Abstract
Purpose
Utility index is a significant outcome in terms of health economics assessment while patient-reported outcome measure (PROMs) evaluates quality of life (QOL) from patient’s perspective. Our objective was to evaluate both utility indices and PROMs using generic and eye specific QOL in glaucomatous patients compared with normal population.
Methods
This is a case-control study. We interviewed normal and glaucomatous participants with the European Quality of Life questionnaire (EQ-5D-5L), the European visual analogue scale (EQ-VAS) and the visual function questionnaire 28 (Thai version) (VFQ-28). The visual function questionnaire utility index (VFQ-UI) and generic utility index from EQ-5D-5L were calculated.
Results
There were 47 normal and 127 glaucomatous participants in this study. Amongst glaucoma group, 35 participants were in the early stage of the disease, 43 were in the moderate stage, 30 normal vision participants were in the severe stage, 14 participants had blindness one eye, and 5 had blindness both eyes. The mean age of the participants in both groups was statistically similar (63.78±6.84 vs 66.30±8.93 years old, respectively, p=0.062). Underlying diseases between groups were also comparable. The EQ-5D-5L utility index score and the EQ-VAS score were not statistically different between normal and glaucomatous groups, respectively (EQ-5D-5L: 0.874±0.122 vs 0.837±0.191, p=0.215; EQ VAS: 76.06±15.07 vs 74.02 ±15.10, p=0.43). By contrast, VFQ-UI of the glaucomatous group was significantly lower than that of the normal group, (VFQ-UI: 0.833±0.147 vs 0.895±0.070, accordingly, p<0.05).
Conclusions
Utility index from the VFQ-UI was a relevant PROMs for evaluating the QOL of glaucomatous patients in terms of visual function specificity and acceptable validity.
Introduction
Glaucoma is a disease that causes visual impairment, thereby hindering one’s quality of life (QOL).Citation1 Several studies have found a link between having glaucoma and an increased risk of experiencing certain negative effects, such as a higher incident of automobile accidents, social withdrawal and even depressive disorders all of which negatively impact QOL.Citation2–Citation4 Due to the lack of visual symptoms in the early stages of glaucoma, the subsequent visual impairment‒affecting both visual acuity and visual field‒becomes apparent only in the later stages of the disease. Without treatment, the disease will usually progress to the end-stage, in which complete blindness can occur.Citation5,Citation6 Fortunately, there are modern glaucoma treatment methods that can effectively halt the progression of the disease.Citation7–Citation10 However, there are also possible negative aspects to these treatment options, including side effects of certain medications,Citation11 cost of treatment options,Citation12 complications, or continuing effects from surgical procedures, which may require long-term care, and other considerations.Citation13 Consequently, glaucoma can impact visual functioning in all areas of life, including work, recreation, and other normal day-to-day activities, activities, thereby significantly decreasing a person’s QOL. At present, the objective of glaucoma management is not only to control the progression of the disease but also to preserve the patient’s QOL as much as possible while utilizing rational treatment options.
The QOL in glaucoma patients can be assessed by using various types of vision-specific, disease-specific, and generic QOL questionnaires. Generic QOL evaluated the general wellbeing of respondents and generated the utility index, for example, the Short-Form Six-Dimension health index (SF-6D), the Medical Outcomes Study 36-item short-form health survey (SF-36) and the EuroQOL’s EQ-5D-5L.Citation14–Citation16 The National eye institute visual functioning questionnaire (NEI-VFQ) is widely used vision-specific QOL questionnaires in ophthalmology.Citation17–Citation19 The glaucoma symptom scale (GSS) and the Glaucoma quality of life-15 (GQL-15) questionnaire are glaucoma disease-specific QOL questionnaires.Citation20,Citation21
QOL is primarily measured by using utility index applied to quality-adjusted life year (QALY) in cost-effectiveness analysis and health economics to assess the burden of diseases in social perspective.Citation22–Citation25 In this work, we compare utility indices and patient-reported outcome measures (PROMs) between glaucomatous patients in various stages of the disease to the general population within a similar age-range by using both generic utility index‒the EuroQOL’s EQ-5D-5L and the European visual analogue scale score (EQ-VAS)Citation26–Citation28 and the vision-specific QOL and vision-specific utility index‒the visual function questionnaire 28-Thai version (VFQ-28)Citation29 and the visual function questionnaire-utility index (VFQ-UI).Citation30,Citation31 Both generic and eye-specific utility indices and PROMs of glaucomatous patients from these data would be beneficial for policymaking and resource allocation, as well as be an incentive for a more patient-based consideration.Citation32
Methods
This was a case–control study which adhered to the tenets of the Declaration of Helsinki and has been approved by the Mettapracharak (Wat Raikhing) research ethics committee in accordance with the international conference on harmonisation good clinical practice (ICH-GCP). Written informed consent was obtained from all participants. Data were collected between June 2015 and June 2016.
Non-glaucomatous participants were invited from both hospital-based eye screening programs for the elderly at the institute hospital and community-based screening mobile unit at Klong Mai subdistrict administrative organization, while glaucomatous participants were recruited from the glaucoma clinic at the institute hospital. The severity classification of glaucomatous participants was determined by referring to Hodapp, Parish and Anderson (H-P-A) glaucoma classification systemCitation33 into early, moderate and severe stages with the different levels of mean deviation (MD) determined from the visual field testing. Sample size estimation in each group was calculated from the infinite population mean formula.
Sample Size=Z21-α/2σ2/d2, where Z is standard normal variate, which was 1.96 (p<0.05), α is 0.05, SD (σ) is the utility index scores’ standard deviation evaluated by the EuroQol’s EQ-5D-5L questionnaire from a pilot study of normal and glaucomatous participants and d is the absolute error, estimated to be 0.05. Estimated participants sample sizes were 29 normal (non-glaucomatous) participants (SD=0.136), 32 early stage glaucoma participants (SD=0.143), 44 moderate stage participants (SD=0.171) and 18 in the severe stages (SD=0.108).
Six-hundreds participants underwent a comprehensive eye examination including visual acuity testing, intraocular pressure measuring, optic nerve evaluating by fundus photograph and standard automated visual field testing to clarify the situation in suspect participants. Non-glaucoma participants were invited to interview with the quality of life questionnaires sequentially followed by a random table number, whereas glaucomatous participants were recruited voluntarily according to the sample size calculation.
Generic QOL was evaluated using the EuroQol’s EQ-5D-5L questionnaire (Thai version) with the outcomes being the utility index and the European visual analogue scale score (EQ-VAS).Citation28,Citation34 The EQ-5D-5L evaluates the health status of respondents with a descriptive system of 5 dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with each dimension having 5 levels (no problems, slight problems, moderate problems, severe problems, and extreme problems). The EQ-VAS represents the respondent’s self-rated health and can be used as a quantitative measure of health as the visual analogue scale (value between 0 and 100). The unique health state of the respondent is defined by scoring in each dimension. Each EQ-5D-5L health state is converted into a single utility index value (value between 0 and 1: value 0 means dead while, 1 means perfectly health) with a country specific dataset that is easily applied in QALY calculation.
Vision-specific QOL was assessed by the visual function questionnaire 28-Thai version (VFQ-28) which was constructed from the National Eye Institute 25-Item Visual Function Questionnaire (NEI VFQ-25) using forward and backward translation. Because its items were created from patients’ perception, this Thai version has been tailored to capture issues relevant to Thai patients.Citation17–Citation19,Citation29 The vision-specific utility index was evaluated by the visual function questionnaire utility index (VFQ-UI).Citation30,Citation31 The VFQ-28 consists of 28 vision targeted questions that can generate vision-targeted sub-scales including, global vision, difficulties with near/distance vision activities, limitations following these axes due to vision: limitations in social functioning, role limitations, dependency on others and mental health, driving difficulties, limitations with peripheral vision and color vision, and ocular pain. The results of these PROMs consist of composite scores (averaging of the vision-targeted sub-scales) and the generated VFQ-28 sub-scales averaging from each items, presented as range between 0 and 100 scores (0 being the worst possible score, 100 being the best).Citation35
The VFQ-UI is a vision-specific utility index that was developed by including 6 items from 6 NEI VFQ-25 sub-scales (near vision activities, distance vision activities, vision-specific social functioning, role difficulties, dependency, and mental health) and 8 health states preference values rather than 15,625 states were estimated. There are 3-steps to generate a VFQ-UI. Firstly, recording the values of each 6 items from respondents by using the scoring system. Secondly, estimating the severity (theta) score from the provided regression equation.
Estimated theta score = 2.6387 + [(−0.8296 * I6R1) + (−0.3246 * I6R2) + (−0.1918 * I6R3) + (−0.1226 * I6R4)] + [(−0.5809 * I11R1) + (−0.3172 * I11R2) + (−0.2629 * I11R3) + (−0.1275 * I11R4)] + [(−0.6473 * I14R1) + (−0.3067 * I14R2) + (−0.2671 * I14R3) + (−0.1742 * I14R4)] + [(−0.5067 * I18R1) + (−0.1751 * I18R2) + (−0.1382 * I18R3) + (−0.0996 * I18R4)] + [(−0.4555 * I20R1) + (−0.2172 * I20R2) + (−0.1932 * I20R3) + (−0.1447 * I20R4)] + [(−0.3692 * I25R1) + (−0.1485 * I25R2) + (−0.1561 * I25R3) + (−0.0924 * I25R4)]
where I = item; R = response category (after step 1 recoding), and its value is determined by I[n]R[k] = 1, when response to item n is k (after step 1 recoding), I[n]R[k] = 0, otherwise. Finally, the theta score is used to estimate the utility index from the equation.
Utility score = 0.87397 + (0.0009 * age) + (−0.10619 * predicted theta) + (−0.11218 * predicted theta squared) + (0.02779 * predicted theta cubed).Citation30,Citation31
Statistical Analysis
The data were analyzed by JASP Version 0.9.0.1. Descriptive statistics were applied to describe demographic data and results. The differences of demographic data, such as sex and other underlying diseases, between the normal and glaucoma groups, and within the glaucoma severity group, were tested by using Chi-squared test and the Fisher’s exact test. The unpaired t-test and one-way analysis of variance (ANOVA) were used to assess differences of mean age, utility index score, EQ-VAS score, VFQ-28 score and VFQ-UI score between the normal and glaucomatous group and within the glaucoma groups. A p<0.05 was considered statistically significant.
Results
A total of 174 participants were included. There were 47 participants without glaucoma and 127 glaucoma participants. The glaucoma group included 35 participants in the early stage, 43 moderate stage, 30 in the severe stage with normal vision, 14 with blindness in one eye, and 5 with blindness in both eyes. The mean age of the normal group was 63.78±6.84 years old, similarly with the mean age in the glaucomatous group, 66.30±8.93 years old (p=0.062). Both males and females participated in equal proportions. Participants’ underlying diseases were comparable ().
Table 1 Demographic Data of Normal and Glaucomatous Group
Results of the VFQ-UI and VFQ-28 were statistically significantly lower in the glaucomatous group as compared with the normal group, whereas the generic utility index and EQ-VAS results were indifferent ().
Table 2 EQ-5D-5L, EQ VAS, VFQ-UI and VFQ-28 Between Normal and Glaucomatous Groups
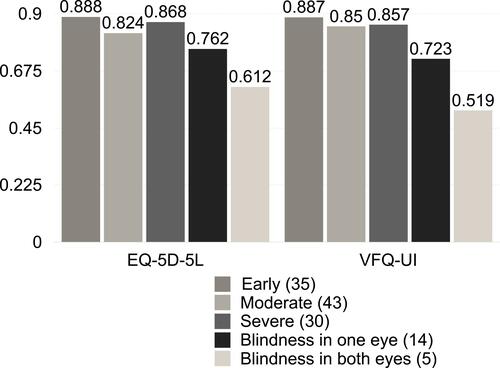
The visually impaired groups showed worse scores as compared to the normal vision group, accordingly: early, moderate, severe, blindness in one eye and blindness in both eyes, respectively ( and ). Due to the central visual field sparing nature of glaucoma diseases, quality of life of visually intact groups was unaffected unless they are in the more advanced stages of the disease.
Table 3 EQ-5D-5L, EQ-VAS, VFQ-UI and VFQ-28 Within Glaucomatous Groups
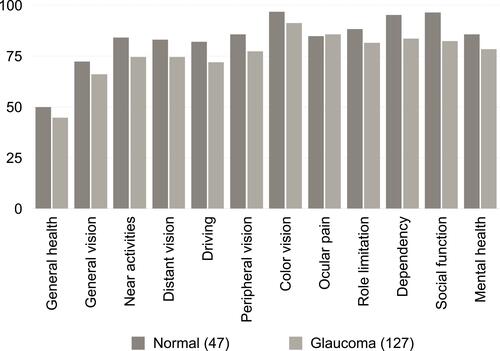
VFQ-28 scale scores were compared between the normal and glaucomatous groups. Almost all visual-related item scores were lower in the glaucomatous group than in the normal group. The glaucomatous group scored worse than the normal group in both dependency and social function items ( and ).
Table 4 VFQ-28 Scale Scores Between Normal and Glaucomatous Groups
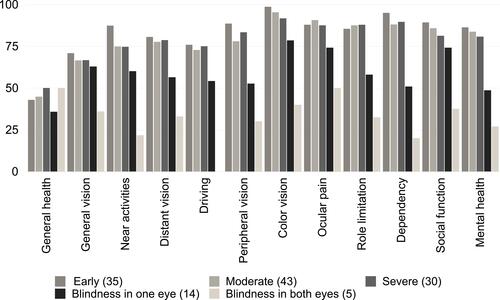
When comparing within the glaucoma group, the visually impaired groups (blindness in one eye and blindness in both eyes) showed significantly worse scores than the visually intact groups ( and ).
Table 5 VFQ-28 Scale Scores Within Glaucomatous Groups
Discussion
A utility index is a significant outcome measurement applied in cost-utility and cost-effectiveness evaluation models. In some countries, these methods are mandatory in the health technology assessment and healthcare decision-making process. However, patient-reported outcome measures (PROMs) that represent a disease’s impact from a patient’s perspective are also equally important. The QOL of glaucoma patients evaluated by EQ-5D-5L and SF-6D, representing the utility index score, showed insignificant differences between early stage glaucoma and healthy participants. However, this ability was increased in visually impaired, constricted visual field, and severe stage respondents.Citation15,Citation36–Citation39 Our results are in good agreement with the above. EQ-5D-5L and EQ VAS results in the glaucoma group were not statistically different from that of the non-glaucoma group. However, when comparing within the glaucoma group, the EQ-5D-5L results were worse for respondents in the severe stages of the disease and the visually impaired. The precision of this test was degraded because central vision sparing was frequently observed even in very late stages of glaucoma.Citation36,Citation40,Citation41 According to its structure, 5 dimensions of the EQ-5D-5L evaluate mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Therefore, the total utility score is indirectly affected by visual impairment. This infers low sensitivity and determination capability of the EQ-5D-5L and EQ-VAS among different stages in visually intact glaucoma respondents, as well as the ability to distinguish between glaucoma and normal participants. McClure et al reported the minimally important difference (MID) range of 0.037 to 0.069 for the EuroQOL’s EQ-5D-5L index scores, and group mean values of 0.069 (0.007) and 0.048 (0.004) for Chinese and Japanese, respectively.Citation42 Although our EQ-5D-5L index score showed a mean difference of 0.037 between glaucoma and non-glaucoma groups, equal to the lowest MID value from the aforementioned studies, the results were not different statistically. This may suggest inadequate power when estimating sample size, in which 0.05 was applied as the absolute error.
In contrast, the vision-specific NEI-VFQ 25 evaluated in chronic eye disease patients, such as those with dry eye syndrome,Citation43 cataracts,Citation44,Citation45 diabetic macular oedema,Citation46,Citation47 age-related macular degeneration,Citation48 as well as glaucoma,Citation17 revealed a negative correlation between the QOL scores and severity of the disease or participants’ remaining vision. In the same way, our VFQ-28ʹ scores (adapted from the NEI-VFQ 25) were also inversely related to the severity of glaucomatous damage. Although the VFQ-28 can efficiently discriminate between normal and glaucomatous participants, we could not evaluate the utility index and utility value from this vision-specific QOL for applications in health economics assessment. Several studies have generated utility index scores by mapping the NEI-VFQ 25 to EuroQol. However, the predictive power of this approach has been proven to be low (squared Spearman correlation coefficient, rs=0.34 and Ordinary Least Square, adjusted R2=0.3349).Citation49,Citation50 Other methods of assessing the utility scores in glaucoma were time trade-off (TTO) and standard gamble (gamble for blindness) methods.Citation51 Lower scores were observed in the more severe stages of the disease.Citation52–Citation54 However, these methods may not be compatible within the Thai cultural context in which death and disability are not openly discussed.Citation55,Citation56
The VFQ-UI would be a potentially useful PROMs in eye diseases, because it can evaluate both the QOL from the patient’s perspective and utility indices for health economics assessment. In this study the VFQ-UI could determine the difference between normal and glaucoma groups. Although the difference of VFQ-UI between severity stages within glaucoma groups was not distinct, the difference was found between the visually intact and visually impaired groups. Goh et al had studied the validity of the VFQ-UI as a measurement of vision-related function and preference-based status in glaucomatous patients.Citation57 Even though they observed good convergent and divergent validity but the limitation of this PROMs was limited by poor targeting, similarly to our results. The discriminating power would increase in the more severe and visually impaired groups.
Over-rating was one of our limitations. According to Thailand’s normative database, the EQ-5D utility index was 0.694 (in stratified age-range of 55–64 years old) and 0.670 (in stratified age-range of 65–74 years old)Citation58 which was lower than our utility index results from both the normal and glaucoma groups. The average age of glaucoma groups was older than that of the non-glaucoma group by 3 years but was not statistically significant (63.78±6.84 vs 66.30±8.93 year-old, p=0.062). In Thailand, the elderly are classified as those over 60 years old as the retirement age. Therefore, the 3 years of difference may not affect the results.
Assessing QOL is significant both from perspectives of patients and the society. The ideal glaucoma-specific QOL is still being developed, as it has been throughout the natural history of the disease. The VFQ-UI is a promising candidate as both utility index and PROMs of glaucoma patients due to the ease of use and acceptable validity.
Conclusions
The impact of glaucomatous disease can evaluate from various perspectives. Eye specific utility index results were significantly lower and more specific in the glaucomatous group than in the normal group. Therefore, the VFQ-UI could potentially be a useful tool for assessing the QOL of glaucomatous patients.
Abbreviations
EQ-5D-5L, European Quality of Life questionnaire; EQ-VAS, European visual analogue scale score; NEI VFQ-25, National Eye Institute 25-Item Visual Function Questionnaire; VFQ-28, visual function questionnaire 28-Thai version; VFQ-UI, visual specific utility index from the visual function questionnaire-utility index; PROMs, patients-reported outcome measures.
Acknowledgments
Thanks to Dr. Puwat Charukamnoetkanok for proofreading this article.
Disclosure
The authors report no conflicts of interest in this work.
References
- Quaranta L, Riva I, Gerardi C, Oddone F, Floriani I, Konstas AGP. quality of life in glaucoma: a review of the literature. Adv Ther. 2016;33(6):959–981. doi:10.1007/s12325-016-0333-6
- Janz NK, Wren PA, Guire KE, Musch DC, Gillespie BW, Lichter PR. Fear of blindness in the collaborative initial glaucoma treatment study: patterns and correlates over time. Ophthalmology. 2007;114(12):2213–2220. doi:10.1016/j.ophtha.2007.02.014
- Skalicky S, Goldberg I. Depression and quality of life in patients with glaucoma: a cross-sectional analysis using the geriatric depression scale-15, assessment of function related to vision, and the glaucoma quality of life-15. J Glaucoma. 2008;17(7):546–551. doi:10.1097/IJG.0b013e318163bdd1
- Kwon M, Huisingh C, Rhodes LA, McGwin G, Wood JM, Owsley C. Association between glaucoma and at-fault motor vehicle collision involvement among older drivers. Ophthalmology. 2016;123(1):109–116. doi:10.1016/j.ophtha.2015.08.043
- Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105(11):2099–2104. doi:10.1016/S0161-6420(98)91133-2
- Heijl A, Bengtsson B, Hyman L, Leske MC. Natural history of open-angle glaucoma. Ophthalmology. 2009;116(12):2271–2276. doi:10.1016/j.ophtha.2009.06.042
- Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505–1516. doi:10.1016/S0140-6736(18)32213-X
- Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388(10052):1389–1397. doi:10.1016/S0140-6736(16)30956-4
- Leske MC, Heijl A, Hyman L, Bengtsson B, Komaroff E. Factors for progression and glaucoma treatment: the early manifest glaucoma trial. Curr Opin Ophthalmol. 2004;15(2):102–106. doi:10.1097/00055735-200404000-00008
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311(18):1901. doi:10.1001/jama.2014.3192
- Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1–9.e2. doi:10.1016/j.ajo.2011.05.033
- Tham CC, Li EY, Chan PP. Cost-effectiveness in the treatment of glaucoma. US Ophthalmic Rev. 2014;07(02):131. doi:10.17925/USOR.2014.07.02.131
- Jung KI, Park CK. Mental health status and quality of life in undiagnosed glaucoma patients: a nationwide population-based study. Medicine (Baltimore). 2016;95(19):e3523. doi:10.1097/MD.0000000000003523
- Wilson MR, Coleman AL, Yu F, et al. Functional status and well-being in patients with glaucoma as measured by the medical outcomes study short form-36 questionnaire. Ophthalmology. 1998;105(11):2112–2116. doi:10.1016/S0161-6420(98)91135-6
- Bozzani FM, Alavi Y, Jofre-Bonet M, Kuper H. A comparison of the sensitivity of EQ-5D, SF-6D and TTO utility values to changes in vision and perceived visual function in patients with primary open-angle glaucoma. BMC Ophthalmol. 2012;12(1):43. doi:10.1186/1471-2415-12-43
- Mills T, Law SK, Walt J, Buchholz P, Hansen J. Quality of life in glaucoma and three other chronic diseases: a systematic literature review. Drugs Aging. 2009;26(11):933–950. doi:10.2165/11316830-000000000-00000
- Parrish RK, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol. 1997;115(11):1447–1455. doi:10.1001/archopht.1997.01100160617016
- Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ field test investigators. Arch Ophthalmol. 1998;116(11):1496–1504. doi:10.1001/archopht.116.11.1496
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi:10.1001/archopht.119.7.1050
- Lee BL, Gutierrez P, Gordon M, et al. The glaucoma symptom scale. A brief index of glaucoma-specific symptoms. Arch Ophthalmol. 1998;116(7):861–866. doi:10.1001/archopht.116.7.861
- Goldberg I, Clement CI, Chiang TH, et al. Assessing quality of life in patients with glaucoma using the glaucoma quality of life-15 (GQL-15) questionnaire. J Glaucoma. 2009;18(1):6–12. doi:10.1097/IJG.0b013e3181752c83
- Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006;21(5):402–408. doi:10.1093/heapol/czl018
- Robinson R. Cost-utility analysis. Br Med J. 1993;307(6908):859. doi:10.1136/bmj.307.6908.859
- Griebsch I. Economic evaluation in health care: merging theory with practise: M Drummond, A McGuire (eds). New York: Oxford University Press, 2001, pp. 286, £26.50 (PB). ISBN: 0-19-263176-4; £ 52.50 (HB) ISBN: 0-19-163177-2. Int J Epidemiol. 2002;31(4):877–878. doi:10.1093/ije/31.4.877-a
- Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21. doi:10.1093/bmb/ldq033
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi:10.1007/s11136-011-9903-x
- Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717–1727. doi:10.1007/s11136-012-0322-4
- J. P. Health-Related Quality of Life Measure (EQ-5D-5L): Measurement Property Testing and Its Preference-Based Score in Thai Population. Bangkok: Mahidol University; 2014.
- Tripop S, Pratheepawanit N, Asawaphureekorn S, Anutangkoon W, Inthayung S. Health related quality of life instruments for glaucoma: a comprehensive review. J Med Assoc Thai. 2005;88 Suppl 9(Suppl 9):S155–162.
- Kowalski JW, Rentz AM, Walt JG, et al. Rasch analysis in the development of a simplified version of the national eye institute visual-function questionnaire-25 for utility estimation. Qual Life Res. 2012;21(2):323–334. doi:10.1007/s11136-011-9938-z
- Rentz AM, Kowalski JW, Walt JG, et al. Development of a preference-based index from the national eye institute visual function questionnaire–25. JAMA Ophthalmol. 2014;132(3):310. doi:10.1001/jamaophthalmol.2013.7639
- National Clinical Guideline Centre. National Institute for Health and Clinical Excellence: Guidance. Patient Experience in Adult NHS Services: Improving the Experience of Care for People Using Adult NHS Services: Patient Experience in Generic Terms. London: Royal College of Physicians (UK) Copyright © 2012, National Clinical Guideline Centre; 2012.
- Hodapp EPRI, Anderson DR. Clinical Decisions in Glaucoma. St Louis: The CV Mosby Co; 1993.
- Agota Szende BJ, Cabases J, ed. Self-Reported Population Health: An International Perspective Based on EQ-5D. Dordrecht: Springer; 2014.
- The national eye institute 25-item Visual Function Questionnaire (VFQ-25); 2000; Available from: https://www.rand.org/content/dam/rand/www/external/health/surveys_tools/vfq/vfq25_manual.pdf.
- Kobelt G, Jonsson B, Bergstrom A, Chen E, Linden C, Alm A. Cost-effectiveness analysis in glaucoma: what drives utility? Results from a pilot study in Sweden. Acta Ophthalmol Scand. 2006;84(3):363–371. doi:10.1111/j.1600-0420.2005.00621.x
- Thygesen J, Aagren M, Arnavielle S, et al. Late-stage, primary open-angle glaucoma in Europe: social and health care maintenance costs and quality of life of patients from 4 countries. Curr Med Res Opin. 2008;24(6):1763–1770. doi:10.1185/03007990802111068
- van Gestel A, Webers CA, Beckers HJ, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye (Lond). 2010;24(12):1759–1769. doi:10.1038/eye.2010.133
- Choi S, Choi JA, Kwon JW, Park SM, Jee D. Utility values for glaucoma patients in Korea. PLoS One. 2018;13(5):e0197581. doi:10.1371/journal.pone.0197581
- Aspinall PA, Johnson ZK, Azuara-Blanco A, Montarzino A, Brice R, Vickers A. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49(5):1907–1915. doi:10.1167/iovs.07-0559
- Pan Y, Varma R. Natural history of glaucoma. Indian J Ophthalmol. 2011;59 Suppl(Suppl1):S19–S23. doi:10.4103/0301-4738.73682
- McClure NS, Sayah FA, Xie F, Luo N, Johnson JA. Instrument-defined estimates of the minimally important difference for EQ-5D-5L index scores. Value Health. 2017;20(4):644–650. doi:10.1016/j.jval.2016.11.015
- Nichols KK, Mitchell GL, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea. 2002;21(6):578–583. doi:10.1097/00003226-200208000-00009
- Javed U, McVeigh K, Scott NW, Azuara-Blanco A. Cataract extraction and patient vision-related quality of life: a cohort study. Eye (Lond). 2015;29(7):921–925. doi:10.1038/eye.2015.70
- Skalicky SE, Martin KR, Fenwick E, Crowston JG, Goldberg I, McCluskey P. Cataract and quality of life in patients with glaucoma. Clin Exp Ophthalmol. 2015;43(4):335–341. doi:10.1111/ceo.12454
- Klein R, Moss SE, Klein BE, Gutierrez P, Mangione CM. The NEI-VFQ-25 in people with long-term type 1 diabetes mellitus: the wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2001;119(5):733–740. doi:10.1001/archopht.119.5.733
- Lloyd AJ, Loftus J, Turner M, Lai G, Pleil A. Psychometric validation of the visual function questionnaire-25 in patients with diabetic macular edema. Health Qual Life Outcomes. 2013;11(1):10. doi:10.1186/1477-7525-11-10
- Orr P, Rentz AM, Margolis MK, et al. Validation of the national eye institute visual function questionnaire-25 (NEI VFQ-25) in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(6):3354–3359. doi:10.1167/iovs.10-5645
- Kay S, Ferreira A. Mapping the 25-item National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) to EQ-5D Utility Scores. Ophthalmic Epidemiol. 2014;21(2):66–78. doi:10.3109/09286586.2014.888456
- Browne C, Brazier J, Carlton J, Alavi Y, Jofre-Bonet M. Estimating quality-adjusted life years from patient-reported visual functioning. Eye (Lond). 2012;26(10):1295–1301. doi:10.1038/eye.2012.137
- Gupta V, Srinivasan G, Mei SS, Gazzard G, Sihota R, Kapoor KS. Utility values among glaucoma patients: an impact on the quality of life. Br J Ophthalmol. 2005;89(10):1241–1244. doi:10.1136/bjo.2005.068858
- Saw SM, Gazzard G, Au Eong KG, Oen F, Seah S. Utility values in Singapore Chinese adults with primary open-angle and primary angle-closure glaucoma. J Glaucoma. 2005;14(6):455–462. doi:10.1097/01.ijg.0000185434.08051.82
- Sun X, Zhang S, Wang N, et al. Utility assessment among patients of primary angle closure/glaucoma in China: a preliminary study. Br J Ophthalmol. 2009;93(7):871–874. doi:10.1136/bjo.2008.139295
- Zhang S, Liang Y, Chen Y, Musch DC, Zhang C, Wang N. Utility analysis of vision-related quality of life in patients with glaucoma and different perceptions from ophthalmologists. J Glaucoma. 2015;24(7):508–514. doi:10.1097/IJG.0000000000000056
- Sakthong P. Measurement of clinical-effect: utility. J Med Assoc Thai. 2008;91 Suppl 2(Suppl 2):S43–52.
- Mahlich J, Dilokthornsakul P, Sruamsiri R, Chaiyakunapruk N. Cultural beliefs, utility values, and health technology assessment. Cost Eff Resour Alloc. 2018;16(1):19. doi:10.1186/s12962-018-0103-1
- Goh RL, Fenwick E, Skalicky SE. The visual function questionnaire: utility index: does it measure glaucoma-related preference-based status? J Glaucoma. 2016;25(10):822–829. doi:10.1097/IJG.0000000000000441
- Pattanaphesaj J, Thavorncharoensap M, Ramos-Goni JM, Tongsiri S, Ingsrisawang L, Teerawattananon Y. The EQ-5D-5L valuation study in Thailand. Expert Rev Pharmacoecon Outcomes Res. 2018;18(5):551–558. doi:10.1080/14737167.2018.1494574