Abstract
Dry eye disease (DED) is a multifactorial disease of the ocular surface and tear film that has gained awareness as a public health problem. Characteristics of DED include tear film instability, hyperosmolarity, and ocular surface inflammation, which can occur independently or may be a sequela of numerous ocular diseases, ocular surgery or contact lens wear. Much has been learned about the impact of the disease to help affected individuals who report symptoms of poor vision, pain, and tearing. Recently, new research highlights the importance of the role of ocular surface inflammation and damage in DED—leading to a vicious cycle of inflammation as well as loss of tear film homeostasis. DED immunopathophysiology is characterized by four stages: initiation, amplification, recruitment, and re-initiation. Cyclosporine is proven to be a valuable ophthalmic therapeutic for DED through its immunomodulatory actions and regulation of the adaptive immune response. Cyclosporine mechanism of action is well described in the published literature and the myriad of benefits in all four stages lend a broad-based immunomodulatory function particularly suitable for addressing DED. Furthermore, cyclosporine has unique goblet cell density improvement capabilities as well as anti-apoptotic properties. Topical formulations of cyclosporine are centered around addressing the highly lipophilic nature of the molecule. The poor aqueous solubility of cyclosporine traditionally presented technical challenges in drug delivery to the ocular surface. Newer formulations such as cationic emulsions and nanomicellar aqueous solutions address formulation, tissue concentration, and drug delivery challenges.
Introduction
Dry eye disease (DED), also known as keratoconjunctivitis sicca (KCS), is a common disorder of the eye affecting more than 16 million people in the US.Citation1 The prevalence of DED ranges from 5% to 33% worldwide with increased prevalence among adult women and Asians.Citation1,Citation2 Symptoms of DED include discomfort, pain, burning, foreign body sensation, and visual disturbances.Citation1 Dry eye negatively affects the patient’s quality of life and results in approximately $4 billion in annual costs in the US.Citation3,Citation4
Dry eye is commonly seen alongside many ocular disorders including glaucoma, cataracts, and refractive errors.Citation5–Citation7 Pharmacological treatments for ocular disorders often contain preservatives such as benzalkonium chloride.Citation8 These preservatives can cause DED signs and symptoms including decreased epithelial cell integrity, increased epithelial cell apoptosis, eye irritation, and increased risk for ocular allergies and delayed hypersensitivity reactions.Citation8,Citation9 Surgical interventions to treat ophthalmic disorders and diseases can also cause dry eye due to the resultant inflammation from the length of time in surgery, incision site disruption to the corneal nerves, and type of post-surgical medication.Citation10–Citation12 Post-trabeculectomy patients have elevated tear film osmolarity and symptoms of dry eye;Citation13 intraoperative use of lubricating substances can decrease post-surgical dry eye symptoms and treatment with dry eye medication post-operatively can help alleviate surgery-induced dry eye.Citation11,Citation14 Dry eye symptoms following cataract surgery occur in 42%, 15%, and 9% of eyes of patients at 1 week, 1 month, and 3 months after surgery, respectively.Citation12 Additionally, new research demonstrates the bilateral impact on the corneal sub-basal nerve complex after cataract surgery, potentially explaining worse dry eye symptoms after cataract surgery on the second eye.Citation10
The Tear Film & Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) developed an evidence-based definition of DED in recognition of its multifactorial nature.Citation5 This key working definition of DED published in 2017 states:
Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.Citation5
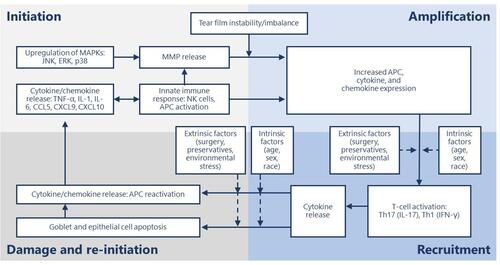
Activation of ocular surface inflammation is characterized by four broad stages: initiation, amplification, recruitment and damage/self-perpetuation.Citation15
In general, the aberrant activation of innate immunity by hyperosmolarity and desiccating stress, along with loss of immunoregulatory controls, results in the conversion to adaptive immunity and sets up a series of vicious circles.Citation15–Citation17 This perpetuating cycle of DED is governed by several immunopathophysiological events starting with hyperosmolarity.Citation18 Tear hyperosmolarity causes damage to the surface epithelium, beginning with an increase in the mitogen-activated protein kinase (MAPK) and nuclear factor kappa B on the ocular surface.Citation18–Citation21 The activation of MAPK triggers the secretion of inflammatory mediators, which facilitate activation of resident dendritic cells and T-cell recruitment to the ocular surface.Citation20,Citation21 Additional inflammatory mediators are released from recruited T cells, which compromises the lacrimal functional unit further, adding to tear hyperosmolarity, which further accentuates cellular damage and loss of epithelial and goblet cells—all leading to a progressive cycle of tear film instability and inflammation ().Citation18,Citation21 Goblet cell loss leads to disturbances in mucin production and loss of immunoregulation from transforming growth factor (TGF)-bCitation22—resulting in epithelial damage and inflammatory cytokine activation. The resultant tear film instability exacerbates ocular surface hyperosmolarity and compounds the vicious cycle.Citation18,Citation19
Figure 1 Stages of dry eye disease and primary effectors.
Cyclosporine A is a neutrally charged hydrophobic molecule with low aqueous solubility, which poses challenges in making a safe and effective ocular drug delivery system.Citation23 Cyclosporine A reduces the underlying inflammation associated with DED that interferes with tear production.Citation24,Citation25 Initially, cyclosporine A ophthalmic solutions were formulated in oil-based solvents such as castor oil or corn oil; however, oils caused side effects such as blurred vision, burning, and stinging, and were poorly tolerated.Citation23 Additionally, these oils provided low bioavailability of cyclosporine A to the ocular targets.Citation26,Citation27 The limitations of oil-based cyclosporine A formulations lead to a need for cyclosporine A formulations with improved tolerability and bioavailability.
This review covers the immunopathophysiology of DED as well as the mechanism of action of cyclosporine A and its role in modulating ocular inflammation, compares the current ophthalmic cyclosporine A formulations, and discusses the limitations and challenges of using cyclosporine A for the treatment of DED.
Ocular Inflammation in the Pathogenesis of DED
The chronic inflammation that accompanies DED involves the innate and adaptive immune response and progresses through four stages: initiation, amplification, recruitment and damage/re-initiation ().Citation15 Throughout the inflammatory response, immune cells release proinflammatory cytokines and chemokines, which recruit more immune cells and eventually results in a vicious cycle of inflammation that does not resolve ().Citation18,Citation20
Table 1 Molecules Involved in the Inflammatory Response of Dry Eye Disease
During the initiation stage, ocular stress increases inflammatory cytokine expression and activates MAPKs, including c-Jun N-terminal kinase (JNK), extracellular signal-related kinase, and p38.Citation28 JNK causes transcription of matrix metalloproteases (MMPs).Citation29 Natural killer (NK) cells, macrophages, and dendritic cells are all involved in this phase.Citation15,Citation18 NK cells promote inflammation, which contributes to DED through a variety of mechanisms, including secreting interferon (IFN)-γ and stimulating antigen-presenting cells (APCs).Citation30 IFN-γ, a pro-inflammatory cytokine, upregulates a cluster of differentiation (CD)4+ T cell-associated chemokines.Citation31 Stimulated APCs expressing major histocompatibility complex class II (MHCII) promote differentiation and mediate the survival of CD4+ T cells.Citation32,Citation33 In a mouse model of DED, NK cell depletion decreased interleukin (IL)-17A-producing CD4+ T cells in the ocular surface on day 5 following desiccant stress exposure and reduced expansion of two subsets of APCs, CD11b+MHCII+ and CD11c+MHCII+.Citation34 This suggests NK cells play a role in the inflammatory response of DED.
Macrophages can function as APCs to T cells and are reciprocally activated by T cells.Citation35 Pro-inflammatory macrophages secrete T cell attracting cytokines (IL-1β, tumor necrosis factor-α [TNF-α], IL-12 and IL-23) as well as chemokines (C-X-C motif chemokine ligand [CXCL] 9 and CXCL10) that induce recruitment of T-helper (Th)1 and Th17 inflammatory T cells which then may lead to tissue damage.Citation36 In an experimental mouse model of DED, macrophage depletion reduced the expression of inflammatory mediators including IL-1β, IL-6, IL-17, and C-C motif chemokine ligand (CCL) 5 and CD4+ T cells.Citation37 CCL5 attracts T cells and has been found to be significantly increased in the tears of patients with DED.Citation38 Similarly, IL-6 expression increases in patients with DED and is associated with the release of MMPs, decreased tear production, cell death, and Th17 cell differentiation.Citation20,Citation38,Citation39
Dendritic cells act as the primary APC, and corneal dendritic cell density increases in DED.Citation40 Dendritic cell maturation is induced by CCL19 and CCL21, two C-C motif chemokine receptor (CCR)7 ligands.Citation41,Citation42 These ligands also enhance the proliferation of T cells.Citation41,Citation42 CCL19 additionally up-regulates co-stimulatory molecules: CD86, CD80, and CD40 on dendritic cells.Citation42 CCR7 expression increases during the maturation of dendritic cells.Citation42 Increased receptor expression allows more ligand binding and facilitates interactions with T cells that also express CCR7 and bind CCL19 and CCL21. In a mouse model of DED, culture of CCR7+CD11b+ dendritic cells with IFN-γ−IL-17−CD4+ T cells stimulated T cell proliferation and T cell IL-17 expression.Citation43 Thereby, CCR7 expression significantly contributes to the inflammatory response in DED. Blockade of CCR7 with a topical solution of rat anti-CCR7 impairs the induction of acute DED and the progression of acute to chronic DED as measured by corneal fluorescein staining.Citation44 This results from reduced inflammatory cytokine expression at the ocular surface and retention of epithelial cell integrity due to decreased expression of MMP-3, TNF-α, IL-1β and Th17 cell secreted IL-17 after CCR7 blockade.Citation44 In an in vivo study of cyclosporine A treated dendritic cells, CCR7 expression was inhibited while maintaining normal expression of CCR1, CCR2, and CCR5 antibodies—resulting in decreased expression of inflammatory cytokines.Citation45 Subsequently, cyclosporine A actively counters the inflammatory response through inhibition of CCR7, disturbance of dendritic cell migration, and interaction with T cells—modulating the adaptive immune response.Citation45
The initiation phase transitions to the amplification stage as pro-inflammatory cytokine expression and T-cell-attracting chemokine expression increase APC activity and production of CD4+ T-cell subsets, resulting in amplification of the immune response.Citation15 The resultant cytokine, chemokine, and immune cell presence drive the inflammatory response to the next stage.
During the recruitment stage, activated T-cells migrate to the conjunctival stroma where they reactivate resident APCs, and the T cells are recruited to the ocular surface.Citation15 Desiccating stress increases the number of CD4+CCR6+IL-17+ and CD4+ C-X-C motif chemokine receptor (CXCR)3+IFN-γ+ Th17 and Th1 cells in an experimental mouse model of DED, respectively.Citation46 Th17 expression of CCR6 and Th1 expression of CXCR3 is necessary for the development of DED; mice that lack these receptors do not develop DED, and IL-6, IL-13, IL-17A, IFN-γ, MMP-3, and MMP-9 expression is inhibited.Citation46 Additionally, the expression of CCL20 and CCR6 ligand at the ocular surface is also decreased in DED.Citation47 Blocking CCL20 inhibits Th17 cell migration to the ocular surface and decreases IL-6, IL-23, MMP-3, TNF-α, and IFN-γ messenger (m)RNA expression in the conjunctiva.Citation47 Th17 cells are resistant to T regulatory cell (Treg) functions and antagonistic to Treg activity.Citation48 Blockade of IL-17, a cytokine produced by Th17 cells, restored Treg function in mice treated with anti-IL-17 antibody compared to control mice.Citation48 Desiccating stress also increases expression of Th1-associated chemokines, CXCL9 and CXCL10, via NK-cell mediated IFN-γ expression.Citation31 Resultantly, Th1 and Th17 cytokines damage the ocular surface and reinitiate the inflammatory cycle.
Throughout the first three stages of the chronic inflammatory response, increased TNF-α and IFN-γ expression cause epithelial cell apoptosis and goblet cell loss, respectively.Citation49,Citation50 Excessive loss of goblet cells and epithelial apoptosis may result in a loss of immunoregulatory mechanisms, allowing the immune response to be amplified.Citation15 In a desiccating stress mouse model of DED, IFN-γ knockout mice had less active caspase 3 and caspase 8, indicating decreased apoptosis.Citation51 Similarly, topical neutralization of IFN-γ with rat anti-mouse IFN-γ IgG1 decreased desiccating stress-induced conjunctival goblet cell loss, reduced epithelial apoptosis, and increased IL-13 expression.Citation52 Goblet cells provide essential immunoregulatory functions by secreting TGF that downregulates dendritic cell expression of MHC class II and co-stimulatory molecules CD80, CD86 and CD40. These actions help to maintain the immature and immunotolerant state of the dendritic cell, which prevents T cell activation.Citation22 Further evidence of the immunoregulatory role of goblet cells is seen in a goblet cell-deficient and SAM-pointed domain epithelial-specific transcription factor (Spdef)-null (sterile α motif pointed domain epithelial-specific transcription factor) mouse model, where findings mimicking DED in human patients was seen with increased macrophages and CD11b+CD11c+ dendritic cellsCitation53 as well as increased expression of proinflammatory IL-1α, IL-1β, TNF-α.Citation54 An increase in macrophages and dendritic cells leads to the recruitment of T cells and supports the continued release of inflammatory mediators as well as tissue damage.
Numerous cytokines and chemokines have altered expression in DED and contribute to the severity of symptoms. In a cohort of patients with DED, CCL2, CCR2, and CXCR4 mRNA levels significantly increased, while CXCL12 levels trended towards an increase.Citation40 All these molecules modulate nociceptive signals and drive nerve pain.Citation40,Citation55 Concentrations of IL-8 (CXCL8), and macrophage inflammatory protein (MIP)-1α (CCL3) were significantly increased in the tears of patients with DED, while epidermal growth factor (EGF) concentrations were significantly decreased.Citation38 The severity of irritation symptoms positively correlated with IL-6 and IL-8 concentrations, and Schirmer scores positively correlated with EGF and inversely correlated with IL-10, IL-8, MIP-1α, IL-1α, IL-1β, IL-6, IL-1Ra, fractalkine (CX3CL1), IP-10 (CXCL10) and vascular endothelial growth factor concentrations.Citation38,Citation56 EGF negatively correlated with corneal fluorescein staining and conjunctival lissamine green staining, but IFN-γ, IL-8, MIP-1α, IL-1α, IL-1β and IL-6 concentrations positively correlated with these scores.Citation38,Citation56
Mechanism of Action of Cyclosporine A
Cyclosporine A was isolated from the fungus Tolypocladium inflatum.Citation57 The immunosuppressive activity of cyclosporine was discovered in 1976, leading to immunological tests and investigations into its structure and synthesis.Citation58 Cyclosporine A was the first immunosuppressive drug that allowed selective immunoregulation of T cells without excessive toxicity and was used in routine organ transplantation to counter graft rejection.Citation59
Topical cyclosporine A was developed to increase tear production in patients with DED who are refractory to conservative treatments such as ocular lubricants and lid hygiene, and its efficacy is well established.Citation60,Citation61 TFOS DEWS II recommends a stepwise approach to DED treatment, starting with education, dietary modification, lid hygiene, lubricating eye drops, and environmental modifications.Citation5,Citation61 If patients with DED are not adequately treated with ocular lubricants or lid hygiene, non-pharmacologic treatment can be used along with prescription medications such as topical cyclosporine A.Citation61,Citation62 However, because of the increasing awareness of the chronic and progressive nature of DED, cyclosporine A is also used for less severe DED in clinical practice.Citation3
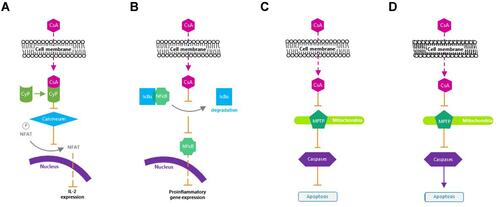
Cyclosporine A is a calcineurin inhibitor that exerts immunomodulatory effects by blocking T cell infiltration, activation, and the subsequent release of inflammatory cytokines.Citation25,Citation63,Citation64 It enters the cytoplasm of T cells, binds to cyclophilin, and forms a cyclosporine A/cyclophilin complex that prevents calcineurin-mediated de-phosphorylation of nuclear factor of activated T cells and the transcription of cytokine genes, including those of IL-2 and IL-4.Citation65 Cyclosporine A additionally inhibits p38 activation and JNK activation, which lead to IL-2 production.Citation66 The subsequent reduction in IL-2 levels further reduces the function of effector T cells.Citation67
The action of cyclosporine A on T cells is the primary mechanism for DED symptom improvement; however, its effects may extend beyond T cell modulation. Twice-daily treatment for 2 weeks with cyclosporine A decreased expression of proinflammatory cytokines and chemokines IL-1β, TNF-α, IL-6, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in a murine model of benzalkonium chloride-induced DED.Citation68 Outside of DED, cyclosporine A affects cytokine expression and MHC expression. In an experimental rat model of autoimmune uveitis, cyclosporine A decreased levels of IL-1, IL-6, and TNF-α relative to the uveitis-induced placebo group.Citation69
Moreover, cyclosporine A protects human conjunctival epithelial cells via its anti-apoptotic action, as well as improves conjunctival goblet cell density and corneal surface integrity via its immunomodulatory activities.Citation25,Citation61 Cyclosporine A blocks mitochondrial permeability transition pore opening in mitochondria, Fas/Fas ligand upregulation, and caspase activation,Citation25 which are considered important aspects of its therapeutic efficacy for ocular inflammation in DED ().Citation25 The reduction in T cell recruitment and activation by cyclosporine A decreases IFN-γ expression, which has been linked to epithelial cell and goblet cell apoptosis.Citation50,Citation70
Figure 2 Mechanism of action of cyclosporine A. (A) Inactivates T cells. (B) Inhibits the release of inflammatory cytokines. (C) Prevents apoptosis of conjunctival epithelial cells. (D) Induces apoptosis of activated T cells.
Cyclosporine A for the Treatment of Surgically Induced Dry Eye
Surgical intervention is sometimes required to address ophthalmic diseases.Citation6,Citation13,Citation61 Glaucoma, a major cause of blindness, is often treated with topical medications, but when medications are ineffective or contraindicated, surgery may be necessary.Citation13 Visually significant cataracts may also require surgical removal.Citation71 These surgical interventions may cause nerve damage or inflammation resulting in symptoms that mimic DED. Moreover, the type of equipment used during surgery can influence the development of dry eye symptoms and the necessity for post-operative dry eye treatment.Citation72 New confocal microscopic evidence from cataract surgeries demonstrates surgical insult to the first eye creates corneal nerve sub-basal changes in both eyes, which may compromise the lacrimal functional unit.Citation10
Cyclosporine A provides additional treatment options for patients with surgically induced dry eye. In a study of DED, dry eye symptoms increased at 1 month following laser-assisted in-situ keratomileusis (LASIK), but the use of topical cyclosporine A 0.05% emulsion improved post-LASIK dry-eye symptoms for up to 1 year, returning symptoms to preoperative baseline levels.Citation73 Similarly, in a study of patients with dry eye following cataract surgery, dry eye symptoms improved from baseline after 3 months of twice-daily treatment with cyclosporine A.Citation14 These findings are supported by an in vivo confocal microscopy study in patients with DED, in which twice-daily cyclosporine 0.05% ophthalmic emulsion administration increased intermediate epithelial cell density and decreased the morphologic markers of inflammation and nerve damage.Citation74
Cyclosporine A for the Treatment of Dry Eye Associated Comorbidities
Chronic DED can often result in mechanical stress on the ocular surface—leading to continuous erosion and damage to corneal and conjunctival tissue.Citation75 In a case series of patients with recurrent corneal erosions and refractory persistent epithelial defects, treatment with cyclosporine A 0.05% improved tear film stability, reduced recurrent corneal erosions, and completely healed areas of previous epithelial loss.Citation75 Moreover, the reduction in recurrent corneal erosions paralleled improvements observed in DED signs and symptoms, indicating the decrease in ocular inflammation by cyclosporine A helped reduce mechanical stressors on the eye and improved epithelial integrity.Citation75 Notably, in a recent review of ophthalmic medications with antiviral properties, the use of cyclosporine A for the potential treatment of coronavirus disease 2019-induced conjunctivitis was suggested based on its shown antiviral activity against hepatitis C virus, flavivirus, and influenza.Citation76 Although treatment for coronavirus-induced conjunctivitis with cyclosporine A is not known, patients with DED experiencing secondary viral infections affecting the ocular surface may benefit from cyclosporine A treatment.
Comparing Ophthalmic Cyclosporine A Formulations
Cyclosporine A has traditionally been formulated as an oil-based preparation due to its high lipophilicity and poor water solubility.Citation23,Citation67 However, oil-based solutions often have poor tolerability and low bioavailability.Citation67 Due to the lipophilic nature of cyclosporine A, it has a greater affinity for an oil-based vehicle than for the target tissues. Therefore, aqueous delivery systems aim to increase cyclosporine A bioavailability and reduce adverse reactions.Citation67
An ophthalmic emulsion of 0.05% cyclosporine A (Restasis®; Allergan, Inc., Irvine, CA, USA) was the first topical cyclosporine A approved by the US Food and Drug Administration (FDA) in 2003 to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with KCS ().Citation23,Citation60,Citation61 It is a preservative-free anionic oil-in-water formulation comprising castor oil with polysorbate 80 and carbomer copolymer acting as an emulsifying and stabilizing agent, respectively.Citation23 In two multicenter, randomized, phase 3 trials, twice-daily administration of cyclosporine A 0.05% emulsion for 6 months significantly improved corneal staining and anesthetized Schirmer’s tear test values from baseline compared with vehicle (P ≤0.05).Citation77 Conjunctival staining in temporal and nasal conjunctival zones improved significantly from baseline in both cyclosporine A 0.05% emulsion and vehicle groups, but there was no significant difference between cyclosporine A 0.05% emulsion and vehicle.Citation77 Cyclosporine A 0.05% emulsion significantly (P <0.001) improved tear film breakup time in patients with DED at month 6 in a phase 4 trial.Citation78 Approximately 43% of treated patients experienced adverse events (AEs), the most frequent being instillation site reactions including burning and pain.Citation78
Table 2 Comparison of Approved Formulations of Cyclosporine A
A cationic nanoemulsion of cyclosporine A (Ikervis®; Santen SAS, Evry, France) () was approved by the European Medical Agency in 2015 for patients with severe keratitis that has not improved with artificial tears.Citation23,Citation79 In a phase 3 study, the cationic emulsion of cyclosporine A significantly improved corneal staining over 6 months compared with vehicle (P = 0.037).Citation80 However, in the combined outcome of improvement of ≥2 grades in corneal staining and improvement of ≥30% in ocular surface disease index, the cationic emulsion was not significantly different than vehicle.Citation80 Furthermore, a greater number of patients in the emulsion group vs vehicle group experienced instillation discomfort (29.2% vs 8.9%) and 10% in the cationic emulsion group discontinued the study.Citation80
OTX-101 0.09% (CEQUA™; cyclosporine A 0.09%; Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA) is a clear, aqueous nanomicellar formulation approved by the FDA in 2018 to increase tear production in patients with KCS ().Citation81,Citation82 Nanomicelles are amphiphilic molecules that self-assemble into typical nanometer-sized supramolecular aggregates above the critical micelle concentration in an aqueous medium. Hydrophobic interactions of core-forming units drive the micelle formation with a water-insoluble or hydrophobic core and an outer water-soluble or hydrophilic shell.Citation82 Thus, the nanomicelles encapsulate hydrophobic cyclosporine A within their hydrophilic cores, and that, in turn, favors dispersion and solubility of cyclosporine A into the precorneal tear film.Citation82,Citation83 The small size of nanomicelles (10–80 nm; average of 22 nm) may also allow diffusion through scleral aqueous pores (20–80 nm in size).Citation83
Phase 2b/3 and 3 clinical studies showed twice-daily administration of OTX-101 0.09% was superior to vehicle in increasing tear production and improving ocular signs, including conjunctival and corneal staining in patients with DED as early as 4 weeks.Citation84–Citation87 In a pooled analysis of phase 2b/3 and 3 trials, OTX-101 0.09% significantly improved total corneal staining on day 28 vs vehicle (P = 0.0008),Citation85 and significantly reduced total conjunctival staining from baseline vs vehicle (P = 0.0316) was seen on day 28.Citation86 This reduction in conjunctival and corneal staining suggests an improvement in ocular surface integrity and DED pathology, as indicated in previous studies involving a different cyclosporine A formulation.Citation77 By day 84, significantly more patients had an increase in Schirmer’s score of ≥10 mm from baseline in the OTX-101 0.09% group vs vehicle (16.6% vs 9.0%, respectively, P <0.0001).Citation88
The majority of enrolled patients on OTX-101 0.09% completed phase 2b/3 and 3 studies (93%), with ≤2.9% withdrawing due to AEs.Citation84,Citation86 During the 3-month treatment period, instillation site pain was the most frequent AE (15.1% and 24.2% of patients in the phase 2b/3 and phase 3 studies, respectively).Citation84,Citation87 Most treatment-emergent AEs (TEAEs) were mild or moderate and resolved without treatment.Citation84 There were no serious TEAEs.Citation84,Citation86,Citation87 In patients receiving OTX-101 0.09%, 3.3% and 3.5% of patients withdrew from treatment due to treatment-emergent AEs in the phase 2b/3 and phase 3 studies, respectively.Citation84,Citation87
A preclinical study in New Zealand white rabbits demonstrated a higher cyclosporine A concentration after a single dosing of OTX-101 0.05% compared with the cyclosporine A 0.05% emulsion in most ocular tissue samples, including the cornea (2.18-fold) and superior bulbar conjunctiva (1.76-fold) with minimal systemic exposure.Citation89 There was a dose-related increase in cyclosporine A with repeat dosing of OTX-101 0.05%, which also resulted in higher concentrations of cyclosporine A in ocular tissues and aqueous humor than in the cyclosporine A 0.05% emulsion.Citation89
Differences in Delivery Vehicles
Cyclosporine A 0.05% ophthalmic emulsion is formulated in a homogenous emulsion of glycerin (2.2%), castor oil (1.25%), polysorbate 80 (1.00%), carbomer copolymer type A (0.05%), purified water (to 100%) and sodium hydroxide for pH adjustment.Citation60,Citation67 Other common formulation strengths include 0.5%, 1%, and 2%.Citation67 Injectable cyclosporine A in artificial tears is often used in lower-dose formulations (0.5% and 1%), while higher-dose (2%) formulations are compounded utilizing the oral solution of cyclosporine A in sterile corn or olive oil.Citation67 Tolerability of injectable cyclosporine A is poor due to the high alcohol content—limiting its use to lower-dose formulations.Citation67 However, oil-based deliveries such as oily vehicles or oil-in-water emulsions may pose similar challenges to tolerability such as irritation and blurred vision.Citation27
Cyclosporine A ophthalmic formulations containing lipids are prepared as emulsions, which can be classified as either macroemulsions, nanoemulsions, or microemulsions based on lipid droplet size.Citation61 Smaller droplet size minimizes adverse effects such as discomfort or blurring upon instillation.Citation61 Consequently, in patients with the aqueous tear-deficient form of dry eye and an increased lipid tear film, lipid-based formulations may cause increased discomfort when applied.
Aqueous vehicle preparations were developed with different variations of nanoparticle-based suspensions and/or micellar or nanomicellar solutions (eg, OTX-101).Citation23,Citation90 Preclinical studies support the superior tolerability and greater bioavailability of cyclosporine A with micellar solutions vs oil-based emulsions; the bioavailability exceeds that of cyclosporine 0.05% ophthalmic emulsion and cationic emulsions.Citation90 Moreover, some cationic emulsions potentially induce corneal epitheliopathy, disrupt corneal barrier function, and increase metabolic stress in rabbit corneas.Citation91 In contrast, aqueous vehicles can deliver therapeutically active cyclosporine A levels to tissues of the anterior and posterior ocular segments.Citation67 Outcomes from this study also suggest higher bioavailability and lower elimination rates compared with cyclosporine 0.05% ophthalmic emulsion.Citation67
Additionally, buffering agents used in cyclosporine A ophthalmic formulations are important to patient comfort and safety as well as pharmacologic activity. Buffers are not only necessary to stabilize a formulation’s pH for optimal solubility, tolerability, and activity but should also simulate the natural system of the tear film.Citation92 To date, the most commonly used buffers in ophthalmic formulations include citrate, phosphate, Tris-HCl, and borate.Citation92 Although AEs with citrate, Tris-HCl, and borate are not commonly reported in the literature, phosphate buffers may induce corneal calcification.Citation92 In a recent study evaluating buffer-induced cytotoxicity in a human corneal epithelial and conjunctival cell model, both citrate and phosphate buffers resulted in significant cytotoxic effects at high concentration levels and long incubation times in a cell model of ocular epithelial tissue.Citation92 For currently approved cyclosporine A formulations, sodium hydroxide and sodium phosphate monobasic dihydrate are used as buffering agents for cyclosporine 0.09% ophthalmic solution (OTX-101),Citation81 while sodium hydroxide is the sole buffering agent in both cyclosporine A 0.05% ophthalmic emulsion and cyclosporine A 0.1% ophthalmic emulsion.Citation60,Citation79
Alternative Current Pharmacologic Treatments for DED
According to TFOS DEWS II, the management of DED should be performed in an individualized, step-wise approach.Citation61 Although ocular lubricants are commonly used in the management of early DED, they fail to address the underlying causes of DED and only deliver palliative care.Citation61 Consequently, many patients who are refractory to over-the-counter lubricants turn to prescription medications including limited-duration topical corticosteroids, topical antibiotics (eg, tetracyclines), lymphocyte function-associated (LFA) antigen-1 antagonist, topical secretagogues, and cyclosporine.Citation61 While topical corticosteroids demonstrate effective interruption of the inflammatory and immune response cycle of DED, long-term use can present complications such as ocular hypertension and opportunistic infections.Citation61,Citation93 Tetracyclines are broad-spectrum antibiotics possessing anti-inflammatory properties that effectively treat disorders associated with DED;Citation61 however, risks associated with long-term use of tetracyclines are unknown and long-term use could lead to potential antibiotic resistance. The topical secretagogue diquafosol tetrasodium is approved for the treatment of DED in Japan and South Korea, though in the US, it did not meet primary and secondary endpoints in phase 3 clinical trial and did not receive FDA approval.Citation61,Citation94 Lifitegrast (Xiidra®, Novartis, Basel, Switzerland), a 5% ophthalmic solution of the LFA antigen-1 antagonist, is approved for the treatment of signs and symptoms of DED.Citation95 Lifitegrast targets inflammation by inhibiting T cell recruitment, T cell activation, and subsequent cytokine release.Citation96
Limitations and Challenges of Cyclosporine A for the Treatment of Dry Eye Disease
Ocular targets for drug delivery in DED include the cornea, conjunctiva, tear film, and lacrimal and meibomian glands.Citation18 However, ocular barriers and dynamic mechanisms limit the bioavailability of topically administered drugs to <5%.Citation97 Structures such as the corneal epithelium, stroma, and blood-aqueous barrier impede drug delivery. In addition, tear turnover rate, dilution into tears, blinking, and tear clearance shorten the exposure to topically administered drugs. Drug permeability into the conjunctiva is further limited by extensive vascular and lymphatic drainage.Citation97 Loss of drug volume can also occur due to gravity and nasolacrimal drainage.Citation82
Conclusions
The complex and chronic nature of DED and its potential for progression require ongoing persistence with treatment. Topical cyclosporine A provides a broad-based approach to DED treatment by decreasing inflammation and improving ocular surface integrity with few systemic effects. In particular, cyclosporine A targets the cycle of chronic inflammation in DED through regulation of the various disease stages including initiation, amplification, recruitment and damage/re-initiation. Through immunomodulatory effects, topical cyclosporine A may prevent activation of conjunctival T cells,Citation98 restore conjunctival goblet cell density,99 reduce transcription of inflammatory cytokines such as IL-2,Citation67 and decrease epithelial cell apoptosisCitation25—thereby, interrupting the ongoing immune reaction and cycle. New cyclosporine A formulations such as aqueous nanomicellar formulations address some of the delivery challenges with topical administration of cyclosporine A and may decrease the time to symptom relief, improve tolerability, and enhance patient persistence with therapy. Future treatment of dry eye will likely move towards the development of noninvasive sustained-release cyclosporine A formulations, providing patients with controlled, long-acting treatment.
Acknowledgments
Writing and editorial support for manuscript preparation were provided by Zehra Gundogan, VMD; and Shavonn Harper, PhD (AlphaBioCom, LLC, King of Prussia, PA); and funded by Sun Pharmaceutical Industries, Inc. (Princeton, NJ). All authors met the International Council of Medical Journal Editors criteria and received neither honoraria nor payment for authorship.
Disclosure
LMP reports research support from Lumenis and Olympic Ophthalmics, is a shareholder for Eyedetec and Visant, and reports consulting fees from Alcon; Allergan; Avellino; Azura; EyeVance; Novartis; Science Based Health; Sight Sciences; Sun Pharmaceutical Industries, Inc.; and TearLab. FSM reports equity from Ocular Science during the conduct of the study, received grants from Allergan, reports personal fees from Shire/Takeda outside the submitted work, and is a consultant for Sun Pharmaceutical Industries, Inc.; Novartis; and Allergan. PMK receives fees from Akorn; Alcon; Aldeyra; Allergan; Allysta; Aurinia; Azura; B+L; BioTissue; Blephex; Cambium; Dompe; Eyevance; Eyegate; ; Johnson & Johnson; Kala; Mallinckrodt; Novaliq; Novartis; Oasis; Ocugen; Ocular Science; Oculus; OcuSoft; Olympic Ophthalmics; Regener-Eyes; Science Based Health; Sight Sciences; Sun Pharmaceutical Industries, Inc.; Surface; Tarsus; TearLab; and Vital Tears. The authors report no other conflicts of interest in this work.
References
- Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi:10.1016/j.ajo.2017.06.033
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
- de Oliveira RC, Wilson SE. Practical guidance for the use of cyclosporine ophthalmic solutions in the management of dry eye disease. Clin Ophthalmol. 2019;13:1115–1122. doi:10.2147/OPTH.S184412
- McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. Ocul Surf. 2016;14(2):144–167. doi:10.1016/j.jtos.2015.11.002
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
- Fahmy RM, Aldarwesh A. Correlation between dry eye and refractive error in Saudi young adults using noninvasive Keratograph 4. Indian J Ophthalmol. 2018;66(5):653–656. doi:10.4103/ijo.IJO_1103_17
- Nichols JJ, Ziegler C, Mitchell GL, Nichols KK. Self-reported dry eye disease across refractive modalities. Invest Ophthalmol Vis Sci. 2005;46(6):1911–1914. doi:10.1167/iovs.04-1294
- Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423. doi:10.1136/bjo.86.4.418
- Lopes NLV, Gracitelli CPB, Chalita MR, de Faria NVL. Ocular surface evaluation after the substitution of benzalkonium chloride preserved prostaglandin eye drops by a preservative-free prostaglandin analogue. Med Hypothesis Discov Innov Ophthalmol. 2019;8(1):52–56.
- Giannaccare G, Bernabei F, Pellegrini M, et al. Bilateral morphometric analysis of corneal sub-basal nerve plexus in patients undergoing unilateral cataract surgery: a preliminary in vivo confocal microscopy study. Br J Ophthalmol. 2020. doi:10.1136/bjophthalmol-2019-315449
- He Y, Li J, Zhu J, Jie Y, Wang N, Wang J. The improvement of dry eye after cataract surgery by intraoperative using ophthalmic viscosurgical devices on the surface of cornea: the results of a consort-compliant randomized controlled trial. Medicine (Baltimore). 2017;96(50):e8940. doi:10.1097/MD.0000000000008940
- Ishrat S, Nema N, Chandravanshi SCL. Incidence and pattern of dry eye after cataract surgery. Saudi J Ophthalmol. 2019;33(1):34–40. doi:10.1016/j.sjopt.2018.10.009
- Lee SY, Wong TT, Chua J, Boo C, Soh YF, Tong L. Effect of chronic anti-glaucoma medications and trabeculectomy on tear osmolarity. Eye (Lond). 2013;27(10):1142–1150. doi:10.1038/eye.2013.144
- Lee JH, Song IS, Kim KL, Yoon SY. Effectiveness and optical quality of topical 3.0% diquafosol versus 0.05% cyclosporine A in dry eye patients following cataract surgery. J Ophthalmol. 2016;2016:8150757. doi:10.1155/2016/8150757
- Periman LM, Perez VL, Saban DR, Lin MC, Neri P. The immunological basis of dry eye disease and current topical treatment options. J Ocul Pharmacol Ther. 2020;36(3):137–146. doi:10.1089/jop.2019.0060
- Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100(3):300–306. doi:10.1136/bjophthalmol-2015-307415
- Geerling G, Baudouin C, Aragona P, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. Ocul Surf. 2017;15(2):179–192. doi:10.1016/j.jtos.2017.01.006
- Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011.
- Baudouin C, Irkec M, Messmer EM, et al. Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018;96(2):111–119. doi:10.1111/aos.13436
- Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. doi:10.3109/08830185.2012.748052
- Zhang X, Qu Y, He X, et al. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci. 2017;18(7):1398. doi:10.3390/ijms18071398
- Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One. 2015;10(3):e0120284. doi:10.1371/journal.pone.0120284
- Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28. doi:10.1016/j.ejpb.2017.03.006
- Du S, Hiramatsu N, Hayakawa K, et al. Suppression of NF-kappaB by cyclosporin a and tacrolimus (FK506) via induction of the C/EBP family: implication for unfolded protein response. J Immunol. 2009;182(11):7201–7211. doi:10.4049/jimmunol.0801772
- Gao J, Sana R, Calder V, et al. Mitochondrial permeability transition pore in inflammatory apoptosis of human conjunctival epithelial cells and T cells: effect of cyclosporin A. Invest Ophthalmol Vis Sci. 2013;54(7):4717–4733. doi:10.1167/iovs.13-11681
- Cholkar K, Patel A, Vadlapudi AD, Mitra AK. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat Nanomed. 2012;2(2):82–95. doi:10.2174/1877912311202020082
- Kuwano M, Ibuki H, Morikawa N, Ota A, Kawashima Y. Cyclosporine A formulation affects its ocular distribution in rabbits. Pharm Res. 2002;19(1):108–111. doi:10.1023/A:1013671819604
- Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45(12):4293–4301. doi:10.1167/iovs.03-1145
- Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45(12):4302–4311. doi:10.1167/iovs.04-0299
- Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon-gamma-secreting NK cells promote induction of dry eye disease. J Leukoc Biol. 2011;89(6):965–972. doi:10.1189/jlb.1110611
- Coursey TG, Bohat R, Barbosa FL, Pflugfelder SC, de Paiva CS. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-gamma in experimental dry eye. J Immunol. 2014;193(10):5264–5272. doi:10.4049/jimmunol.1400016
- Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186(8):1223–1232. doi:10.1084/jem.186.8.1223
- Lemos MP, Fan L, Lo D, Laufer TM. CD8alpha+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J Immunol. 2003;171(10):5077–5084. doi:10.4049/jimmunol.171.10.5077
- Zhang X, Volpe EA, Gandhi NB, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One. 2012;7(5):e36822. doi:10.1371/journal.pone.0036822
- Zhou D, Chen YT, Chen F, et al. Critical involvement of macrophage infiltration in the development of Sjogren’s syndrome-associated dry eye. Am J Pathol. 2012;181(3):753–760. doi:10.1016/j.ajpath.2012.05.014
- Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi:10.3389/fimmu.2014.00491
- Schaumburg CS, Siemasko KF, De Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187(7):3653–3662. doi:10.4049/jimmunol.1101442
- Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205e191. doi:10.1016/j.ajo.2008.08.032
- Kothari P, Pestana R, Mesraoua R, et al. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunol. 2014;192(1):349–357. doi:10.4049/jimmunol.1301906
- Nicolle P, Liang H, Reboussin E, et al. Proinflammatory markers, chemokines, and enkephalin in patients suffering from dry eye disease. Int J Mol Sci. 2018;19(4):1221. doi:10.3390/ijms19041221
- Gollmer K, Asperti-Boursin F, Tanaka Y, et al. CCL21 mediates CD4+ T-cell costimulation via a DOCK2/Rac-dependent pathway. Blood. 2009;114(3):580–588. doi:10.1182/blood-2009-01-200923
- Marsland BJ, Battig P, Bauer M, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22(4):493–505. doi:10.1016/j.immuni.2005.02.010
- Ji YW, Seo Y, Choi W, et al. Dry eye-induced CCR7+CD11b+ cell lymph node homing is induced by COX-2 activities. Invest Ophthalmol Vis Sci. 2014;55(10):6829–6838. doi:10.1167/iovs.14-14744
- Kodati S, Chauhan SK, Chen Y, et al. CCR7 is critical for the induction and maintenance of Th17 immunity in dry eye disease. Invest Ophthalmol Vis Sci. 2014;55(9):5871–5877. doi:10.1167/iovs.14-14481
- Chen T, Guo J, Yang M, et al. Cyclosporin A impairs dendritic cell migration by regulating chemokine receptor expression and inhibiting cyclooxygenase-2 expression. Blood. 2004;103(2):413–421. doi:10.1182/blood-2003-07-2412
- Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine receptors CCR6 and CXCR3 are necessary for CD4(+) T cell mediated ocular surface disease in experimental dry eye disease. PLoS One. 2013;8(11):e78508. doi:10.1371/journal.pone.0078508
- Dohlman TH, Chauhan SK, Kodati S, et al. The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci. 2013;54(6):4081–4091. doi:10.1167/iovs.12-11216
- Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182(3):1247–1252. doi:10.4049/jimmunol.182.3.1247
- García-Posadas L, Contreras-Ruiz L, Soriano-Romaní L, Dartt DA, Diebold Y. Conjunctival goblet cell function: effect of contact lens wear and cytokines. Eye Contact Lens. 2016;42(2):83–90. doi:10.1097/ICL.0000000000000158
- Pflugfelder SC, De Paiva CS, Moore QL, et al. Aqueous tear deficiency increases conjunctival interferon-gamma (IFN-gamma) expression and goblet cell loss. Invest Ophthalmol Vis Sci. 2015;56(12):7545–7550. doi:10.1167/iovs.15-17627
- Zhang X, Chen W, De Paiva CS, et al. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011;52(9):6279–6285. doi:10.1167/iovs.10-7081
- Zhang X, De Paiva CS, Su Z, Volpe EA, Li DQ, Pflugfelder SC. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res. 2014;118:117–124. doi:10.1016/j.exer.2013.11.011
- Ko BY, Xiao Y, Barbosa FL, de Paiva CS, Pflugfelder SC. Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight. 2018;3(3). doi:10.1172/jci.insight.98222
- Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013;183(1):35–48. doi:10.1016/j.ajpath.2013.03.017
- Li XQ, Zhang ZL, Tan WF, Sun XJ, Ma H. Down-regulation of CXCL12/CXCR4 expression alleviates ischemia-reperfusion-induced inflammatory pain via inhibiting glial TLR4 activation in the spinal cord. PLoS One. 2016;11(10):e0163807. doi:10.1371/journal.pone.0163807
- Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873.
- Bushley KE, Raja R, Jaiswal P, et al. The genome of tolypocladium inflatum: evolution, organization, and expression of the cyclosporin biosynthetic gene cluster. PLoS Genet. 2013;9(6):e1003496. doi:10.1371/journal.pgen.1003496
- Borel JF, Feurer C, Magnee C, Stahelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977;32(6):1017–1025.
- Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;4:Cd005161.
- RESTASIS® (cyclosporine ophthalmic emulsion) 0.05% for topical ophthalmic use. Full prescribing information. Irvine, CA: Allergan; 2017.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
- Kymionis GD, Bouzoukis DI, Diakonis VF, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. 2008;2(4):829–836.
- Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopinski P. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol. 2016;41(2):195–208. doi:10.5114/ceji.2016.60995
- Meyer S, Kohler NG, Joly A. Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-kappaB activation. FEBS Lett. 1997;413(2):354–358. doi:10.1016/S0014-5793(97)00930-7
- Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2–3):119–125. doi:10.1016/S0162-3109(00)00192-2
- Matsuda S, Moriguchi T, Koyasu S, Nishida E. T lymphocyte activation signals for interleukin-2 production involve activation of MKK6-p38 and MKK7-SAPK/JNK signaling pathways sensitive to cyclosporin A. J Biol Chem. 1998;273(20):12378–12382. doi:10.1074/jbc.273.20.12378
- Ames P, Galor A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidence. Clin Investig (Lond). 2015;5(3):267–285. doi:10.4155/cli.14.135
- Bang SP, Yeon CY, Adhikari N, et al. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS One. 2019;14(11):e0224805. doi:10.1371/journal.pone.0224805
- Demir T, Godekmerdan A, Balbaba N, Turkcuoglu P, Ilhan F, Demir N. The effect of infliximab, cyclosporine A and recombinant IL-10 on vitreous cytokine levels in experimental autoimmune uveitis. Indian J Ophthalmol. 2006;54(4):241–245. doi:10.4103/0301-4738.27948
- Ghasemi H, Djalilian A. Topical calcineurin inhibitors: expanding indications for corneal and ocular surface inflammation. J Ophthalmic Vis Res. 2019;14(4):398–399. doi: 10.18502/jovr.v14i4.5435
- Davis G. The Evolution of Cataract Surgery. Mo Med. 2016;113(1):58–62.
- Salomao MQ, Ambrosio R Jr., Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. 2009;35(10):1756–1760. doi:10.1016/j.jcrs.2009.05.032
- Kanellopoulos AJ. Incidence and management of symptomatic dry eye related to LASIK for myopia, with topical cyclosporine A. Clin Ophthalmol. 2019;13:545–552. doi:10.2147/OPTH.S188521
- Iaccheri B, Torroni G, Cagini C, et al. Corneal confocal scanning laser microscopy in patients with dry eye disease treated with topical cyclosporine. Eye (Lond). 2017;31(5):788–794. doi:10.1038/eye.2017.3
- Napoli PE, Braghiroli M, Iovino C, Demarinis G, Fossarello M. A study of refractory cases of persistent epithelial defects associated with dry eye syndrome and recurrent corneal erosions successfully treated with cyclosporine A 0.05% eye drops. Drug Des Devel Ther. 2019;13:2001–2008. doi:10.2147/DDDT.S207453
- Napoli PE, Mangoni L, Gentile P, Braghiroli M, Fossarello M. A panel of broad-spectrum antivirals in topical ophthalmic medications from the drug repurposing approach during and after the coronavirus disease 2019 era. J Clin Med. 2020;9(8):2441. doi:10.3390/jcm9082441
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 study group. Ophthalmology. 2000;107(4):631–639. doi:10.1016/S0161-6420(99)00176-1
- Stonecipher KG, Torkildsen GL, Ousler GW 3rd, Morris S, Villanueva L, Hollander DA. The IMPACT study: a prospective evaluation of the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual performance in patients with dry eye. Clin Ophthalmol. 2016;10:887–895. doi:10.2147/OPTH.S101627
- IKERVIS® (ciclosporin ophthalmic emulsion) 1 mg/mL, for topical ophthalmic use. Full prescribing information. Evry, France: Santen SAS; 2015.
- Leonardi A, Van Setten G, Amrane M, et al. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016;26(4):287–296. doi:10.5301/ejo.5000779
- CEQUATM (cyclosporine ophthalmic solution 0.09%). Full prescribing information. Cranbury, NJ: Sun Pharmaceutical Industries, Inc.; 2018.
- Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(5):422–437. doi:10.1002/wnan.1272
- Cholkar K, Gilger BC, Mitra AK. Topical, aqueous, clear cyclosporine formulation design for anterior and posterior ocular delivery. Transl Vis Sci Technol. 2015;4(3):1. doi:10.1167/tvst.4.3.1
- Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A Phase 3, randomized, double-masked study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease. Ophthalmology. 2019;126(9):1230–1237. doi:10.1016/j.ophtha.2019.03.050
- Malhotra R, Devries DK, Luchs J, et al. Effect of OTX-101, a novel nanomicellar formulation of Cyclosporine A, on corneal staining in patients with keratoconjunctivitis sicca: a pooled analysis of Phase 2b/3 and Phase 3 studies. Cornea. 2019;38(10):1259–1265. doi:10.1097/ICO.0000000000001989
- Smyth-Medina R, Johnston J, Devries DK, et al. Effect of OTX-101, a novel nanomicellar formulation of Cyclosporine A, on conjunctival staining in patients with keratoconjunctivitis sicca: a pooled analysis of Phase 2b/3 and 3 clinical trials. J Ocul Pharmacol Ther. 2019;35(7):388–394. doi:10.1089/jop.2018.0154
- Tauber J, Schechter BA, Bacharach J, et al. A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–1929. doi:10.2147/OPTH.S175065
- Sheppard J, Kannarr S, Luchs J, et al. Efficacy and safety of OTX-101, a novel nanomicellar formulation of Cyclosporine A, for the treatment of keratoconjunctivitis sicca: pooled analysis of a Phase 2b/3 and Phase 3 study. Eye Contact Lens. 2020;46(Suppl 1):S14–s19. doi:10.1097/ICL.0000000000000636
- Weiss SL, Kramer WG. Ocular distribution of cyclosporine following topical administration of OTX-101 in New Zealand White Rabbits. J Ocul Pharmacol Ther. 2019;35(7):395–402. doi:10.1089/jop.2018.0106
- Mandal A, Gote V, Pal D, Ogundele A, Mitra AK. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of Cyclosporine (Cequa®) for dry eye disease. Pharm Res. 2019;36(2):36. doi:10.1007/s11095-018-2556-5
- Pinheiro R, Panfil C, Schrage N, Dutescu RM. Comparison of the lubricant eyedrops Optive®, Vismed Multi®, and Cationorm® on the corneal healing process in an ex vivo model. Eur J Ophthalmol. 2015;25(5):379–384. doi:10.1089/jop.2015.0054
- Schuerer N, Stein E, Inic-Kanada A, et al. Implications for ophthalmic formulations: ocular buffers show varied cytotoxic impact on human corneal-limbal and human conjunctival epithelial Cells. Cornea. 2017;36(6):712–718. doi:10.1097/ICO.0000000000001199
- Abidi A, Shukla P, Ahmad A. Lifitegrast: a novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194–198. doi:10.4103/0976-500X.195920
- Chao W, Belmonte C, Benitez Del Castillo JM, et al. Report of the Inaugural Meeting of the TFOS i(2) = initiating innovation Series: targeting the Unmet Need for Dry Eye Treatment. Ocul Surf. 2016;14(2):264–316. doi:10.1016/j.jtos.2015.11.003
- Xiidra® (lifitegrast ophthalmic solution) 5%, for topical ophthalmic use. Full prescribing information. Lexington, MA: Shire; 2016.
- Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14(2):207–215. doi:10.1016/j.jtos.2016.01.001
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi:10.1208/s12248-010-9183-3
- Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118(11):1489–1496. doi:10.1001/archopht.118.11.1489
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330–337. doi:10.1001/archopht.120.3.330
- Jerkins GW, Pattare GR, Kannarr SR. A Review of Topical Cyclosporine A Formulations-A Disease-Modifying Agent for Keratoconjunctivitis Sicca. Clin Ophthalmol. 2020;14):481–489. doi:10.2147/OPTH.S228070.
