Abstract
Purpose:
To evaluate the intraocular pressure (IOP)-lowering efficacy of goniosynechialysis (GSL) for advanced chronic angle-closure glaucoma (CACG) using a simplified slit-lamp technique.
Patients and methods:
Patients with CACG with one severely affected eye with best-corrected visual acuity below 20/200 and a mildly or functionally unaffected fellow eye were enrolled in this study. All patients underwent ophthalmologic examinations including measurement of visual acuity, best-corrected visual acuity, and IOP; biomicroscopy; specular microscopy; fundus examination; and gonioscopy followed by anterior chamber paracentesis and GSL for nasal peripheral anterior synechiae in the eye with severe CACG.
Results:
Thirty patients (18 men, 12 women) were identified as having CACG with an initial mean IOP of 47.1 ± 6.7 mmHg (range 39–61 mmHg) in the severely affected eye. One week after GSL, the mean IOP of the treated eyes decreased to 19.3 ± 2.8 mmHg (range 14–26 mmHg) without antiglaucoma medication (average decrease 27.7 ± 6.5 mmHg; range 16–41 mmHg), which was significant (P < 0.00001) compared with baseline. After an average follow-up period of 36.6 ± 1.0 months (range 35–38 months), the mean IOP stabilized at 17.4 ± 2.2 mmHg (range 12–21 mmHg). The nasal angle recess did not close again in any one of the patients during the follow-up period. The average significant (P < 0.00001) decrease in corneal endothelial cell density in the treated eyes was 260 ± 183 cells/mm2 (range 191–328 cells/mm2).
Conclusions:
Anterior chamber paracentesis and GSL lowers IOP in advanced CACG, though it may lead to mild corneal endothelial cell loss.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Glaucoma, the second leading cause of blindness worldwide, affects about 66.8 million people and causes bilateral blindness in 6.7 million individuals.Citation1 In Asia, primary angle-closure glaucoma is the major form of the disease. Chronic angle-closure glaucoma (CACG) is highly prevalent in China, India, and other South-East Asian countries, in which about 40% of the world’s population lives.Citation2–Citation15
Peripheral anterior synechiae (PAS) around the circumference of the anterior chamber (AC) angle is thought to cause CACG, which blocks aqueous humor outflow in the trabecular meshwork-Schlemm’s canal pathway and the uveoscleral pathway.Citation16–Citation23 As PAS formation is a quiet and gradual process, intraocular pressure (IOP) in the affected eye may increase slowly. As a result, most patients with CACG are asymptomatic and tolerate the elevated IOP well until a very late stage of the disease. When patients notice any problem, they may already have severe visual disability, accounting for the high rate of blindness.Citation6,Citation9,Citation18
Compared with primary open-angle glaucoma, the etiology of CACG seems simpler and more like an anatomic disorder. Adhesion between the peripheral iris and AC angle wall mechanically blocks the aqueous humor outflow from entering the trabecular meshwork and/or ciliary band, leading to IOP elevation and glaucomatous neuropathy.Citation6,Citation8,Citation9,Citation11,Citation14,Citation16,Citation18,Citation19,Citation21–Citation24 Etiologic treatment, one of the most fundamental and important principles in medicine, has not been applied fully in the treatment of CACG, due to technical difficulties. Although it is widely accepted that PAS formation causes CACG, PAS dissection and reopening of the closed angle have not been emphasized as essential in the management of CACG. The treatment protocols for CACG are similar to those of primary open-angle glaucoma, which has far more complicated etiology and pathogenesis, except for peripheral laser iridotomy (PI) to relieve pupillary block and/or laser peripheral iridoplasty (LPI) to widen the AC angle before medication or surgery.Citation6,Citation7,Citation9,Citation11,Citation13,Citation17,Citation18,Citation22,Citation24,Citation25 Antiglaucoma medication is prescribed routinely after laser therapy if IOP exceeds 21 mmHg or glaucomatous neuropathy or visual defect are confirmed. For advanced cases, filtration surgery, mostly trabeculectomy, is performed, because medication alone cannot decrease the elevated IOP to the target level.
PAS dissection has not been highlighted in the treatment of CACG, because of a few factors. First, PAS develops in the circumference of the AC angle and is difficult to access during surgery. In clinical practice, visualization of the AC angle can be achieved only with a goniolens, which covers the cornea and prevents intraocular procedures. To dissect PAS in the AC angle through the cornea, a modified goniolens is required, which aids the visualization of the angle but interferes with the manipulation of the dissection. This barrier might prevent surgeons from considering dissection of PAS as a treatment for CACG. Second, it is unclear whether the synechiae between the iris root and angle wall leads to functional impairment of the trabecular meshwork. If this is the case, PAS dissection will not benefit aqueous humor outflow and will be meaningless as a management strategy. Finally, many currently available antiglaucoma medications, ie, carbonic anhydrase inhibitors, β-blockers, and prostaglandin analogs, are effective in patients with CACG,Citation24–Citation29 and that success has dampened the enthusiasm to develop more rational treatments for CACG.
The goal of the current study was to evaluate the IOP-lowering effect of PAS dissection on CACG with a novel slit-lamp procedure that treated 180° of PAS.
Materials and methods
Patients
CACG was defined as glaucomatous optic neuropathy with a compatible visual field defect or visual disability and at least 180° of synechial angle closure on dynamic gonioscopy. CACG is a bilateral ocular disorder, although the morbidity and manifestations are usually asymmetric. It is common in clinical practice for a patient with CACG to have one severely damaged eye, referred to as the glaucomatous eye in the current study, while the fellow eye is mildly or not functionally affected at the initial diagnosis, referred to as the fellow eye in the current study.
The inclusion criteria included a best-corrected visual acuity (BCVA) of less than 20/200 in the glaucomatous eye. The exclusion criteria included a history of ocular trauma, inflammation, intraocular surgery, laser peripheral iridoplasty, an episode of acute angle-closure glaucoma, a ciliary cyst or tumor identified by ultrasound biomicroscopy, and age below 18 years or above 80 years.
Surgical intervention
In the current study, we introduced an innovative slit-lamp procedure, AC paracentesis and goniosynechialysis (GSL), as a surgical intervention. It is derived and developed from paracentesis, which is an established technique for lowering elevated IOP in acute angle-closure glaucoma, a similar ocular disorder secondary to a narrow or closed angle.
Before the procedure, the patients underwent detailed ophthalmologic examinations that included measurement of the VA, BCVA, biomicroscopy, and IOP; specular microscopy, fundus examination; gonioscopy; ultrasound biomicroscopy; and Humphrey visual field analysis if applicable. The width of the AC angle recess was graded in all four quadrants using Shaffer’s classification system with dynamic gonioscopy.
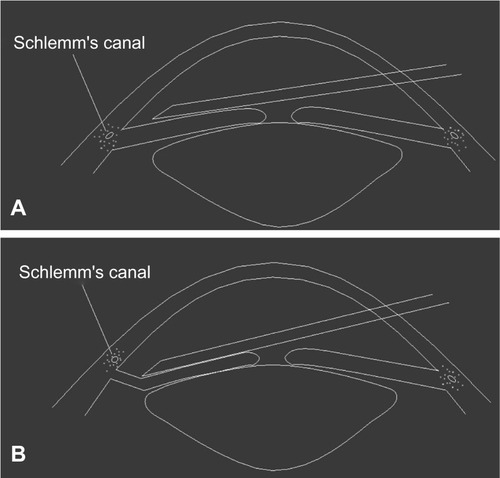
In AC paracentesis and GSL, the needle was inserted into the AC and advanced to the opposite periphery over the iris plane. To avoid interpersonal surgical skill bias, the same doctor (GQ) performed AC paracentesis-guided GSL. To prepare the eyes for surgery, two drops of tobramycin 0.3% and two drops of sterile pilocarpine 2% were instilled in the surgical eye twice at an interval of 5 minutes. Three drops of sterile Alcaine (proparacaine, Alcon, Fort Worth, TX) was applied as topical anesthesia. The patient sat in front of the slit lamp with the beam focused on the nasal AC and iris. A 26-gauge needle with a syringe was inserted into the AC through the peripheral cornea of the inferotemporal quadrant. The needle tip was advanced to the inferior AC angle over the iris plane. To dissect the PAS, the needle tip was placed on the iris root with the bevel at the front side, which was visualized through the slip-lamp biomicroscope (). The surgeon then pushed the iris root backward to drag it down from the angle wall and trabecular meshwork surface (). The procedure began inferiorly at the 6 o’clock position and moved clockwise to the 9 o’clock position nasally. To separate the superonasal quadrant, the needle was withdrawn and reinserted into the AC at the peripheral cornea of the superotemporal quadrant. PAS dissection in the superonasal quadrant started at the 9 o’clock position and moved clockwise to the 12 o’clock position superiorly. Care must be taken to avoid touching the crystalline lens when pushing the nasal iris root near the 9 o’clock position. Four to five pushes were done in each quadrant to dissect the PAS. At the completion of the procedure, tobramycin and dexamethasone ointment (Tobradex, Alcon) were instilled into the conjunctival sac before the eye was patched. Postoperative care included instillation of tobramycin and dexamethasone eye drops four times daily from postoperative days 1 to 7.
Figure 1 (A) A 26-gauge needle is inserted into the anterior chamber angle in front of the iris before peripheral anterior synechiae dissection. (B) The surgeon pushed the iris root backward to drag it down from the angle wall and trabecular meshwork surface.
The patients were examined on postoperative days 1 and 7, 1 month, and every 6 months. PI was performed in both the glaucomatous eye and the fellow eye to relieve pupillary block at the second follow-up visit 1 week after GSL. Antiglaucoma medication was prescribed if the postoperative IOP exceeded 21 mmHg.
Primary outcomes
The follow-up examinations included measurement of VA, BCVA, and IOP; biomicroscopy; gonioscopy at each visit; and measurement of the corneal endothelial cell count at the last visit. Postoperative IOP, corneal endothelial cell density, and angle status on gonioscopy were the primary outcome measurements.
Statistical analysis
The means of postoperative IOP 1 week later and corneal endothelial cell density counted at the last follow-up visit were compared with baseline with a paired sample t-test. The significance level was set at 5%. All statistical analyses were carried out using Statistical Package for the Social Sciences version 12.0 (SPSS, Chicago, IL).
Clinical trial registration
Clinical trial registration of this study was done on the Chinese Clinical Trial Registry website (http://www.chictr.org), where the registration information is publicly available.
Statement of ethics
This study was conducted in Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing, China, after the institutional review board and ethics committee approved the study. All patients provided informed consent after detailed information about the procedure was provided.
Results
Thirty patients (18 men, 12 women) were diagnosed with CACG and enrolled from November 2007 to February 2008 at the Beijing Tongren Eye Center. Each patient had a glaucomatous eye that was severely affected and a fellow eye that was mildly affected or functionally normal. The average patient age was 53.1 ± 12.6 years (range 28–74 years). At the initial diagnosis, the average IOP values of the glaucomatous eyes and the fellow eyes were 47.1 ± 6.7 mmHg (range 39–61 mmHg) and 19.3 ± 4.8 mmHg (range 11–29 mmHg), respectively. After limited GSL, the IOP of the glaucomatous eyes decreased dramatically to 24.5 ± 5.0 mmHg (range 17–38 mmHg) on postoperative day 1 and 19.3 ± 2.8 mmHg (range 14–26 mmHg) 1 week later. The average decrease in IOP of the glaucomatous eyes after treatment was 27.7 ± 6.5 mmHg (range 16–41 mmHg), which was significantly different compared with that at diagnosis (P = 0.000, paired samples t-test). At the last follow-up visit (average 36.6 ± 1.0 months; range 35–38 months), the mean IOP of the glaucomatous eyes was 17.4 ± 2.2 mmHg (range 12–21 mmHg). Nine patients were receiving pilocarpine as an adjunctive medication, because the follow-up IOP increased to 21 mmHg or higher. Following the medical treatment, the IOP decreased into the teens. Daily pilocarpine eye drops were also prescribed for patient 7, who had the highest IOP of 21 mmHg at the last visit. She had had stable postoperative IOP below 21 mmHg, but IOP increased to 21 mmHg only at the last visit.
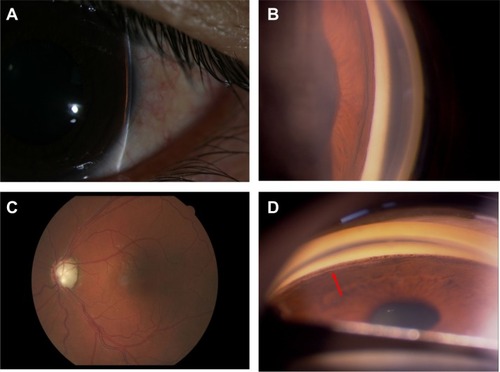
Synechial angle closure of 360° was seen in the AC angle of all eyes that underwent GSL in which the trabecular meshwork band was invisible on dynamic gonioscopy. After AC paracentesis and GSL were performed for 180° nasally, the trabecular meshwork band in the nasal quadrants was exposed. Remnant pigment granules were commonly seen on Schwalbe’s line and the anterior trabecular meshwork surface (). No recurrence of PAS formation or angle closure was discerned in the nasal angle of the treated eyes during the study period.
Figure 2 (A) Peripheral anterior chamber angle width assessment using the Von Herrick method on biomicroscopy in the left eye of patient 3. (B) Dynamic gonioscopy shows that the nasal angle is closed due to massive peripheral anterior synechiae. (C) The cup of the optic nerve head in the left eye is enlarged to the edge of the disc (vertical cup/disc ratio of 1.0). (D) The trabecular meshwork is exposed after the procedure (arrow), with remnant pigment granules on the Schwalbe’s line and trabecular meshwork.
No cataract formation or decrease in VA or BCVA was revealed in any one of the treated eyes posterior to limited GSL. The mean pretreatment corneal endothelial cell density of the glaucomatous eyes was 2318 ± 509 cells/mm2 (range 1423–2980 cells/mm2), which decreased to 2060 ± 468 cells/mm2 (range 1167–2865 cells/mm2) at follow-up 36.6 ± 1.0 months (range 35–38 months) later. The average decrease in the corneal endothelial cell density was 260 ± 183 cells/mm2 (range 16–754 cells/mm2), which was statistically significant (P = 0.000, t = 7.753, paired samples t-test). The average pre-PI and post-PI corneal endothelial cell counts of the fellow eyes were 2513 ± 251 cells/mm2 (range 1477–3012 cells/mm2) and 2511 ± 267 cells/mm2 (range 1478–3010 cells/mm2), respectively, which did not differ significantly.
Intermittent ocular pain was the most common symptom in the glaucomatous eyes among the enrolled patients. Twenty-two of 30 (73.3%) patients reported ocular pain before the procedure. The pain resolved in all cases during the postoperative follow-up period when the IOP decreased.
AC bleeding was a common intraoperative complication, occurring in 25 (83.3%) of 30 patients. Because bleeding was usually minimal and easily stopped with application of pressure to the outer upper eyelid when the needle was withdrawn, the procedure was completed in all patients. The bleeding did not require special care or medication and was resolved within a few days. No other complications or safety problems developed intraoperatively or during the follow-up period.
Discussion
We evaluated the IOP-lowering effect of PAS dissection and reopening of the closed angle on CACG through a new and uncomplicated procedure, paracentesis-guided limited GSL, performed with a slit lamp in 30 eyes of 30 patients with CACG. The current results were impressive, in that all treated eyes achieved a substantial average decrease in IOP of 27.7 ± 6.5 mmHg (range 16–41 mmHg). No safety problems were observed with this procedure except for mild corneal endothelial cell loss.
PAS dissection and/or closed AC angle widening had been tried as a treatment for CACG but were usually conducted as adjunct procedures with other intraocular surgeries, mostly phacoemulsification.Citation30–Citation34 As a result, it was hard to evaluate the IOP-lowering efficacy of PAS dissection or angle-widening procedures on CACG. LPI is a simpler and more direct laser therapy intended to reopen the closed angle by dissecting the PAS through peripheral iris contraction.Citation18,Citation22,Citation35–Citation37 The laser burns in the peripheral iris result in iris contraction that pulls the iris posteriorly away from the trabecular meshwork and angle wall and opens the closed angle. As the iris tissue contraction is usually too weak to dissect established PAS, LPI has limited efficacy in patients with CACG.Citation22,Citation38
The use of AC paracentesis and GSL in the current study is a novel way to dissect PAS by separating the peripheral iris from the anterior AC angle wall by pushing the iris root back. It is superior to LPI laser burns in power and has showed excellent efficiency in dissecting PAS and reopening the angle recess, as shown by the gonioscopy results. Except for dissecting the PAS and reopening the closed angle, there were no other side effects of this slit-lamp procedure on the AC structures. Therefore, it was easier to evaluate the IOP-lowering efficacy of GSL on CACG. Considering that the IOP decreased dramatically in all treated eyes and only the nasal 180° of the angle was reopened, we concluded that PAS dissection effectively lowered IOP in patients with CACG, and that synechial angle closure in CACG does not necessarily result in functional impairment of the trabecular meshwork or the aqueous humor outflow pathway.
We tested our hypothesis in eyes with advanced CACG, because, if GSL works in patients with end-stage CACG, it is likely that it will be effective in mild cases, because PAS is usually wider and more established in severe cases. Conversely, if PAS dissection lowers the IOP in patients with early-stage CACG, that does not necessarily mean that the procedure will be effective in cases with advanced or end-stage CACG. The latter would have had more serious trabecular meshwork damage and functional impairment if PAS formation does lead to pathological changes and functional disability to aqueous humor outflow pathway.
In the current study, no serious intraoperative or postoperative complications developed except for minimal bleeding in the AC, which did not prevent completion of the surgery and resolved spontaneously during the first postoperative days. Although it turned out to be an effective and successful method to dissect PAS in patients with CACG, and no serious complications like endophthalmitis and cataract formation were seen in the operated eyes, we still want to warn that AC paracentesis-guided GSL is a potentially risky intraocular procedure. The inherent risks for slit-lamp procedures, such as it being difficult to maintain a sterile field, difficult to control the patient’s head position, and difficult to adjust focus during the performance, may impact on the procedure’s generalization. In this study, we used it just as a resolution for PAS dissection. And it is not one of our purposes to recommend or generalize this slit-lamp procedure for other surgeons.
To the best of our knowledge, AC paracentesis-guided GSL has never been reported in the literature as a treatment for CACG. There is no standard method of performing this novel technique. We developed this procedure from AC paracentesis and mastered the technique after performing it numerous times in CACG patients. It is not problematic to perform it from the temporal side of the cornea and separate the nasal angle. However, separating the temporal angle from the nasal cornea is much more difficult because the patient’s nose may interfere with the surgical manipulations during the procedure. Considering the difficulties of the procedure and uncertainties of the results, we dissected the PAS only in the nasal 180° of the angle, which was unlikely to cause safety issues if the treatment did not reduce the IOP in the affected eyes.
A limitation of the current study was that it was a noncomparative pilot study. The long-term outcome remains unclear, as ten patients were prescribed adjunctive pilocarpine eye drops due to an IOP increase after 3 years of follow-up. Further research, especially a randomized, multicenter, prospective, controlled study, is needed before this new slit-lamp procedure can be accepted as a treatment for CACG.
Conclusion
In summary, based on the current study, we concluded tentatively that GSL through PAS dissection effectively lowers IOP in patients with CACG, though it may cause mild corneal endothelial cell loss.
Acknowledgements
Meeting presentation: oral presentation at the World Ophthalmology Congress in Abu Dhabi, UAE, February 20, 2012. This work was supported by the Beijing Municipal Health Bureau High-level Medical Professionals Promotion Project (2011-3-044), Ministry of Science and Technology of China grant (2010IM030800), and National Nature Science Foundation of China grants (30921064, 90820307).
Disclosure
The authors declare no conflict of interest.
References
- HensonDBThampyRPreventing blindness from glaucomaBMJ2005331750912012116020831
- WangYXXuLYangHJonasJBPrevalence of glaucoma in North China: the Beijing Eye StudyAm J Ophthalmol2010150691792420970107
- SongWSunXShaoZPrevalence and causes of visual impairment in a rural North-east China adult population: a population-based survey in Bin County, HarbinActa Ophthalmol201188666967419900201
- HuangSZhengYFosterPJHuangWHeMPrevalence and causes of visual impairment in Chinese adults in urban southern ChinaArch Ophthalmol2009127101362136719822854
- LiZCuiHLiuPZhangLYangHZhangLPrevalence and causes of blindness and visual impairment among the elderly in rural southern Harbin, ChinaOphthalmic Epidemiol200815533433818850470
- HeMFosterPJJohnsonGJKhawPTAngle-closure glaucoma in East Asian and European people. Different diseases?Eye (Lond)200620131215688051
- SihotaRAgarwalHCProfile of the subtypes of angle closure glaucoma in a tertiary hospital in north IndiaIndian J Ophthalmol199846125299707844
- SoodDSoodNNAngle closure and IndiaIndian J Ophthalmol200654314714816921209
- ChewPTAungTPrimary angle-closure glaucoma in AsiaJ Glaucoma2001105 Suppl 1S7S811890283
- FosterPJOenFTMachinDThe prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar districtArch Ophthalmol200011881105111110922206
- KumarRSBaskaranMChewPTPrevalence of plateau iris in primary angle closure suspects an ultrasound biomicroscopy studyOphthalmology2008115343043417900691
- KumarRSTantiseviVWongMHPlateau iris in Asian subjects with primary angle closure glaucomaArch Ophthalmol2009127101269127219822841
- SeeJLChewPTGlaucoma in SingaporeJ Glaucoma200413541742015354082
- ShenSYWongTYFosterPJThe prevalence and types of glaucoma in malay people: the Singapore Malay eye studyInvest Ophthalmol Vis Sci20084993846385118441307
- SimDHGohLGHoTGlaucoma pattern amongst the elderly Chinese in SingaporeAnn Acad Med Singapore199827681982310101557
- NongpiurMEKuJYAungTAngle closure glaucoma: a mechanistic reviewCurr Opin Ophthalmol20112229610121252671
- NazmNGandhiMDubeySPeguJAngle closure glaucomaOphthalmology2009116122478 author reply 2478–2479.19948280
- AmerasingheNAungTAngle-closure: risk factors, diagnosis and treatmentProg Brain Res2008173314518929100
- HeMLuYLiuXYeTFosterPJHistologic changes of the iris in the development of angle closure in Chinese eyesJ Glaucoma200817538639218703949
- FriedmanDSVedulaSSLens extraction for chronic angle-closure glaucomaCochrane Database Syst Rev20063CD00555516856103
- SalmonJFSharmaTChronic angle-closure glaucomaOphthalmology200511210184416199271
- RosmanMAungTAngLPChewPTLiebmannJMRitchRChronic angle-closure with glaucomatous damage: long-term clinical course in a North American population and comparison with an Asian populationOphthalmology2002109122227223112466163
- AungTLimMCChanYHRojanapongpunPChewPTConuration of the drainage angle, intraocular pressure, and optic disc cupping in subjects with chronic angle-closure glaucomaOphthalmology20051121283215629816
- AungTChanYHChewPTDegree of angle closure and the intraocular pressure-lowering effect of latanoprost in subjects with chronic angle-closure glaucomaOphthalmology2005112226727115691562
- SihotaRSaxenaRAgarwalHCGulatiVCrossover comparison of timolol and latanoprost in chronic primary angle-closure glaucomaArch Ophthalmol2004122218518914769594
- ChengJWCaiJPLiYWeiRLA meta-analysis of topical prostaglandin analogs in the treatment of chronic angle-closure glaucomaJ Glaucoma200918965265720010242
- HowACKumarRSChenYMA randomised crossover study comparing bimatoprost and latanoprost in subjects with primary angle closure glaucomaBr J Ophthalmol200993678278619336424
- ChenMJChenYCChouCKHsuWMComparison of the effects of latanoprost and travoprost on intraocular pressure in chronic angle-closure glaucomaJ Ocul Pharmacol Ther200622644945417238812
- SakaiHShinjyoSNakamuraYNakamuraYIshikawaSSawaguchiSComparison of latanoprost monotherapy and combined therapy of 0.5% timolol and 1% dorzolamide in chronic primary angle-closure glaucoma (CACG) in Japanese patientsJ Ocul Pharmacol Ther200521648348916386090
- LaiJSThamCCChuaJKLamDSEfficacy and safety of inferior 180 degrees goniosynechialysis followed by diode laser peripheral iridoplasty in the treatment of chronic angle-closure glaucomaJ Glaucoma20009538839111039740
- LaiJSThamCCLamDSThe efficacy and safety of combined phacoemulsification, intraocular lens implantation, and limited goniosynechialysis, followed by diode laser peripheral iridoplasty, in the treatment of cataract and chronic angle-closure glaucomaJ Glaucoma200110430931511558816
- KiuchiYTsujinoCNakamuraTOtoriYMochizukiHPhacoemulsification and trabeculotomy combined with goniosynechialysis for uncontrollable chronic angle-closure glaucomaOphthalmic Surg Lasers Imaging201141334835420507020
- PapamatheakisDGDevauxACordahiGHarasymowyczPJChronic angle-closure glaucoma secondary to a suprachoroidal effusion induced by central retinal vein occlusionOphthalmic Surg Lasers Imaging200738324824917552395
- TeekhasaeneeCRitchRCombined phacoemulsification and goniosynechialysis for uncontrolled chronic angle-closure glaucoma after acute angle-closure glaucomaOphthalmology19991064669674 discussion 674–675.10201585
- SawadaAAoyamaAYamamotoTTakatsukaNLong-term therapeutic outcome of acute primary angle closure in JapaneseJpn J Ophthalmol200751535335917926112
- LamDSThamCCLaiJSLeungDYCurrent approaches to the management of acute primary angle closureCurr Opin Ophthalmol200718214615117301617
- LaiJSThamCCChuaJKPoonASLamDSLaser peripheral iridoplasty as initial treatment of acute attack of primary angle-closure: a long-term follow-up studyJ Glaucoma200211648448712483091
- AgarwalHCKumarRKalraVKSoodNNArgon laser iridoplasty: a primary mode of therapy in primary angle closure glaucomaIndian J Ophthalmol199139387901841898