Abstract
Purpose
To evaluate the intraocular pressure (IOP)-lowering effect of adding dorzolamide 1.0%/timolol 0.5% fixed combination (DTFC) to prostaglandin analogs (PGAs) as monotherapy in patients with normal tension glaucoma.
Methods
A prospective, clinical, case-controlled study of patients with normal tension glaucoma. Patients had been on a once-daily night dose of prostaglandins (PGs) as monotherapy and then received DTFC added to PGs for 8 weeks. The IOP was measured at 9 am, week 0 (baseline), week 4, and week 8.
Results
The baseline IOP of 40 patients who had previously been treated by prostaglandin monotherapy was 15.6 ± 2.0 mmHg at baseline. The IOPs at 4 and 8 weeks after adding DTFC to PGs were 13.5 ± 2.1 mmHg and 13.7 ± 2.2 mmHg, respectively. Significant decrease of the IOP was observed at each time point of measurement as compared with the baseline IOP before adding DTFC (P = 0.01). The percent IOP reduction from the baseline IOP at week 4 and week 8 was 13.5% ± 12.3% and 11.7% ± 13.1%, respectively. The percentage of patients who achieved 10% or more IOP reduction from the baseline IOP at week 8 was 62.5%. The baseline IOP was significantly correlated with the percent IOP reduction at week 8 (P = 0.03, r = 0.34).
Conclusion
DTFC therapy added to PGAs as glaucoma monotherapy is effective in patients with normal tension glaucoma.
Introduction
Elevated intraocular pressure (IOP) is a known risk factor for glaucoma progression and reduction of IOP can prevent the progression of glaucomatous optic nerve damage and maintain the visual field.Citation1,Citation2 Normal tension glaucoma (NTG) is a disease characterized by progression of glaucomatous disorders despite the maintenance of IOP within the normal range. Nonetheless, reduction of the IOP is also important in the treatment of NTG, and it has been reported that the target reduction rate of IOP should be set at 30%.Citation1,Citation2
Numerous reports have indicated that prostaglandin analog (PGA) therapies are effective in reducing IOP in NTG.Citation3–Citation12 However, since PGAs produce only approximately a 20% reduction of IOP,Citation3–Citation12 it is considered that the use of prostaglandin (PG) alone may be insufficient for obtaining the required 30% reduction of IOP, according to the reports of the Collaborative Normal-Tension Glaucoma Study Group.Citation1,Citation2 The majority of patients eventually require more than one medication to control their IOP. When IOP control is inadequate with PGAs alone, the addition of one of several therapies, including carbonic anhydrase inhibitor, timolol maleate 0.5%, and fixed combination therapies, has been shown to provide additional IOP control.Citation13–Citation17 However, it is yet unclear which of these therapies are superior in IOP control.
Treatment that is inconvenient or causes undesirable side effects encourages nonadherence in patients with glaucoma. By contrast, reducing the number of products and the number of required daily instillations increases patients’ adherence.Citation18–21 In recent years, various fixed combinations have been introduced in the market. Combining two medications in one bottle may reduce the time required to administer drops, the frequency or total number of drops, and the number of medication bottles. Therefore, the combination formulation may improve the rate of compliance and consequently improve IOP control.
There are many reports that have demonstrated the efficacy of these drugs in lowering the IOP in patients with primary open-angle glaucoma.Citation13–Citation17 However, there are no reports on the effect of the fixed combination of dorzolamide 1.0%/timolol maleate 0.5% (DTFC) (Cosopt®; MDS/Santen, Tokyo/Osaka, Japan) in NTG patients. In addition, there are also no reports on the additional IOP-lowering effect of DTFC in NTG patients treated with PGAs.
Therefore, in this study, the additive IOP-lowering effect of DTFC on PGAs monotherapy as the first-line therapy in Japanese patients with NTG was investigated.
Patients and methods
This was a prospective, clinical, case-controlled study undertaken from December 1, 2010 to May 30, 2011. The study followed the tenets of the Declaration of Helsinki and had the approval of the ethics committee of Mizoguchi Eye Clinic. Written informed consent was obtained from each patient before the start of the study.
Patients with NTG satisfying all of the following inclusion criteria were eligible for the study: (1) having had received PG as first-line glaucoma monotherapy; (2) in cases of bilateral NTG, the eye with higher IOP or the more advanced eye was selected; (3) aged 40 years or over; (4) refractive error of more than −6 dpt; (5) no history of previous treatment of glaucoma.
The exclusion criteria were: (1) patients with exfoliation syndrome; (2) patients with diabetic retinopathy; (3) refractive error of less than or equal to −6 dpt; (4) patients with a history of ocular trauma, ocular inflammatory disease, glaucoma surgery, vitrectomy, and laser trabeculoplasty; (5) patients with conjunctivitis, dry eye or periocular cutaneous disease, possibly affecting the results of the investigation; (6) currently pregnant or nursing women, or women considering pregnancy were also excluded, as were patients with a history of noncompliance, or patients who had participated in another therapeutic drug study within 1 month; (7) history of cerebrovascular and hepatic disease.
NTG was diagnosed when glaucoma hemifield test results were outside the normal limits; the standard deviation had a P-value <0.05; or there was a cluster of three points or more in the pattern of the deviation plot, in a single hemifield, with a P-value <0.05, one of which had to have a P-value <0.01 on the Humphrey Swedish Interactive Thresholding Algorithm 24-2 test (Humphrey Visual Field Analyzer, model 750, Humphrey Instruments, San Leandro, CA) and/or a nerve fiber layer defect combined with a corresponding optic disk change. In addition, the IOP measured by Goldmann applanation tonometer (Haag-Streit, Koniz, Switzerland) was consistently less than 21 mmHg. Gonioscopy excluded angle closure, rubeosis, and secondary glaucoma.
Patients who had previously received PG as glaucoma monotherapy, and needed to receive additive medications on PGAs monotherapy because of inadequate IOP reduction, were enrolled in the study. Twenty-four eyes were given tafluprost 0.005% (TAPROS®; Santen Pharmaceutical Co Ltd, Osaka, Japan), ten eyes were given latanoprost 0.005% (Xalatan®; Pfizer, Inc, New York, NY), and six eyes were given travoprost 0.004% (TRAVATANZ®, Alcon Laboratories Inc, Fort Worth, TX). Patients received PG at a dose of one drop every evening (9 pm) to the study eye. IOP at the start of the study served as the baseline IOP. Once-daily administration (at 9 pm) of PG as monotherapy was continued and DTFC was added twice daily (9 am and 9 pm) for 8 weeks after the baseline visit. All patients enrolled in the study underwent a comprehensive ocular examination, including measurement of best-corrected visual acuity, slit lamp examination, and biomicroscopic fundus examination. Static gonioscopy was performed using Goldmann 2-mirror lens (Haag-Streit) at the first visit or before cataract surgery. IOPs were measured three times at 9 am using a Goldman applanation tonometer. If the difference in IOP between any two of the three measurements was greater than 3 mmHg, the median value was used. If it was less than 3 mmHg, the mean value was used.
Statistical analysis
Baseline and post-treatment values were compared by a one-way repeated-measures analysis of variance test. The difference in IOP was assessed by a paired t-test. Correlation between the baseline IOP and percent IOP reduction was analyzed by Spearman’s correlation coefficient by rank test.
A P-value of <0.05 (two-tailed) was regarded as statistically significant. Statistical analysis was performed using Statistical Analysis System (v 8; SAS Institute Inc, Tokyo, Japan) software.
Results
In total, 44 patients (44 test eyes) were enrolled in this study. One patient dropped out of the study prematurely because of change of residence and discontinued their visit to the clinic. In three patients, treatment was discontinued because of the development of conjunctival hyperemia and ocular irritation. After excluding these four cases, 40 patients were included in the final analysis.
The demographic features are summarized in . All patients were Japanese. The mean age was 69.8 ± 12.0 years (range: 43–85 years). There were four males and 36 females.
Table 1 Demographic characteristics of the participants in the study
shows the IOP and the percent IOP reduction from the baseline IOP at weeks 4 and 8. Significant decrease of the IOP was observed at weeks 4 and 8 as compared with the baseline IOP (P = 0.01). The percent reduction from the baseline IOP at weeks 4 and 8 was 13.5% ± 12.3% and 11.7% ± 13.1%, respectively.
Table 2 The mean intraocular pressure (IOP) and percent reduction (%) at each time point from baseline IOP
shows the IOP and percent IOP reduction at each time point of measurement of the patient with a baseline IOP of 15 mmHg or higher (the high teens group) and those with a baseline IOP of less than 15 mmHg (the low teens group). In the high teens group, significant additional IOP reduction was observed at each time point of measurement as compared with the baseline IOP (P = 0.01). However, in the low teens group, there was no significant difference in the IOP from baseline IOP at any time point (P = 0.182). The percent reduction at week 8 from the baseline in the high teens group and the low teens group was 14.7% ± 11.7% and 6.6% ± 14.2%, respectively.
Table 3 The mean intraocular pressure (IOP) and percent reduction (%) at each time point from baseline IOP in the subgroups
and show the number and percentage of patients who achieved 10% or more, 20% or more, or 30% or more IOP reduction from baseline at week 8. The percentage of patients whose IOP reduction was 10% or more, 20% or more, or 30% or more was 62.5%, 22.5%, and 5%, respectively (). In the high teens group and low teens group, those were 72% and 53%, 32% and 13%, and 8% and 0%, respectively ().
Table 4 The number of subjects who experienced a percentage decrease of intraocular pressure (IOP) at week 8 from baseline IOP
Table 5 The number of subjects who experienced a percentage decrease of intraocular pressure (IOP) at week 8 from the baseline IOP in the subgroups
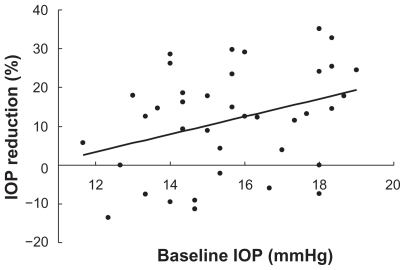
shows the correlation between the baseline IOP and percent IOP reduction using Spearman’s correlation coefficient by rank test. The baseline IOP was significantly correlated with the percent IOP reduction (P = 0.03, r = 0.34).
Figure 1 The correlation between the baseline intraocular pressure (IOP) and percent IOP reduction by using Spearman’s correlation coefficient by rank test.
The most frequent adverse events related to the study medications were blurred vision, eye irritation, ocular hyperemia, and eye itching (). There were no systemic adverse events.
Table 6 Adverse events
Discussion
A number of reports have indicated that DTFC might be effective in lowering the IOP in patients with glaucoma.Citation13–Citation17 In addition, there are reports that the IOP-lowering effect of the dorzolamide/timolol fixed combination may be equivalent to that of concomitant dorzolamide/timolol therapy, and other reports suggesting that the fixed combination therapy has a significant IOP-lowering effect.Citation14,Citation16,Citation17 All these studies were conducted in patients with primary open-angle glaucoma who have elevated IOPs. There have been no reports on the efficacy of DTFC in NTG patients, reported for the first time in this study. The reported IOP-lowering effect of PG is approximately 20% in NTG patients, which suggests that PGA alone is insufficient for obtaining the target 30% or more reduction of IOP,Citation3–Citation11 according to the report of the Collaborative Normal-Tension Glaucoma Study Group.Citation1,Citation2 Glaucoma progression was seen significantly less frequently in the group in which a 30% or more IOP reduction was achieved, as compared with that in the group in which such reduction failed to be achieved.Citation1,Citation2 If the targeted IOP reduction can not be obtained with PGA monotherapy, additional medications are considered necessary. In this study, the IOP decreased significantly and remained low for 2 months with DTFC as second-line therapy, even in patients with NTG who had previously received IOP-lowering therapy with PGA. In 80% of the cases, DTFC have the capability to lower IOP even in patients with NTG. Thus, DTFC was found to have a favorable IOP-lowering effect. In addition, the drug combination produced an approximate additional 13% lowering of the IOP, as compared with the baseline IOP, suggesting that the administration of DTFC can be expected to produce an additional effect in patients in whom the target reduction rate (30%) of IOP has not been achieved by administration of PG alone. Konstas et alCitation15 have reported that the administration of latanoprost and DTFC had the strongest IOP-lowering effect in patients with primary open-angle glaucoma, suggesting that administration of the DTFC in addition to PG might also produce a strong IOP-lowering effect in patients with NTG. However, it was also found that the administration of DTFC had no significant IOP-lowering effect in patients with IOP levels of <15 mmHg prior to the start of treatment, and no effect can be expected in such patients. The IOP was significantly reduced after additional instillation in NTG patients with baseline IOP of >15 mmHg prior to the additional instillation, therefore, additional instillation is considered to be effective in NTG patients with relatively high IOP levels. In addition, a significant correlation was found between the baseline IOP prior to the additional instillation and the percent IOP reduction from the baseline IOP; that is, the higher IOP was prior to the start of the additional administration, the higher the percent IOP reduction from the baseline IOP. These results suggest that additional administration of DTFC is effective, and that it can improve the achievement rate of the target IOP in NTG patients, of which the targeted IOP could not be obtained with PGA monotherapy as first-line therapy.
It is considered important to determine the most effective PGs as the first-line medicine. If the target IOP cannot be achieved with PG monotherapy, additional instillation becomes necessary. It is reported that adherence to glaucoma medication is often poor.Citation18–Citation20 Multiple factors related to poor adherence have been identified, including more frequentCitation18 and complex dosing.Citation20 Therefore, the number of medications should be reduced as much as possible for nonadherence patients. If IOP is inadequate with PGA as first-line medication, fixed combination should be added as second-line medication, because the therapy is less inconvenient.
With regard to safety, DTFC treatment is generally well tolerated. The most common adverse events in this study are eye irritation and blurred vision. All of the adverse events were mild. In this study, there was little change in the eyes after treatment compared with condition at the baseline period, suggesting that adverse events are unlikely to pose a significant clinical problem during use of DTFC.
Conclusion
This study suggests that a significant IOP-lowering effect can be obtained by instillation of DTFC in NTG patients in whom the target IOP has not been achieved by PG instillation alone. Furthermore, the higher the IOP prior to the additional administration, the higher the rate of reduction of IOP became. Taking treatment adherence into consideration, it was considered that DTFC was useful as a second choice drug for use in elderly patients with NTG after treatment with a PG preparation. However, the number of cases in this study was small, and further studies with a larger number of cases are considered necessary.
Disclosure
The authors have no proprietary or commercial interest in any materials discussed in this article.
References
- The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucomaCollaborative Normal-Tension Glaucoma Study GroupAm J Ophthalmol19981264985059780094
- Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressuresCollaborative Normal-Tension Glaucoma Study GroupAm J Ophthalmol19981264874979780093
- RuloAHGreveELGeijssenHCHoyngPFReduction of intraocular pressure with treatment of latanoprost once daily in patients with normal-pressure glaucomaOphthalmology1996103127612828764799
- DranceSMCrichtonAMillsRPComparison of the effect of latanoprost 0.005% and timolol 0.5% on the calculated ocular perfusion pressure in patients with normal-tension glaucomaAm J Ophthalmol19981255855929625541
- HarrisAMigliardiRRechtmanEColeCNYeeABGarzoziHJComparative analysis of the effects of dorzolamide and latanoprost on ocular hemodynamics in normal tension glaucoma patientsEur J Ophthalmol200313243112635671
- TomitaGAraieMKitazawaYTsukaharaSA three-year prospective, randomized and open comparison between latanoprost and timolol in Japanese normal-tension glaucoma patientsEye (Lond)20041898498915037889
- DriksMSNoeckerRJEarlMRohSSilversteinSMWilliamsRDA 3-month clinical trial comparing the IOP-lowering efficacy of bimatoprost and latanoprost in patients with normal-tension glaucomaAdv Ther20062338539416912020
- KiuchiTMotoyamaYOshikaTInfluence of ocular hypotensive eyedrops on intraocular pressure fluctuation with postural change in eyes with normal-tension glaucomaAm J Ophthalmol200714369369517386282
- QuarantaLPizzolanteTRivaITwenty-four-hour intraocular pressure and blood pressure levels with bimatoprost versus latanoprost in patients with normal-tension glaucomaBr J Ophthalmol2008921227123118586898
- SuhMHParkKHKimDMEffect of travoprost on intraocular pressure during 12 months of treatment for normal-tension glaucomaJpn J Ophthalmol200953182319184304
- KuwayamaYKomemusiSIntraocular pressure lowering effect of 0.0015% tafluprost as compared to placebo in patients with normal tension glaucoma: randomized, double-blind, multicenter, phase III studyJ Jpn Ophthalmol Soc2010114436443 Japanese
- AngGSKerseyJPSheptoneLBroadwayDCThe effect of travoprost on daytime intraocular pressure in normal tension glaucoma: a randomised controlled trialBr J Ophthalmol2008921129113318511540
- KonstasAKozobolisVPLallosNChristodoulakisEStewartJAStewartWCDaytime diurnal curve comparison between the fixed combinations of latanoprost 0.005%/timolol maleate 0.5% and dorzolamide 2%/timolol maleate 0.5%Eye (Lond)2004181264126915218522
- ChoudhriSWandMShieldsMBA comparison of dorzolamide-timolol combination versus the concomitant drugsAm J Ophthalmol200013083283311124307
- KonstasAGMikropoulosDDimopoulosATMoumtzisGNelsonLAStewartWCSecond-line therapy with dorzolamide/timolol or latanoprost/timolol fixed combination versus adding dorzolamide/timolol fixed combination to latanoprost monotherapyBr J Ophthalmol2008921498150218703549
- StrohmaierKSnyderEDuBinerHAdamsonsIThe efficacy and safety of the dorzolamide-timolol combination versus the concomitant administration of its components. Dorzolamide-Timolol Study GroupOphthalmology1998105193619449787367
- HutzelmannJOwensSSheddenAAdamsonsIVargasEComparison of the safety and efficacy of the fixed combination of dorzolamide/timolol and the concomitant administration of dorzolamide and timolol: a clinical equivalence study. International Clinical Equivalence Study GroupBr J Ophthalmol199882124912539924327
- NordstromBLFriedmanDSMozaffariEQuigleyHAWalkerAMPersistence and adherence with topical glaucoma therapyAm J Ophthalmol200514059860616226511
- OkekeCOQuigleyHAJampelHDAdherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid studyOphthalmology200911619119919084273
- TsaiJCMcClureCARamosSESchlundtDGPichertJWCompliance barriers in glaucoma: a systematic classificationJ Glaucoma20031239339814520147