Abstract
Purpose
To assess the stability, safety, predictability, and efficacy of topography-guided myopic Femto-LASIK with two different treatment protocols.
Setting
Ebsar Eye center, Benha, Qalyopia, Egypt.
Design
Single-center, retrospective, COHORT control study.
Methods
A total of 330 eyes enrolled in the study in group A and 322 eyes enrolled in group B underwent uncomplicated primary bilateral topography-guided Femto-LASIK. Group A was treated with the subjective clinical refraction; however, group B was treated with the modified refraction according to ALCON protocol.
Results
The mean preoperative refractive spherical equivalent (MRSE) was −4.85±1.90D and −5.0±1.93D in group A and B, respectively (P = 0.86), and a cylinder of −0.95±0.80 D and −0.92±0.81D, respectively. At the 12 months’ postoperatively, the residual manifest SE within ± 0.5D was achieved by 82.86% of eyes in group A compared to 83.93% in group B. Of eyes, 92.06% had ≤0.5 astigmatism dioptre, while 100% of eyes had ≤1.0 astigmatism dioptre in group A (315 eyes); however, 91.80% of eyes had ≤0.5 astigmatism dioptre, while 100% of eyes had ≤1.0 astigmatism dioptre in group B.
Conclusion
Topographic modification of the magnitude and axis of astigmatism treated using ALCON protocol when different from the clinical refraction may offer good refractive outcomes when we apply the Alcon precalculation considerations.
Introduction
Laser-assisted in situ keratomileuses (LASIK) has been established over the past 20 years as a safe and efficient surgery practiced worldwide.Citation1,Citation2 Femtosecond laser-assisted LASIK (Femto-LASIK) has become a familiarized transformation over the last 10 years over the standard LASIK technique.Citation3,Citation4 LASIK procedure using the excimer lasers to correct refractive inaccuracy has been improving as well, with most excimer laser machines currently using flying spot technology of very high repetition rates and advanced tracking systems to track intra-operatively the pupil and some even to provide iris registration for cyclorotation modulation.Citation5–Citation7 Aspherical ablation profiles have been used with some excimer laser machines to reduce spherical aberration associated with laser vision corrections, so some LASIK operations are performed nowadays as optimize aspheric treatments.Citation8–Citation10 Since most of the ocular aberrations come from the cornea, the topography-guided treatments (T-CAT) have been used as a method of customization to correct corneal irregularities that affect clear vision.Citation11,Citation12 Topography-guided Femto-LASIK refractive outcomes have been improved both refractive visual outcomes and high-order aberrations.Citation1,Citation5,Citation13–Citation15 The manifest refraction has been used in many excimer laser vision correction protocols. Thus, the modification of the clinical refraction with topographic data: T-CAT modified refraction was prescribed by some investigators for better treatment of astigmatism.Citation16 The United States Food and Drug Administration approved the WaveLight® Contoura topographic-guided ablation (Contoura) (WaveLight, Erlangen, Germany), ophthalmologists now can choose between the excimer laser treatment using the clinical refraction or the Contoura modified refraction for T-CAT laser correction. The Contoura modified refraction is resulted from the systematic analysis of the anterior surface of the cornea with a WaveLight® algorithm and sometimes it is different from the clinical refraction that may result in a refractive mystery for ophthalmologists. This ambiguity is alleviated by Alcon clinical crew who are teaching the refractive surgeons how to use measured Contoura correction with the LYRA (Layer Yolked Reduction of Astigmatism) or ALCON Protocol (Contoura training cards).Citation17,Citation18
This study aims to compare the refractive and visual outcomes when using the ALCON protocolCitation18 to the standard manifest refraction in myopic Femto-LASIK.
Patients and Methods
This is a single-center, retrospective, cohort control study. The study was conducted in Ebsar eye center (Benha city, Egypt) on myopic patients who are scheduled for T-CAT Femto-LASIK procedure, between February and April 2019.
330 eyes enrolled in the study in group A and 322 eyes enrolled in group B, subjected to primary bilateral topography-guided Femto-LASIK by the same surgeons (SMA & MAE) on the same refractive surgery machines (VisuMax® femtosecond laser, Carl Zeiss Meditec, Jena, GermanCitation19 and WaveLight EX500 Excimer laser, Alcon Laboratories, Inc.Citation20). The relative eye number per each surgeon remained constant during the study. The flap thickness was 100 µm, and the diameter was 7.9 mm. The treatment protocol used for group A was the subjective manifest refraction, but for group B the treatment was the ALCON protocolCitation18 of refraction modification.
A full and thorough ophthalmologic examination was carried out before surgery including the assessment of cycloplegic and clinical (manifest) refraction, uncorrected distance visual acuity (UDVA), best-corrected distance visual acuity (CDVA), intraocular pressure, pupil size, corneal tomography (Pentacam Oculus), corneal topography (Topolyzer VARIO) and posterior segment examination. During postoperative follow-up visits, the patients were assessed for CDVA and UDVA and expressed in LogMAR visual acuity, refraction, and corneal tomography. The study protocol had followed and was adhered to the belief and principles of the statement of Helsinki and was confirmed by the Benha University Faculty of Medicine Research Ethical Committee that it meets national and international guidelines for research on humans. Informed consent and permission to use their data were acquired from each patient before surgery.
The inclusion criteria were spherical equivalent up to −9.0 D, age from 21 years to 50 years, refraction stability for at least one year, rigid contact lenses not used for 3 weeks, and soft ones not used for one week before the operation, the minimum permitted corneal pachymetry was 500 μm, and a minimum residual stromal thickness of 300 μm.
The exclusion criteria were presence of eye diseases such as keratitis, iritis, glaucoma, dry eye, autoimmune diseases, and thyrotoxicosis. Females who were breastfeeding or pregnant, and any patient with clinical sphere only (no clinical cylinder) or cases that had intraoperative complications.
During measurement acquisition, at least four to eight corneal topography images were taken on undilated pupils with the WaveLight Topolyzer VARIO machine (Alcon Laboratories, Inc.). The accepted images were exported to the T-CAT software of the Allegretto EX500 machine. The T-CAT measured anterior corneal astigmatism (ACA) is calculated from the Zernike polynomials C3 (oblique astigmatism) and C5 (vertical astigmatism) and then converted to the astigmatism magnitude in diopters and axis.Citation21
T-CAT Surgical Planning
In group A: The clinical refraction sphere and astigmatism power and axis were entered into the modified refraction cells of the allegretto EX500 software to be treated as T-CAT Femto-LASIK.
In group B: the clinical refraction sphere and astigmatism power and axis were modified according to ALCON protocolCitation18 (the Contoura training card), and then entered into the modified refraction cells of the allegretto EX500 software to be treated as T-CAT Femto-LASIK (Contoura Femto-LASIK). Precalculation considerationsCitation18 which is; consider recommending wavefront-optimized™ Ablation to your patient if any of the following conditions apply:
A difference of >1.25 D between the refraction cylinder and the measured cylinder.
A difference of >5° between the refraction axis and measured axis if the refraction cylinder ≥2.00 D.
A difference of >10° between the refraction axis and measured axis if the refraction cylinder <2.00 D.
Artificial intelligence (AI); is outlined as “hardware or computer code that exhibits behaviour that seems intelligent”.Citation22
The coauthor of this research article professor Abdelmonem Hamed has developed some artificial intelligence (Excel sheet) calculator, which was named Contoura calculator (Beta version). The Contoura calculator (Supplement 1) facilitates calculating the modified refraction according to Alcon protocolCitation18 and prevents human errors throughout the manual calculation as well. Furthermore, this Contoura calculator is going to be uploaded with this research article to be downloaded and evaluated by ophthalmologists. An illustrating attachment (Supplement 2) and illustrating movie (Supplementary Video S1) for the Contoura calculator are going to be attached as well.
Statistical Analysis
The outcomes were analyzed. Data from the 12-month visit were used for analysis. Unpaired Student’s t-test was used to calculate the statistical significance.
Microsoft Excel master sheet 2010 (Microsoft Corporation, Redmond, WA) was used for statistical analysis. A P-value lesser than 0.05 was statistically significant.
Results
This study included 330 eyes in group A and 322 eyes in group B. The mean age of the patients in group A was 38.84±8.35 years, with a range of 22–48 years, however in group B was 39.48±9.36 years, with a range of 21–49 years. The mean preoperative refractive spherical equivalent (MRSE) was −4.85±1.90 D and −5.0±1.93D in group A and B, respectively (P = 0.86), and a cylinder of −0.95±0.80 D and −0.91±0.81 D, respectively (P = 0.85). shows the biodata data for the study population (groups A & B).
Table 1 Preoperative Demographic and Refractive Characteristics of the Included Patients
Uncorrected Distant Visual Acuity (UDVA) Outcomes and Stability
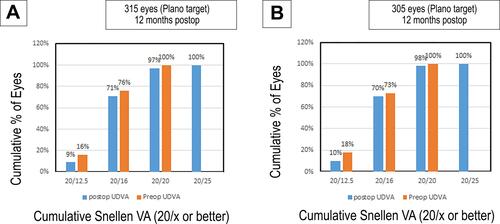
At the 12 months’ postoperative visit, 97% and 71% of the eyes demonstrated a UDVA of 20/20 and 20/16 or better, respectively, in group A []. Whereas in group B, 98% and 70% of the eyes presented with a UDVA of 20/20 and 20/16 or better, respectively (P = 0.92) [].
Safety
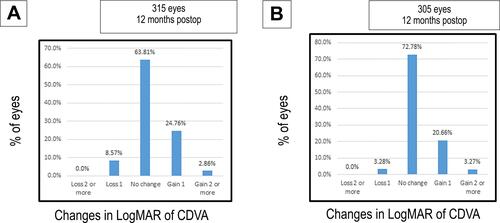
The gain or loss of corrected distance visual acuity (CDVA) at 12 months’ follow-up indicated that 24.76% of the eyes in group A versus 20.66% in group B gained one line (P=0.07), and 2.86% of the eyes in group A versus 3.27% in group B gained 2 lines (P=0.08). While 63.81% in group A showed no change in CDVA while 72.78% in group B showed no change in CDVA, and 8.57% in group A showed a loss of 1 line of CDVA while 3.28% in group B showed a loss of 1 line of CDVA. [ and ].
Predictability
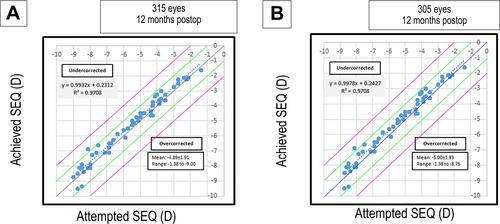
Attempted versus achieved spherical equivalent rectification and shows the scatter plot analysis comparing attempted and achieved spherical equivalent rectification in group A and group B, respectively. The degree of definition between the attempted and achieved MRSE was comparable between group A (R2 = 0.97) and group B (R2 = 0.97) groups (P = 0.80). The higher R2 value which equals 0.97 on the attempted versus the achieved spherical equivalent scatter plot in group A and group B suggests that both groups led to a predictable outcome.
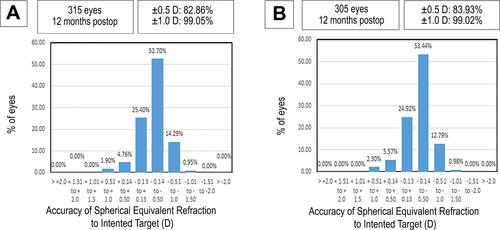
The accuracy and predictability of the residual manifest SE within ± 0.5D was 82.86% of eyes in group A, and 83.93% in group B (P = 0.10, and ).
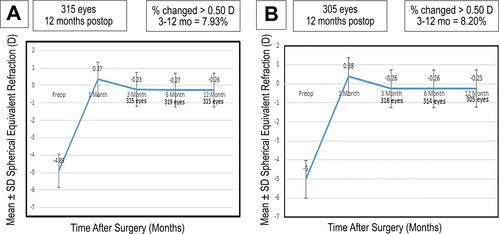
Stability
and shows the long-term stability in both groups between three and 12 months. Only 7.93% and 8.20% had a change greater than 0.5 D in groups A and B, respectively (P= 0.8). However, the spherical equivalent had insignificant change from −0.23 ± 0.41 D at 3rd month to −0.26 ± 0.33 D at 12th month in group A and from −0.26 ± 0.40 D at 3rd month to −0.25 ± 0.33 D at 12th month in group B (P-value: 0.62).
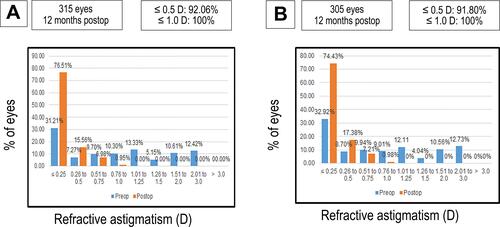
Astigmatism Analysis
and show refractive astigmatism at 12 months postoperatively were 92.06% of eyes were ≤0.5 astigmatism dioptre, while 100% of eyes were ≤1.0 astigmatism dioptre in group A (315 eyes); however, 91.80% of eyes were ≤0.5 astigmatism dioptre, while 100% of eyes were ≤1.0 astigmatism dioptre in group B (305 eyes) (P-value: 0.72).
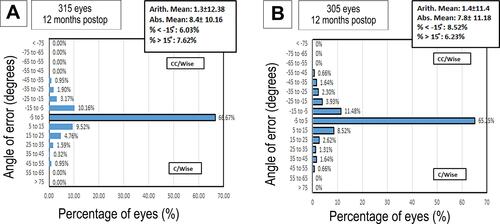
and ; show the vector analysis results at 12 months postoperatively, the arithmetic mean was 1.3±12.38 in group A, and 1.4±11.4 in group B; however, the absolute mean was 8.4±10.16 in group A, and 7.8±11.18 in group B. The percentage of refractive astigmatism angle error < −15° was 6.03% in group A, and 8.52% in group B; however, the percentage of refractive astigmatism angle error ˃ −15° was 7.62% in group A and 6.23% in group B. The mean of the target induced astigmatism (TIA) was 0.95±0.80, and the surgically induced astigmatism (SIA) was 0.77±88 in group A, and TIA was 0.91±0.81, and SIA was 0.74±0.88 in group B (P = 0.6).
Discussion
The combined VisuMax® femtosecond laser, Carl Zeiss Meditec, Jena, GermanyCitation19 and WaveLight EX500 Excimer laser, Alcon Laboratories, Inc.Citation20 excimer laser, and some topography and tomography instruments were used clinically.Citation21
Clinical outcomes of previous WaveLight excimer laser machines have been studied by previous investigators.Citation12
The EX500 laser employs a 1050 Hz multidimensional active tracker with an estimated response time of 2 ms, able to track pupil size from 1.5 mm to 8 mm.Citation23
The Allegretto EX500 excimer laser machine shows the capability to import the topographic data from the Vario-WaveLight® to the treatment mode, and then customize the excimer laser treatment to each patient’s cornea (T-CAT treatment).Citation12 Previous studies have reported the applications of T-CAT treatments in irregular and virgin eyes with these Allegretto excimer laser machines.Citation24–Citation26
Clinical and anterior corneal astigmatism rarely match in power and axis. The ocular residual astigmatism (ORA) after refractive surgery means the difference between anterior corneal and clinical astigmatism.Citation27 ORA represents a refractive problem that needs more research. It has an important surgical problem which is; to what astigmatism power and axis to treat by topography-guided treatment to get rid of the ORA after surgery. T-CAT treatments precisely capture the measured anterior corneal astigmatism power and axis and it combines that data into the treatment profile, resulting in a smoother cornea. Many trials have been done to replace the conventional strategies for treating clinical astigmatism.Citation28,Citation29 Both treatment protocols, either manifest refractive or measured ACA, accomplished superb efficacy, accuracy, and safety, with a small benefit in eyes treated on the clinical astigmatism protocol.Citation29
The 12 months clinical results show magnificent refractive outcome, predictability, and stability with both treatment protocols. The modification of the manifest refraction according to Alcon protocolCitation18 led to superior visual function especially with UDVA, CDVA, and spherical equivalent. The only detected defect in ALCON protocolCitation18 was it does not consider what the refractive surgeon can do if there is no clinical cylinder and the measured one has a magnitude and axis. This point needs detailed research to advise the surgeons what to do in such cases (either the surgeon enters the clinical sphere with T-CAT or optimize treatment or the surgeon enters the measured cylinder according to Alcon protocol with T-CAT treatment). The mystery of treating on clinical astigmatism versus measured ACA in eyes with significant ORA was previously studied by investigators to ameliorate refractive surgical outcomes.Citation30–Citation32
Conclusion
Topographic modification of the magnitude and axis of astigmatism treated using ALCON protocol when different from the clinical refraction may offer comparable outcomes in topography-guided myopic Femto-LASIK using clinical refraction protocol if we applied the Alcon precalculation considerations.Citation18 These feedbacks may alter the current clinical paradigm of using subjective refraction used in excimer laser machines for vision correction. However, more detailed research studies are required to evaluate the ALCON T-CAT protocol.
Disclosure
This is to certify that the authors did not extradite any monetary backing from any sources. The authors have no monetary or proprietary avails in a product, method, or material described in this research article. The authors report no conflicts of interest for this work.
References
- Kugler LJ, Wang MX. Lasers in refractive surgery: history, present, and future. Appl Opt. 2010;49(25):F1–F9. doi:10.1364/AO.49.0000F1
- Lukenda A, Martinović ZK, Kalauz M. Excimer laser correction of hyperopia, hyperopic and mixed astigmatism: past, present, and future. Acta Clin Croat. 2012;51(2):299–304.
- Reggiani-Mello G, Krueger RR. Comparison of commercially available femtosecond lasers in refractive surgery. Expert Rev Opthalmol. 2011;6(1):55–56. doi:10.1586/eop.10.80
- Salomão MQ, Wilson SE. Femtosecond laser in laser in situ keratomileusis. J Cataract Refract Surg. 2010;36(6):1024–1032. doi:10.1016/j.jcrs.2010.03.025
- Vega-Estrada A, Alió JL, Arba Mosquera S, Moreno LJ. Corneal higher order aberrations after LASIK for high myopia with a fast repetition rate excimer laser, optimized ablation profile, and femtosecond laser-assisted flap. J Refract Surg. 2012;28(10):689–696. doi:10.3928/1081597X-20120921-03
- Winkler von Mohrenfels C, Khoramnia R, Lohmann CP. Comparison of different excimer laser ablation frequencies (50, 200, and 500 Hz). Graefes Arch Clin Exp Ophthalmol. 2009;247(11):1539–1545. doi:10.1007/s00417-009-1102-x
- Iseli HP, Mrochen M, Hafezi F, Seller T. Clinical photoablation with a 500-Hz scanning spot excimer laser. J Refract Surg. 2004;20(6):831–834. doi:10.3928/1081-597X-20041101-12
- de Ortueta D, Magnago T, Triefenbach N, Arba Mosquera S, Sauer U, Brunsmann U. In vivo measurements of thermal load during ablation in high-speed laser corneal refractive surgery. J Refract Surg. 2012;28(1):53–58. doi:10.3928/1081597X-20110906-01
- Aslanides IM, Kolli S, Padron S, Arba Mosquera S. Stability of therapeutic retreatment of corneal wavefront customized ablation with the SCHWIND CAM: 4-year data. J Refract Surg. 2012;28(5):347–352. doi:10.3928/1081597X-20120410-01
- Smadja D, Reggiani-Mello G, Santhiago MR, Krueger RR. Wavefront ablation profiles in refractive surgery: description, results, and limitations. J Refract Surg. 2012;28(3):224–232. doi:10.3928/1081597X-20120217-01
- Kanellopoulos AJ. Topography-guided custom retreatments in 27 symptomatic eyes. J Refract Surg. 2005;21(5):S513–S518. doi:10.3928/1081-597X-20050901-19
- Kanellopoulos AJ. Topography-guided hyperopic and hyperopic astigmatism femtosecond laser-assisted LASIK: long-term experience with the 400 Hz eye-Q excimer platform. Clin Ophthalmol. 2012;6:895–901. doi:10.2147/OPTH.S23573
- Zheng H, Song LW. Visual quality of Q-value-guided LASIK in the treatment of high myopia. Yan Ke Xue Bao. 2011;26(4):208–210.
- Alio JL, Vega-Estrada A, Piñero DP. Laser-assisted in situ keratomileusis in high levels of myopia with the amaris excimer laser using optimized aspherical profiles. Am J Ophthalmol. 2011;152(6):954–963. doi:10.1016/j.ajo.2011.05.009
- El Awady HE, Ghanem AA, Saleh SM. Wavefront-optimized ablation versus topography-guided customized ablation in myopic LASIK: comparative study of higher order aberrations. Ophthalmic Surg Lasers Imaging. 2011;42(4):314–320. doi:10.3928/15428877-20110421-01
- Kanellopoulos AJ. Topography-modified refraction (TMR): adjustment of treated cylinder amount and axis to the topography versus standard clinical refraction in myopic topography-guided LASIK. Clin Ophthalmol. 2016;10:2213–2221. doi:10.2147/OPTH.S122345
- Motwani M. The use of WaveLight(R) Contoura to create a uniform cornea: the LYRA Protocol. Part 2: the consequences of treating astigmatism on an incorrect axis via excimer laser. Clin Ophthalmol. 2017;11:907–913. doi:10.2147/OPTH.S133840
- Contoura® Vision Training Card en-us/Rev.00/19-01-22 Item No.: 6675 2022. © 2019 Novartis 3/19 GL-WVL-19-MK-0214. ALCON; A Novartis division.
- Blum M, Kunert K, Gille A, Sekundo W. LASIK for myopia using the Zeiss VisuMax femtosecond laser and MEL 80 excimer laser. J Refract Surg. 2009;25(4):350–356. doi:10.3928/1081597X-20090401-01
- Kanellopoulos AJ, Asimellis G. Correlation between central corneal thickness, anterior chamber depth, and corneal keratometry as measured by oculyzer II and waveLight OB820 in preoperative cataract surgery patients. J Refract Surg. 2012;22:1–6.
- Wallerstein A, Gauvin M, Qi SR, et al. Primary topography-guided LASIK: treating manifest refractive astigmatism versus topography-measured anterior corneal astigmatism. J Refract Surg. 2019;35(1):15–23. doi:10.3928/1081597X-20181113-01
- Graham N. Artificial intelligence, Vol. 1076. In: Blue Ridge Summit: Tab Books. Philadelphia, PA, USA; 1979.
- Matsuura T, Ikeda H, Idota N, Motokawa R, Hara Y, Annaka M. Anisotropic swelling behavior of the cornea. J Phys Chem B. 2009;113(51):16314–16322. doi:10.1021/jp907232h
- Han DC, Chen J, Htoon HM, Tan DT, Mehta JS. Comparison of outcomes of conventional WaveLight(®) Allegretto Wave(®) and Technolas(®) excimer lasers in myopic laser in situ keratomileusis. Clin Ophthalmol. 2012;6:1159–1168.
- Celik HU, Alagöz N, Yildirim Y, et al. Accelerated corneal crosslinking concurrent with laser in situ keratomileusis. J Cataract Refract Surg. 2012;38(8):1424–1431. doi:10.1016/j.jcrs.2012.03.034
- Kanellopoulos AJ. Long-term safety and efficacy follow-up of prophylactic higher fluence collagen cross-linking in high myopic laser-assisted in situ keratomileusis. Clin Ophthalmol. 2012;6:1125–1130. doi:10.2147/OPTH.S31256
- Alpins N. A new method of analyzing vectors for changes in astigmatism. J Cataract Refract Surg. 1993;19(4):S24–S33. doi:10.1016/S0886-3350(13)80617-7
- Stulting RD, Fant BS. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. J Cataract Refract Surg. 2016;42(1):11–18. doi:10.1016/j.jcrs.2015.08.016
- Adding CONTOURA vision, topography-guided LASIK to the refractive armamentarium. Eyetube.net, Alcon; 2017.
- Motwani M. The use of WaveLight ((R)) Contoura to create a uniform cornea: the LYRA protocol: part 3: the results of 50 treated eyes. Clin Ophthalmol. 2017;11:915–921. doi:10.2147/OPTH.S133841
- Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg. 1997;23(1):65–75. doi:10.1016/S0886-3350(97)80153-8
- Arbelaez MC, Alpins N, Verma S, Stamatelatos G, Arbelaez JG, Arba-Mosquera S. Clinical outcomes of laser in situ keratomileusis with an aberration-neutral profile centered on the corneal vertex comparing vector planning with manifest refraction planning for the treatment of myopic astigmatism. J Cataract Refract Surg. 2017;43(12):1504–1514. doi:10.1016/j.jcrs.2017.07.039