Abstract
Purpose
This study aimed to compare the effect of trabectome surgery in patients with and without intolerance to their medication and with preoperatively sufficiently controlled, insufficiently controlled, and uncontrolled intraocular pressure (IOP) on the surgical outcome.
Patients and Methods
A total of 155 eyes (133 patients) with different forms of open angle glaucoma with or without intolerance to their glaucoma medication undergoing trabectome surgery alone (AIT) or combined with phacoemulsification (phaco-AIT) were included in this retrospective monocentric study. Patients were corresponding to IOP ≤ 18 mmHg (controlled but glaucoma progression or intolerance, group 1), 19–26 mmHg (insufficiently controlled, group 2), and ≥ 26 mmHg (not controlled, group 3), respectively. Pre- and postoperative IOP and the number of IOP-lowering medications were registered over 12 months. Surgical success was defined as a postoperative IOP of ≤18mmHg and/or reduction of the topical treatment demand after 1 year.
Results
Of the 155 included eyes, 79 received AIT and 76 received phaco-AIT. Sixty-nine eyes had a preoperatively sufficiently controlled IOP, 63 had an insufficiently controlled IOP, and 23 had an uncontrolled IOP. In all groups, the IOP significantly dropped by 6 and 12 months after surgery (p < 0.001). Surgical success war similar in all groups [47.8% (group 1), 38.1 (group 2) and 34.8% (group 3); p= 0.47]. The effect of AIT on IOP and glaucoma medication independent of intolerance to the anti-glaucoma medication and type of surgery (AIT/phaco-AIT).
Conclusion
Independently of the preoperative IOP, a satisfying surgical success was achieved using AIT. In instances that do not qualify for filtrating surgery, trabectome surgery alone or in combination with phacoemulsification thus represents a safe and effective minimally invasive glaucoma surgery technique regardless of an intolerance to the topical medication.
Introduction
Ab interno trabeculectomy (AIT) or trabectome surgery uses electro-ablation of the trabecular meshwork and the roof of Schlemm’s canal with the Trabectome® under gonioscopic control over up to four clock hours. This technique has been on the European market since 2009.Citation1 The device was approved by the Food and Drug Administration (FDA) in 2004 and has gained widespread use based on its minimally invasive nature, which makes it ideal for ambulant outpatient surgery.Citation2 Since its launch, several studies have proven the safety and efficacy of this procedure. With clear-cornea access, the short surgical duration under topical anesthesia, and a low complication rate, AIT has definite advantages compared with other glaucoma surgical techniques. Different studies have reported satisfying outcomes, specifically if trabectome operation is combined with cataract surgery (phaco-AIT).Citation1,Citation2
Here, we compare the outcome of AIT and phaco-AIT regarding their IOP-lowering effect and postoperative treatment demand in patients with a sufficiently controlled, an insufficiently controlled, and an uncontrolled preoperative IOP, and with or without intolerance to their topical medication.
Patients and Methods
In this retrospective study, we included a consecutive series of 155 eyes from 133 patients (between 2015 and 2017) under pre-operative anti-glaucoma therapy qualifying for minimally invasive surgery, but not for filtrating glaucoma surgery, based on a mild to moderate glaucoma disease with a postoperative IOP target pressure in the mid to high teens and a preoperatively controlled IOP but progressing nerve fiber layer thinning (15–18 mmHg; group 1, n = 69), moderately uncontrolled IOP (19–26 mmHg; group 2, n = 63), or an uncontrolled IOP (> 26 mmHg; group 3, n = 23) with or without intolerance to their glaucoma medication. To be eligible for this analysis, patients had to have a confirmed diagnosis of open-angle glaucoma (significant thinning of the nerve fiber layer based on optical coherence tomography [OCT] compared with the normative database provided by the manufacturer, Heidelberg Instruments, Inc, Heidelberg, Germany), an IOP of ≥ 15 mmHg under treatment, gonioscopically visible trabecular meshwork according to Shaffer grade 2 or more, intolerance to their medication, and/or a demand of a minimum of three different anti-glaucoma drugs to control the IOP at the time of inclusion. Eyes with angle closure and neovascular glaucoma, a history of anterior segment trauma and anterior segment surgery other than cataract surgery, a history of vitrectomy with the use of silicone oil, any form of uveitis requiring treatment, a myopia of ≥ 6 diopters, as well as patients with systemic inflammatory diseases were excluded from the analysis.
All surgeries were performed in a single center by one surgeon. In short, trabectome surgery included a temporal 20-gauge paracentesis, anterior chamber lavage with lidocaine 0.5% before introduction of the trabectome into Schlemm’s canal. Under gonioscopic visualization, the trabecular meshwork was removed by electrocauterization and suction to remove the debris in the 3.5 to 4 clock hours. In combined procedures, trabectome surgery was performed before phacoemulsification and intraocular lens implantation. Fifty eyes (32.3%) had previously undergone selective laser trabeculoplasty (SLT). The primary outcome was surgical success, defined as a postoperative IOP ≤ 18 mmHg and a significant reduction in the topical medication demand 12 months after surgery without a secondary intervention. Surgical success rates were compared between eyes with at baseline sufficiently controlled, an insufficiently controlled, and an uncontrolled IOP.
Best-corrected visual acuity (BCVA) and the IOP were registered along with the number of topical and systemic IOP-lowering medications, prior to surgery: 1 day; 1 week; and 1, 3, 6, and 12 months. An intolerance to topical medication preoperatively was assumed in patients with significant and symptomatic surface irritation and conjunctival hyperemia that persisted after switching to preservative-free treatment and the addition of monodosed hyaluronic-acid-containing artificial tears.
This analysis was approved in advance by the Bern University Institutional Ethics Committee (registration number 2018–01874) based on the informed consent of all included patients to use their coded data for this retrospective analysis. The analysis followed Good Clinical Practice and strictly adhered to the current version of the Declaration of Helsinki. The coded data from this analysis are available upon request to the corresponding author.
All statistical analyses were performed using the SPSS Statistics 23.0 software package (IBM Corp., Armonk, NY, USA) and R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria). Because the data were not normally distributed, the non-parametric Mann–Whitney U-test was performed for intergroup comparisons of continuous variables. For intragroup changes, the Wilcoxon signed-rank test was applied. To test whether two categorical variables are associated, we used the chi-square test for association. The level of significance was set at p < 0.05. Data are presented as mean ± standard deviation (SD) as well as median and interquartile range (IQR).
Results
Of 155 eyes (133 patients), 69 (58 patients) had a preoperatively sufficiently controlled IOP (15–18 mmHg; group 1), 63 eyes (53 patients) had an insufficiently controlled IOP (19–26 mmHg; group 2), and 23 eyes (22 patients) had an uncontrolled IOP (> 26 mmHg; group 3). The underlying diagnoses were primary open angle glaucoma (POAG; n = 94), pseudoexfoliation glaucoma (PXF; n = 49), and other forms of open angle glaucoma (n = 12). Because our sample included 22 patients from whom both eyes were included in the sample, we explored how much variation in our main outcome variable (IOP) was due to the inter-eye correlation within the same subject. This was performed using the intraclass correlation coefficient (ICC) ρ, which measures the degree of correlation between observations within a cluster. We found an ICC of ρ = 0.001, meaning that 0.01% of the variation in IOP in our series is explained by the dependency of the data from both eyes in the same patient. Based on the simulation of Musca et al,Citation3 we interpreted this as negligible, which is why we decided not to apply multilevel modeling for the analysis.
All three groups were comparable regarding gender and age at baseline, as well as the number of topical and systemic glaucoma medications and the frequency of intolerance to their glaucoma medication ().
Table 1 Baseline Characteristics
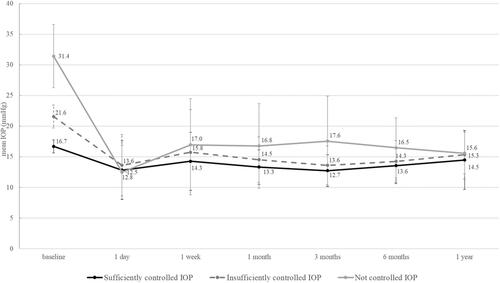
Per the aforementioned definition, the groups differed in the mean IOP before surgery (). In all three groups, the IOP dropped by 6 and 12 months after surgery (p < 0.001). In correlation with the preoperative level, the postoperative IOP differed between group 1 (a sufficiently controlled IOP) and group 3 (an uncontrolled IOP) at 1, 3, and 6 months after surgery. This difference was lost by 12 months after surgery, with a similar IOP in all groups (, ).
Table 2 Evolution of Intraocular Pressure (IOP; mmHg)
Due to 49% of AIT surgeries being combined with phacoemulsification (), in groups 1 and 2 the BCVA improved 12 months after surgery, and it remained stable in group 3, where only 26% of eyes received a combined surgery (). The strongest improvement was observed in eyes with a sufficiently controlled IOP (group 1: +6.2 ± 15.7, median +5.9, IQR 1.0 to 11.4, Wilcoxon signed rank test: p < 0.001). In group 2 and 3 eyes, visual acuity increased moderately (group 2: +1.1 ± 13.0, median +1.9, IQR −3.3 to 8.2; group 3: +1.4 ± 15.4, median +4.4, IQR −6.1 to 6.8, Wilcoxon signed rank test: p < 0.001 and p = 0.014, respectively).
We observed no difference between phaco-AIT and AIT alone regarding their IOP-lowering effect during the 12-month postoperative observation period (), except for IOP at baseline, where eyes receiving a combined procedure had a lower IOP (p = 0.017). Of the 69 eyes with a sufficiently controlled IOP, 25 (36.2%) had an intolerance to their topical IOP-reducing medication compared with 24 of the 63 eyes (38.1%) with an insufficiently controlled IOP, and 11 of 23 eyes (47.8%) with an uncontrolled IOP (p = 0.61). As expected, the effect of AIT on IOP was not linked to the presence or absence of intolerance to the anti-glaucoma medication during the first year after surgery ().
Table 3 Evolution of Intraocular Pressure (IOP; mmHg) and Type of Surgical Procedure
Table 4 Impact of Intolerance to Glaucoma Medication on Intraocular Pressure (IOP)
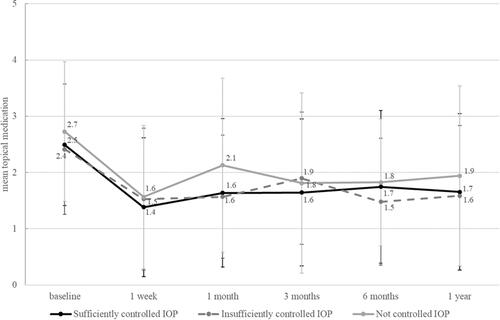
There was no difference in the number of IOP-lowering medications between the three groups at any time point (, ). The mean treatment demand was reduced from 2.5 to 1.7 drugs, representing a 32% reduction in the number of anti-glaucoma drugs. Groups 1 and 2 experienced a significant reduction in their topical glaucoma medication at 6 and 12 months after surgery (p < 0.001), group 3 (an uncontrolled IOP) did not achieve a significant reduction in topical glaucoma medication after 12 months (p = 0.09). The reduction in the number of IOP-lowering drugs was stronger in patients without an intolerance 12 months after surgery (Mann–Whitney U-test: p=0.020). Surgical success (defined as an IOP ≤ 18 mmHg along with a reduction in the number of IOP-lowering medications 12 months after surgery without a secondary intervention) was similar in the three groups: Success was observed in 41.9% of all eyes, thereof in 47.8% of eyes with a sufficiently controlled IOP (group 1), in 38.1% of eyes with an insufficiently controlled IOP (group 2), and in 34.8% of eyes with an uncontrolled IOP (group 3; p = 0.47).
Table 5 Number of Topical Glaucoma Medications
Revision surgery was required in 19 cases of the total sample. From 69 eyes with sufficiently controlled IOP, 5 (7.2%) needed a revision surgery within 12 months compared with 7 of 63 eyes (11.1%) with an insufficiently controlled IOP (p = 0.55) and 7 out of 23 eyes (30.4%) with an uncontrolled IOP (p = 0.009 compared with group 1 and p = 0.047 compared with group 2). A mild early postoperative anterior chamber bleeding 1 day after surgery was found in 18 cases, persisting for 1 week in 14 cases prior to spontaneous resolution. No further complications were observed.
Discussion
Our study revealed that independently of the preoperative IOP and treatment, a target IOP of < 16 mmHg and a reduction in the number of glaucoma medications from 2.5 to 1.7 was achieved, resulting in a surgical success in 42% of the eyes. Given the minimally invasive nature of AIT, this figure justifies its application in eyes that do not undoubtedly qualify for filtering surgery, which has a less favorable safety profile.Citation4 The outcomes in our patients are consistent with several recent reports.Citation5 Though being a simple and safe outpatient procedure with topical anesthesia, trabectome surgery has not found broad acceptance in the community due to a low predictability of success in a given case and the absence of randomized clinical trials confirming its effect and success rates.Citation6 Because the effect of AIT in one eye of a given patient seems predictive of the second eye,Citation7 it has to be assumed that as yet undefined individual rather than local factors drive the outcome. A 32% reduction (from 2.5 to 1.7) of anti-glaucoma medications, by contrast, is possibly not sufficiently meaningful to justify the use of AIT as a standard treatment in patients with intolerance to their medication if the IOP is sufficiently controlled. However, if cataract surgery is indicated, a combined phaco-AIT might well be worth considering. Given the increasing incidence of ocular surface problems in the context of glaucoma treatment,Citation8 this reduction may also attract clinical attention.
Several reports in recent years have described a relevant IOP reduction after AIT.Citation1,Citation5,Citation9,Citation10 Esfandiari et alCitation10 reported an IOP decrease from 20.0 ± 5.6 mmHg at baseline to 15.6 ± 4.6 mmHg at 5-year follow-up after phaco-AIT (p= 0.001), and a reduction in IOP-lowering medications from 1.8 ± 1.2 to 1.0 ± 1.2 medications at year five. Surgical success was stronger in PXF glaucoma,Citation10 which meets our experience, but was not in the scope of this study and is consistent with other studies.Citation1,Citation11–Citation13 A stronger effect in PXF may be linked to the pathogenesis of PXF, namely a clogging of the trabecular meshwork (TM) with fibrillar material, which is removed along with a TM strip with the trabectome.Citation11
A significant impact on IOP after AIT and phaco-AIT is in line with the results of Avar et al,Citation1 who reported a 28% decrease in IOP in POAG and 26% in PXF, along with a reduction in the number of IOP-lowering medications of 32% for POAG and 29% for PXF.Citation1 This compares well to a reduction of 32% in our patients, but cannot be generalized since the IOP-lowering effect seems to be closely linked to the preoperative IOP as our data demonstrate. The previously reported rate of 4.3% (4/9) of re-surgeries in combined phaco-AIT after a follow up time of 60 monthsCitation7 compares well to our outcomes within 12 months (5.3%, 4/76), whereas the number of revision surgeries in AIT alone in our series was higher (19.0%, 15/79, p = 0.013).
Another study revealed that phaco-AIT shows an equal IOP-lowering effect and a similar number of glaucoma medications at 1-year post-intervention compared with the much more traumatizing combined trabeculectomy with mitomycin C and cataract surgery (phaco-Trab) in POAG,Citation14 whereas severe complications were only observed in phaco-Trab. This also represents our experience in phaco-Trab, whereas only one revision surgery in their phaco-AIT groupCitation14 seems surprisingly low compared with our series.
The risk of postoperative complications after AIT is generally low. One study revealed minimal effects to corneal endothelial cells after trabectome surgery.Citation15 Although early postoperative hyphema is common, it does not require a specific treatment. Other complications are generally rare and similar to those seen in cataract surgery, resulting in a safety profile that is favorable compared to other glaucoma surgeriesCitation2,Citation16 and is in line with the lack of severe complication observed in our series of 155 eyes.
Changes to the ocular surface induced by long-term anti-glaucoma treatment, especially by preservatives, are a major concern associated with glaucoma eye drops.Citation17 Furthermore, ocular side effects to topical medications often have an impact on treatment compliance. Trabectome surgery may be beneficial not only for IOP reduction, but also for improving ocular surface conditions and visual acuity in response to a reduction in the number of IOP-lowering eye drops.Citation18 One third of our patients underwent surgery because of intolerance to IOP-lowering medication. It has been reported that intolerance occurs in up to 50% of patients with IOP-lowering therapy, and 10% of these patients have severe manifestations.Citation8 The use of preservatives in glaucoma drops may cause ocular surface disease (OSD), but preservatives considerably extend the shelf-life of medications and patients are able to administer their drops in a convenient way.Citation8 Intolerance most often presents with hyperemia and ocular discomfort, which are associated with dissatisfaction.Citation8,Citation19 In many patients, this dissatisfaction leads to reduced compliance with glaucoma therapy. Preservative-free eye drops are an alternative, but they cost much more than the equivalent eye drops with preservatives and can lead to OSD, as reported in several articles.Citation19,Citation20 It is important to discuss options for patients experiencing OSD. As a minimally invasive procedure, AIT could be an interesting option in a given case that does not limit other filtering options in the further course if they are needed; this is especially important for those who need to minimize exposure to both topical agents and preservatives, as demonstrated in our patients.
The limitations of our study include the small sample size, the rather short follow-up period, and the retrospective nature of this consecutive case series. While there are studies with larger number of patients and longer follow-up, but most of these studies did not compare the effect of AIT in association with the baseline IOP and do not provide information about intolerance to IOP-lowering medications or the number of postoperative revision surgeries. In the vast majority of these studies, a controlled IOP and need for IOP-lowering medications was reported beyond 1 year,Citation10,Citation13 which further supports our findings and indicates that the 1-year results predict the long-term outcomes. While in eyes with a preoperative IOP < 18 mmHg the IOP-lowering effect is not relevant, an IOP reduction of close to 50% was achieved for a baseline IOP > 25 mmHg. A higher risk of revision surgeries may be the price for this benefit.Citation21 Differences in the baseline IOP may well explain why some studies enthusiastically report about the IOP-lowering effect of AIT whereas others do not see a relevant impact. In general, a relevant IOP-lowering effect must not be expected at a baseline IOP below 20 mmHg. Based on published evidence, it seems that the cases that ideally qualify for a significant drop in IOP (ie, with a preoperative IOP above 30 mmHg) are not scheduled for AIT, but for filtering surgical techniques.Citation22 Our study was not sufficiently powered to assess other possible outcome factors such as axial length and state after SLT.Citation23
Conclusion
AIT represents an effective, safe, and minimally invasive intervention in glaucoma therapy, to achieve a similar surgical success, independently of the preoperative IOP. While the IOP-lowering effect of 13.2% was not so strong in patients with a preoperative IOP of ≤ 18 mmHg, there was a 29.2% reduction in eyes with a preoperative IOP of 19–25 mmHg, and a 50.3% reduction in eyes with an IOP of ≥ 26 mmHg. In eyes with intolerance to their IOP-lowering medications, AIT has a limited effect (−0.8 medications or 32% reduction) on the postoperative treatment demand.
Disclosure
Juliana Wons and Nadine Mihic share the first authorship. JGG acts as advisor for several pharmaceutical companies including Novartis, Bayer, Chengdu-Kanghong, and Allergan, and contributes to several international industry-sponsored clinical studies in the fields of retinal disease and uveitis. This manuscript is independent of these activities. None of the authors received direct or indirect support for this study nor do they have conflicting interests with the data that are presented herein.
References
- Avar M, Jordan JF, Neuburger M, et al. Long-term follow-up of intraocular pressure and pressure-lowering medication in patients after ab-interno trabeculectomy with the trabectome. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):997–1003. doi:10.1007/s00417-019-04259-5
- Polat JK, Loewen NA. Combined phacoemulsification and trabectome for treatment of glaucoma. Surv Ophthalmol. 2017;62(5):698–705. doi:10.1016/j.survophthal.2016.03.012
- Musca SC, Kamiejski R, Nugier A, Méot A, Er-Rafiy A, Brauer M. Data with hierarchical structure: impact of intraclass correlation and sample size on type-I error. Front Psychol. 2011;2:74. doi:10.3389/fpsyg.2011.00074
- Ahmed SF, Bhatt A, Schmutz M, Mosaed S. Trabectome outcomes across the spectrum of glaucoma disease severity. Graefes Arch Clin Exp Ophthalmol. 2018;256(9):1703–1710. doi:10.1007/s00417-018-4023-8
- Tojo N, Abe S, Hayashi A. Factors that influence of trabectome surgery for glaucoma patients. J Glaucoma. 2017;26(9):835–844. doi:10.1097/IJG.0000000000000743
- Hu K, Shah A, Virgili G, Bunce C, Gazzard G. Ab interno trabecular bypass surgery with trabectome for open-angle glaucoma. Cochrane Database Syst Rev. 2021;2:CD011693. doi:10.1002/14651858.CD011693.pub3
- Kiessling D, Rennings C, Hild M, et al. Predictability of ab-interno trabeculectomy success in the subsequent eye: a contralateral eye comparison study. Clin Exp Ophthalmol. 2021;49(3):242–250. doi:10.1111/ceo.13905
- Bresson-Dumont H. [Ocular intolerance to antiglaucoma medications is underestimated]. Bull Soc Belge Ophtalmol. 2010;315:47–53. French.
- Esfandiari H, Taubenslag K, Shah P, et al. Two-year data comparison of ab interno trabeculectomy and trabecular bypass stenting using exact matching. J Cataract Refract Surg. 2019;45(5):608–614. doi:10.1016/j.jcrs.2018.12.011
- Esfandiari H, Shah P, Torkian P, et al. Five-year clinical outcomes of combined phacoemulsification and trabectome surgery at a single glaucoma center. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):357–362. doi:10.1007/s00417-018-4146-y
- Okeke CO, Miller-Ellis E, Rojas M. Trabectome success factors. Medicine. 2017;96(24):e7061. doi:10.1097/MD.0000000000007061
- Mosaed S. The first decade of global trabectome outcomes. Eur Ophth Rev. 2014;8(2):113–119. doi:10.17925/EOR.2014.08.02.113
- Pahlitzsch M, Davids AM, Zorn M, et al. Three-year results of ab interno trabeculectomy (Trabectome): berlin study group. Graefes Arch Clin Exp Ophthalmol. 2018;256(3):611–619. doi:10.1007/s00417-017-3882-8
- Ting JLM, Rudnisky CJ, Damji KF. Prospective randomized controlled trial of phaco-trabectome versus phaco-trabeculectomy in patients with open angle glaucoma. Can J Ophthalmol. 2018;53(6):588–594. doi:10.1016/j.jcjo.2018.01.033
- Kasahara M, Shoji N, Matsumura K. The influence of trabectome surgery on corneal endothelial cells. J Glaucoma. 2019;28(2):150–153. doi:10.1097/IJG.0000000000001128
- Bendel RE, Patterson MT. Long-term effectiveness of trabectome (ab-interno trabeculectomy) surgery. J Curr Glaucoma Pract. 2018;12(3):119–124. doi:10.5005/jp-journals-10028-1256
- Detry-Morel M. Side effects of glaucoma medications. Bull Soc Belge Ophtalmol. 2006;299:27–40.
- Kashiwagi K, Matsubara M. Reduction in ocular hypotensive eyedrops by ab interno trabeculotomy improves not only ocular surface condition but also quality of vision. J Ophthalmol. 2018;2018:8165476. doi:10.1155/2018/8165476
- Ramli N, Supramaniam G, Samsudin A, Juana A, Zahari M, Choo MM. Ocular surface disease in glaucoma: effect of polypharmacy and preservatives. Optom Vis Sci. 2015;92(9):e222–e226. doi:10.1097/OPX.0000000000000542
- Pérez-Bartolomé F, Martínez-de-la-casa JM, Arriola-Villalobos P, Fernández-Pérez C, Polo V, García-Feijoó J. Ocular surface disease in patients under topical treatment for glaucoma. Eur J Ophthalmol. 2017;27(6):694–704. doi:10.5301/ejo.5000977
- Tojo N, Hayashi A. The outcomes of trabectome surgery in patients with low, middle, and high preoperative intraocular pressure. Clin Ophthalmol. 2020;14:4099–4108. doi:10.2147/OPTH.S285883
- Gillmann K, Mansouri K. Minimally invasive glaucoma surgery: where is the evidence? Asia Pac J Ophthalmol. 2020;9(3):203–214. doi:10.1097/APO.0000000000000294
- Kuusniemi A-M, Lindbohm N, Allinen P, Koskinen M, Harju M. Ab interno trabeculotomy: key prognostic factors. J Glaucoma. 2020;29(3):211–216. doi:10.1097/IJG.0000000000001432