study population CI=0.79), and particularly within the WTR astigmatism subgroup given the CI (0.73) and FE (0.14D). Collectively, these findings suggest increasing the magnitude of astigmatic reduction could improve overall efficacy of the nomogram. Specifically, with the CoA of 1.26 on total population analysis, increasing the SIA in future cases by 26% may improve future outcomes. Methods of increasing SIA include utilizing larger arc lengths, pairing LRI incisions, or moving LRI placement centrally.Citation5
The consistent placement of the main corneal incision temporally was used to control the main corneal incisions’ astigmatic effect from confounding the effect of the intrastromal LRIs. Therefore, the differences in SIA represented the changes induced by the LRIs. The purpose of the study was to ascertain the astigmatic changes under the current nomogram in order to identify areas for nomogram refinement. Although the consistent temporal incisions may have contributed to a reduction in astigmatism in the ATR group, the aggregate analyses demonstrated that this group remained undercorrected. Therefore, increasing SIA by increasing the intrastromal LRI magnitude of effect may help to further improve patients’ refractive outcomes.
Examination of individual CIs (), however, illustrated that approximately one third of patients corrected>125%. ATR astigmatism was a predictor of overcorrection, which – as previously discussed – may be a result of the additive effect of the temporal main corneal incision for all patients. Arc length was also a predictor of overcorrection, with 30º-arcs proportionally having greater rates of overcorrection than other arc lengths. This study’s nomogram, which was based on the surgeon group’s previous clinical experience as a starting point, set the lower limit to 0.2D Kcyl in order to reflect the group’s anticipated low astigmatic changes induced by single arc 30º intrastromal LRIs. The overcorrection demonstrated in this study, however, was correlated with lower preoperative astigmatism values, suggesting that although the nomogram undercorrected overall, caution to avoid overcorrections should be considered when correcting preoperative astigmatism <0.7D ().
Of the few studies that have investigated femtosecond laser AK or LRI efficacy, all reported their nomograms undercorrected.Citation7,Citation20,Citation21 Both Baharozian et al’s anterior penetrating transepithelial LRIs and Day et al’s paired intrastromal AKs significantly reduced corneal astigmatism, although their nomograms systematically undercorrected.Citation7,Citation20 In Day et al’s study, however, this was the intended outcome as they aimed to provide 70% astigmatism magnitude correction in order to avoid overcorrections.Citation7 Chan et al also found their personal nomogram of single, non-paired, femtosecond anterior penetrating AKs undercorrected overall, but overcorrections were seen when preoperative astigmatism <1.0DCitation21 – a finding similar to the current study.
The arithmetic mean AoE approached zero in the total population, suggesting that there was no systematic trend of error on the LRIs. However, a wide distribution of AoE was found. Variability in AoE is a common finding reported in other studies of manual and femtosecond laser incisionsCitation7,Citation21,Citation22 and may be a result of imperfect intrastromal LRI placement,Citation7 inconsistency in aligning the incisions to the steepest meridian,Citation21 eye movement or clinically significant cyclotorsion during FLACS,Citation23 or keratometer measurement error. However, iris registration technology in femtosecond laser systems allow for automatic cyclotorsion compensation, which assist in achieving accurate and precise LRIs placement at the intended meridian. Therefore, it is the authors’ opinion that keratometer measurement error of axis may represent the strongest contributing factor of the variability in AoE. This measurement error may have affected the study at both the preoperative surgical planning phase as well as the postoperative outcome analysis phase. It is well-known that rotational error can negatively affected astigmatism treatment.Citation24 As such, in order to improve the predictability of astigmatic change, future studies should explore and compare the AoE in a treatment population between keratometric devices and manifest values in order to determine whether a true pattern of rotational error exists and elucidate methods of improving accurate LRI placement. In addition, the WTR astigmatism subgroup demonstrated a slight clockwise trend in AoE (). Due to the wide distribution of AoE, larger sample sizes in the WTR subgroup would be prudent to determine whether this clockwise trend was due to random effects or systematic treatment error.
Although the current nomogram’s efficacy may be improved by increasing SIA, overcorrections were seen at all arc lengths (). This finding suggests that narrower ranges for arc length selection may be useful for avoiding overcorrections and improving precision of the LRIs’ effects. For example; the personal nomogram utilized by Day et al increased intrastromal AK arc length by 5º every 0.25D increase in preoperative astigmatism, and demonstrated superior results with their nomogram.Citation7
Age and CCT were not associated with overcorrections in the current study. However, clinical factors that could improve predictability if added to the nomogram should be explored in the future. Other reported nomograms have utilized age, astigmatism class, and CCT to yield more predictable astigmatic reduction when performing LRIs.Citation25–Citation27 In addition to age and astigmatism class, Day et al examined intrastromal AKs and found that preoperative measurements of corneal hysteresis and corneal resistance factor were predictors of SIA.Citation28 Therefore, expanding the current understanding of the effects of femtosecond laser incisions on corneal biophysical properties may provide useful information for nomogram refinement.
As seen in , there was a high variance in CI between eyes. This was in part expected as previous studies with manual AKs demonstrated similar variance and unpredictability.Citation5,Citation29 The variance in this study may be a result of the variance in AoE, device measurement error, or excessively wide ranges for arc length selection. Furthermore, as alluded to by Visco et al,Citation30 unaccounted posterior corneal astigmatism may cause error in predicting residual refractive astigmatism and therefore may be an additional cause of CI variance. As such, the influence of LRIs on posterior corneal astigmatism should be investigated in the future.
A strength of this study was the 3-month postoperative follow-up. This follow-up was longer than other AK analyses reported in literature.Citation7,Citation20,Citation21 Lim et al found that keratometric astigmatism continued to change until 10 weeks, but then remained stable from 10-weeks to 3-years.Citation31 Therefore, the 3-month postoperative follow-up in the current study may have allowed for LRIs to stabilize, yielding more valid measurements over the long-term.Citation32 Another strength of the study was the standardization of surgical technique across all patients. By standardizing the corneal incisions, the LRI arc length was the sole independent variable changed between patients. Therefore, the difference between preoperative and postoperative Kcyl was a direct metric of the LRI arc length.
There are a number of limitations to this study. Firstly, its retrospective design precluded randomization to control for potential confounding variables and may have introduced selection bias. Secondly, different devices were used for calculating preoperative and postoperative astigmatism, which may have affected the validity of comparisons between these values. Agreement and interchangeability between IOLMaster and Pentacam cylinder and axis measurements have shown mixed results in the literature, although most studies suggest significant differences in corneal power measurements between the two devices.Citation33–Citation37 The rationalization of using IOLMaster preoperatively was to reflect real-world applicability of which device surgeons use most commonly, and one in which would also ultimately guide LRI titration for the majority. To ensure this comparison was valid, the authors performed an analysis comparing preoperative and postoperative Pentacam values, which did not demonstrate differences in outcomes compared to the primary analysis with IOLMaster (). In addition, preoperative Kcyl and steep axis values by IOLMaster and Pentacam did not significantly differ (), further suggesting that the difference in devices was unlikely to have had a major influence. However, further research should attempt to ensure consistency in keratometry devices used throughout study time points for improved accuracy and validity. A third limitation was the low sample sizes for 50º and 60º arc lengths, which created challenges when drawing conclusions about the results for these LRIs. Nomogram adjustments following this analysis suggests that greater arc lengths, such as 50º and 60º arcs, should be attempted. Follow-up astigmatism analysis post-adjustments should better report the efficacy of these arc lengths.
In conclusion, intrastromal LRIs can be conveniently performed during FLACS and appear safe. With the exception of the ATR astigmatism class, the current nomogram of single-arc incisions did not demonstrate a significant reduction in corneal astigmatism 3-months postoperatively. This was largely due to systematic undercorrection. Recommended nomogram adjustments include increasing the SIA with a target of 26% increase in magnitude of astigmatic reduction, exercising caution when treating astigmatism <0.7D to avoid overcorrections, and applying narrower ranges of preoperative astigmatism for each arc length to potentially reduce risk of overcorrection and improve predictability of correction. Future nomogram refinements can be achieved through subsequent astigmatism analysis following these nomogram adjustments and elucidating clinical factors to improve predictability if added to the nomogram.
Ethics and Consent Statement
This study was approved by the Research Ethics Board of William Osler Health System (Brampton, Ontario, Canada, Reference number 18-0023). We confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Author Contributions
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed.
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Steve A. Arshinoff, MD, FRCSC for his support through a medical student scholarship provided by The Eye Foundation of Canada, Toronto, Ontario, Canada. American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting, San Diego, USA, May 2019; Canadian Ophthalmology Society (COS) Annual Meeting, Quebec City, Canada, June 2019.
Disclosure
Dr Sohel Somani works as a consultant for Bayer and Novartis, outside the submitted work. We confirm that there are no other known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
References
- Hayashi K, Manabe S, Yoshida M, Hayashi H. Effect of astigmatism on visual acuity in eyes with a diffractive multifocal intraocular lens. J Cataract Refract Surg. 2010;36(8):1323–1329. doi:10.1016/j.jcrs.2010.02.016
- Watanabe K, Negishi K, Kawai M, Torii H, Kaido M, Tsubota K. Effect of experimentally induced astigmatism on functional, conventional, and low-contrast visual acuity. J Refract Surg. 2013;29(1):19–24. doi:10.3928/1081597X-20121211-01
- Hoffmann PC, Hütz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36(9):1479–1485. doi:10.1016/j.jcrs.2010.02.025
- Guan Z, Yuan F, Yuan Y-Z, Niu W-R. Analysis of corneal astigmatism in cataract surgery candidates at a teaching hospital in Shanghai, China. J Cataract Refract Surg. 2012;38(11):1970–1977. doi:10.1016/j.jcrs.2012.07.025
- Price FW, Grene RB, Marks RG, Gonzales JS. Astigmatism reduction clinical trial: a multicenter prospective evaluation of the predictability of arcuate keratotomy. Evaluation of surgical nomogram predictability. ARC-T Study Group. Arch Ophthalmol. 1995;113(3):277–282. doi:10.1001/archopht.1995.01100030031017
- Mastropasqua L, Toto L, Mastropasqua A, et al. Femtosecond laser versus manual clear corneal incision in cataract surgery. J Refract Surg. 2014;30(1):27–33. doi:10.3928/1081597X-20131217-03
- Day AC, Lau NM, Stevens JD. Nonpenetrating femtosecond laser intrastromal astigmatic keratotomy in eyes having cataract surgery. J Cataract Refract Surg. 2016;42(1):102–109. doi:10.1016/j.jcrs.2015.07.045
- Venter J, Blumenfeld R, Schallhorn S, Pelouskova M. Non-penetrating femtosecond laser intrastromal astigmatic keratotomy in patients with mixed astigmatism after previous refractive surgery. J Refract Surg. 2013;29(3):180–186. doi:10.3928/1081597X-20130129-09
- Alpins NA. A new method of analyzing vectors for changes in astigmatism. J Cataract Refract Surg. 1993;19(4):524–533. doi:10.1016/S0886-3350(13)80617-7
- Alpins N. Astigmatism analysis by the Alpins method. J Cataract Refract Surg. 2001;27(1):31–49. doi:10.1016/S0886-3350(00)00798-7
- Alpins NA, Goggin M. Practical astigmatism analysis for refractive outcomes in cataract and refractive surgery. Surv Ophthalmol. 2004;49(1):109–122. doi:10.1016/j.survophthal.2003.10.010
- Alpins N, Stamatelatos G. Outcomes analysis in a clinical setting. CRST Europe. 2015;10(2).
- Alpins NA. Vector analysis of astigmatism changes by flattening, steepening, and torque. J Cataract Refract Surg. 1997;23(10):1503–1514. doi:10.1016/S0886-3350(97)80021-1
- Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(1):17. doi:10.1186/1751-0473-3-17
- Gauvin M, Wallerstein A. AstigMATIC: an automatic tool for standard astigmatism vector analysis. BMC Ophthalmol. 2018;18(1). doi:10.1186/s12886-018-0920-1
- Hoffart L, Proust H, Matonti F, Conrath J, Ridings B. Correction of postkeratoplasty astigmatism by femtosecond laser compared with mechanized astigmatic keratotomy. Am J Ophthalmol. 2009;147(5):779–787, 787.e1. doi:10.1016/j.ajo.2008.12.017
- Monaco G, Scialdone A. Long-term outcomes of limbal relaxing incisions during cataract surgery: aberrometric analysis. Clin Ophthalmol. 2015;9:1581–1587. doi:10.2147/OPTH.S89024
- Kim DH, Wee WR, Lee JH, Kim MK. The short term effects of a single limbal relaxing incision combined with clear corneal incision. Korean J Ophthalmol. 2010;24(2):78–82. doi:10.3341/kjo.2010.24.2.78
- Sharma BR, Kumar A. Preliminary experiences with limbal relaxing incision for treatment of astigmatism during phacoemulslfication. Nepal J Ophthalmol. 2009;1(2):90–94. doi:10.3126/nepjoph.v1i2.3681
- Baharozian CJ, Song C, Hatch KM, Talamo JH. A novel nomogram for the treatment of astigmatism with femtosecond-laser arcuate incisions at the time of cataract surgery. Clin Ophthalmol. 2017;11:1841–1848. doi:10.2147/OPTH.S141255
- Chan TCY, Cheng GPM, Wang Z, Tham CCY, Woo VCP, Jhanji V. Vector analysis of corneal astigmatism after combined femtosecond-assisted phacoemulsification and arcuate keratotomy. Am J Ophthalmol. 2015;160(2):250–255.e2. doi:10.1016/j.ajo.2015.05.004
- Roberts HW, Wagh VK, Sullivan DL, Archer TJ, O’Brart DPS. Refractive outcomes after limbal relaxing incisions or femtosecond laser arcuate keratotomy to manage corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2018;44(8):955–963. doi:10.1016/j.jcrs.2018.05.027
- Hummel CD, Diakonis VF, Desai NR, Arana A, Weinstock RJ. Cyclorotation during femtosecond laser-assisted cataract surgery measured using iris registration. J Cataract Refract Surg. 2017;43(7):952–955. doi:10.1016/j.jcrs.2017.04.034
- Ma JJK, Tseng SS. Simple method for accurate alignment in toric phakic and aphakic intraocular lens implantation. J Cataract Refract Surg. 2008;34(10):1631–1636. doi:10.1016/j.jcrs.2008.04.041
- Nichamin LD. Nomogram for limbal relaxing incisions. J Cataract Refract Surg. 2006;32(9):1408;author reply 1408. doi:10.1016/j.jcrs.2006.03.046
- Kaufmann C, Peter J, Ooi K, et al. Limbal relaxing incisions versus on-axis incisions to reduce corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2005;31(12):2261–2265. doi:10.1016/j.jcrs.2005.08.046
- Eliwa TF, Abdellatif MK, Hamza II. Effect of limbal relaxing incisions on corneal aberrations. J Refract Surg. 2016;32(3):156–162. doi:10.3928/1081597X-20160121-02
- Day AC, Stevens JD. Predictors of femtosecond laser intrastromal astigmatic keratotomy efficacy for astigmatism management in cataract surgery. J Cataract Refract Surg. 2016;42(2):251–257. doi:10.1016/j.jcrs.2015.09.028
- Agapitos PJ, Lindstrom RL, Williams PA, Sanders DR. Analysis of astigmatic keratotomy. JCRS. 1989;15(1):13–18. doi:10.1016/S0886-3350(89):80134-8
- Visco DM, Bedi R, Packer M. Femtosecond laser-assisted arcuate keratotomy at the time of cataract surgery for the management of preexisting astigmatism. J Cataract Refract Surg. 2019;45(12):1762–1769. doi:10.1016/j.jcrs.2019.08.002
- Lim R, Borasio E, Ilari L. Long-term stability of keratometric astigmatism after limbal relaxing incisions. J Cataract Refract Surg. 2014;40(10):1676–1681. doi:10.1016/j.jcrs.2014.01.045
- Vickers LA, Gupta PK. Femtosecond laser-assisted keratotomy. Curr Opin Ophthalmol. 2016;27(4):277–284. doi:10.1097/ICU.0000000000000267
- Lee BW, Galor A, Feuer WJ, et al. Agreement between Pentacam and IOLMaster in patients undergoing toric IOL implantation. J Refract Surg. 2013;29(2):114–120. doi:10.3928/1081597X-20130117-06
- Huang J, Liao N, Savini G, et al. Comparison of anterior segment measurements with Scheimpflug/Placido photography-based topography system and IOLMaster partial coherence interferometry in patients with cataracts. J Ophthalmol. 2014;2014:540760. doi:10.1155/2014/540760
- Karunaratne N. Comparison of the Pentacam equivalent keratometry reading and IOLMaster keratometry measurement in intraocular lens power calculations. Clin Experiment Ophthalmol. 2013;41(9):825–834. doi:10.1111/ceo.12124
- Utine CA, Altin F, Cakir H, Perente I. Comparison of anterior chamber depth measurements taken with the Pentacam, Orbscan IIz and IOLMaster in myopic and emmetropic eyes. Acta Ophthalmol. 2009;87(4):386–391. doi:10.1111/j.1755-3768.2008.01278.x
- Dong J, Tang M, Zhang Y, et al. Comparison of anterior segment biometric measurements between Pentacam HR and IOLMaster in normal and high myopic eyes. PLoS One. 2015;10(11):e0143110. doi:10.1371/journal.pone.0143110