Abstract
Gene therapy has emerged as a research topic of choice in recent years. The eye in particular is one of few organs of the body for which gene therapy has received Food and Drug Administration approval, and it remains a field of great interest for gene therapy development. However, its associated immune and inflammatory reactions may render the treatment ineffective or harmful, which are of particular concern for the eyes due to their susceptibility to inflammation. The severity of immune and inflammatory reactions depends on the choice of vector and its route of administration. Furthermore, most preclinical and clinical studies have shown that the dose of vectors is correlated with the degree of humoral response and ocular inflammation. The route of administration directly impacts the degree of immune and inflammatory reaction. Subretinal delivery produces a weaker humoral response than the intravitreal route. However, some studies have demonstrated that the subretinal delivery induces a stronger inflammatory reaction. On the other hand, several instances of vision loss due to severe late onset intraocular inflammation were reported in a clinical trial involving intravitreal delivery of viral vectors. When compared with the intravitreal route, suprachoroidal gene delivery has been shown to produce weaker humoral response. However, unlike the subretinal space, the suprachoroidal space is not known to have immune privilege status. Inflammatory reactions following ocular gene therapy are typically mild and most clinical and preclinical studies have shown that they can be controlled with topical, local or systemic steroids. However, severe inflammatory responses may occur and require aggressive management to avoid permanent vision loss. Further investigations are required to elucidate and expand our knowledge of inflammatory reactions, and their optimal management, following ocular gene therapy.
Introduction
Gene therapy has become an emerging treatment modality for both inherited and acquired diseases. Therapeutic genes can be delivered into the nuclei of target cells via viral and non-viral vectors. The viral vector approach utilizes the natural ability of viruses to infect human cell genomes. Viral pathologic genetic sequences are replaced by the desired therapeutic genes, and target cells are then infected with the modified viruses leading to incorporation of the therapeutic material into the nuclei. The non-viral vector approach uses different chemical and physical methods to deliver the therapeutic genetic material.Citation1
While gene therapy is used to treat inherited diseases with loss of function mutations, it can also be used to treat acquired diseases. Target cells are infected with therapeutic genes which encode for specific drugs, so that the infected cells can produce the desired drugs in vivo.Citation2 This concept has considerable potential; patients can be treated once, and their tissues are transformed into bio-factories that produce the medications indefinitely.
The eye is considered a good candidate for gene therapy; it is small and compartmentalized, requires relatively small numbers of vectors/gene copies, and has special immune response features that can favor viral-mediated gene therapy.Citation3 Gene therapy for ocular diseases has already been approved by the United States Food and Drug Administration (FDA) to treat pediatric patients with Leber congenital amaurosis harboring a particular gene deficiency, named RPE65.Citation4 Multiple promising clinical trials are currently being conducted for many other ocular diseases, described below.
Similar to every novel approach, gene therapy has its own set of challenges. Insertional oncogenesis, an inadvertent activation of oncogenes by insertion of transduced genetic material near proto-oncogenes, is a potential limiting factor.Citation5 The irreversibility and unpredictable longevity of gene therapy effects highlights the lack of control once the treatment is administered.Citation6
Different vector types and subtypes induce variable immune and inflammatory responses. These responses can nullify the effect of gene therapy or prevent repeated therapy in the same patient. In addition, different modes of delivery, whether intravitreal, subretinal or suprachoroidal, induce variable immune and inflammatory reactions. Given the etiological complexity of these responses and their detrimental effect on gene therapy efficacy, many studies have tried to analyze the factors that influence these responses.
Recently available data from clinical trials have shown that ocular gene therapy has been associated with severe ocular inflammation with resultant vision loss. Therefore, in this review, we would like to discuss ocular gene therapy with special focus on the resultant immune and inflammatory reactions in the light of the recent updates.
Different Types of Vectors for Ocular Gene Therapy
Viral vectors that are used for ocular gene therapy include adenovirus (AV), adeno-associated virus (AAV), and lentivirus. Non-viral vectors utilize different chemical and physical methods to deliver naked genetic material into the cells.Citation1
Viral Vectors
Adenovirus
Adenovirus is a double-stranded deoxyribonucleic acid (DNA) virus that can efficiently transduce dividing and non-dividing cells. It can induce a high amount of protein production by inserting numerous copies of the same gene into a cell.Citation7 Adenovirus offers multiple advantages, including broad range of tissue tropism (transduction of both retina and anterior segment), a well-characterized genome, ease of genetic manipulation, capacity of carrying large genes, non-replicative nature in a host, and producibility at a large scale.Citation3,Citation7
Although adenovirus was the first vector to be evaluated in clinical trials, it is not currently used in ocular gene therapy clinical trials except for one trial studying retinoblastoma. Adenovirus has fallen out of favor due to the resulting robust immune response that causes inflammation and elimination of the transduced cells.Citation8 Severe side effects have been reported using adenovirus, as severe as death of a patient with systemic fever and liver damage in a clinical trial for metabolic disease.Citation9 Although it does not usually replicate inside hosts, a replication-competent virus can be inadvertently created by combination of adenoviral derived vectors with pre-existing adenoviral genome in the targeted cells, causing active systemic adenoviral infection in the patient.Citation10
Adeno-Associated Virus
Adeno-associated virus (AAV) is the most commonly used vector for ocular gene therapy trials. AAV is a small (25 nm), replication defective, single-stranded DNA, non-enveloped virus belonging to the Parvoviridae family. It has been evaluated as a gene therapy vector for metabolic, hematological, ophthalmological, muscular, infectious disorders, and cancers.Citation11 Currently, 13 different AAV serotypes have been identified in primates. They differ in their capsid components and display variable cellular tropism, transduction efficiency, and immunogenicity.Citation7
Bennett et al have reported that AAV2 and AAV8 can infect retinal cells from the vitreous, but it was limited to the inner retina.Citation12 AAV2 has also been used effectively through a subretinal injection to transduce retinal pigment epithelium (RPE) in a number of gene therapy trials and animal models.Citation3,Citation4 For AAV8, subretinal delivery leads to efficient photoreceptor transduction.Citation13,Citation14 AAV2 is commonly used in ocular gene therapy trials and is the vector used in the first FDA-approved ocular gene therapy voretigene neparvovec-rzyl (Luxturna®) for Leber congenital amaurosis.Citation4
AAVs deliver the genetic material as an extragenomic circular episome and do not integrate it into the human genome. This mechanism significantly decreases the risk of insertional oncogenesis. Similar to adenovirus, AAVs can also infect dividing and non-dividing cells.Citation15,Citation16 AAVs’ limited capacity to carry large-sized genetic material restricts their use in gene delivery for some diseases that are coded by large genes such as Usher syndrome.Citation17
Unlike adenoviruses, AAVs generate relatively mild innate and adaptive immune responses, which allow for stable long-term transgene expression.Citation13,Citation15,Citation18,Citation19 These characteristics make AAV vectors particularly suited for applications in a variety of chronic ocular diseases.
Around 70% of the normal population have preexisting antibodies (Abs) against AAV2. On the other hand, AAV8 was isolated from non-human primates (NHPs). Therefore, there is a lower percentage of humans with Abs against it (around 38%).Citation20 The presence of antiviral Abs has been correlated with minimal or absent gene expression following gene therapy.Citation21 Thus, the use of AAV8-based vector therapy has the potential to be more effective than AAV2. However, there is still cross-reactivity of anti-AAV Abs against different serotypes.Citation21
AAVs can be rapidly eliminated by the humoral immune response in patients who have previously been exposed to the virus.Citation22 The immune reactions include induction of neutralizing antibodies that reduce the number of capsids reaching the target cells, innate immune pathways silencing the gene cassette within the host cell, and cell-mediated T-cell immune responses against foreign protein expression.Citation18
Lentivirus
Lentivirus is a complex, single-stranded ribonucleic acid (RNA) retrovirus that has been studied as a vector. Similar to the two previous vectors, it possesses the ability to induce a stable transduction in both dividing and non-dividing cells in a broad range of target organs.Citation23 Lentiviral vectors are derived from primate lentiviruses human immunodeficiency virus (HIV), equine infectious anemia virus (EIAV) and simian immunodeficiency virus.Citation3 Lentiviruses integrate their complementary DNA (reverse-transcribed RNA) into the chromosome of target cells enabling sustained gene expression but, like all integrative systems, can increase the risk of insertional mutagenesis and oncogenesis.Citation7,Citation24 They can be manufactured in high numbers and their genome can be deleted to reduce the inflammatory response. They also have high transgene carrying capacity allowing delivery of large-sized therapeutic genes that cannot be packed into AAV vectors. Such viral composition is particularly useful for diseases with large causative genes such as Stargardt disease and Usher syndrome.Citation22,Citation25,Citation26
Lentiviruses are possibly more immunogenic than AAVs.Citation27 Binley et al found that 5 out of 6 non-human primates that were injected with EIAV in their subretinal space developed a peripheral perivascular retinal whitening. However, this whitening (which probably represented a form of intraocular inflammation) was transient and resolved with treatment.Citation28 In general, multiple studies have shown that lentiviruses are relatively safe and can result in effective and sustained gene transduction for a significant time.Citation28–Citation30
Non-Viral Gene Therapy
Non-viral vectors were developed as an alternative to viral vectors to avoid their associated immune responses. Non-viral vectors are used to transfer large DNA-like plasmids, small DNA (eg, oligodeoxynucleotides), and RNA molecules through different chemical and physical methods.Citation31
Chemical methods include lipid-based delivery systems, polymers, and cell-penetrating peptides. They form nano-complexes with the genetic material that can either penetrate the cell membrane or be endocytosed. These complexes also protect genetic material from premature degradation. Nanoparticles carrying genetic material have been shown to effectively transduce the outer retina if injected subretinally.Citation6,Citation32
Physical methods are more variable and technically utilize different approaches to facilitate the genetic material’s delivery into the cells. Examples are electrical pulses, ultrasound, magnetic fields, gene guns, and lasers. High voltage short electrical pulses (electrotransfection) are highly efficient in transducing the ciliary body to produce the desired drug autonomously.Citation6,Citation32,Citation33
The main advantages of non-viral methods include the lower risk of immune stimulation and insertional mutagenesis (as most do not integrate with chromosomes), the ability to deliver a large amount of genetic materials, and the ease of production. Short-term expression of the transduced genetic material can be considered as a major disadvantage of this method.Citation6,Citation32
Applications of Gene Therapy in Different Ocular Diseases
The eye constitutes an excellent site for gene therapy due to its anatomy, ease of access, immune-privileged state, which limits the immune responses and inflammatory reactions against the delivered genetic product, and tight blood-ocular barriers that limit the systemic exposure of the drug. Its relatively small size also grants a minor volume of drug to have effective results. In addition, the assessment of treatment implications can be performed non-invasively via diagnostic imaging, and the second eye can be used as a control group.Citation34 Several studies are focusing on possible gene therapies for an array of ocular diseases from the cornea to the retina. A summary of completed and/or current ocular gene therapy clinical trials can be found in .
Table 1 Ocular Gene Therapy Clinical Trials*
Corneal Diseases
Gene therapy has been studied as a potential treatment option for both inherited and non-inherited corneal diseases. Examples of studied non inherited diseases for gene therapy were Herpes simplex keratitis (HSK), dry eye – Sjogren’s syndrome (SS), corneal graft rejection, and corneal neovascularization.Citation35 Mucopolysaccharidosis (MPS), Meesmann epithelial corneal dystrophy, ectrodactyly-ectodermal dysplasia-clefting (EEC) syndrome, aniridia, and Fuchs endothelial corneal dystrophy are among the inherited diseases where corneal gene therapy is applied in several animal studies.Citation35
Glaucoma
As 72% of the cases with primary open-angle glaucoma are hereditary and there is a monogenic inheritance component in juvenile-onset open-angle glaucoma, gene therapy may be beneficial.Citation36 Preclinical and Phase I studies have shown that gene therapy using small interference RNA (siRNA) that suppresses ß2-adrenergic-receptor synthesis, via topical drops, might be effective in lowering intraocular pressure (IOP) in glaucoma patients. Phase II studies have also been conducted.Citation37–Citation39
Retinal Disorders
X-Linked Retinoschisis (XLRS)
Because the retina is fragile in patients with X-linked Retinoschisis XLRS, the risk for subsequent retinal detachments increases following gene therapy if performed via the subretinal route. Therefore, the intravitreal route is preferred for the transfer of vectors in this condition.Citation40 There are currently two clinical trials in which the AAV vector expressing RS1 gene is delivered intravitreally to XLRS patients. The preliminary results were reported that the gene product was well tolerated, and ocular adverse events, including dose-related inflammation, resolved using topical and oral corticosteroids.Citation41
Stargardt Disease
Stargardt disease is caused by ABCA4 mutations leading to accumulation of lipofuscin pigment inside the RPE causing degeneration of both RPE and photoreceptor cells.Citation42 A phase I/II clinical trial investigated the utility of EIAV-vector carrying the ABCA4 gene that is delivered through the subretinal route, but it was terminated in 2015.Citation43 Other clinical trials for Stargardt Disease are being planned.
Choroideremia
Choroideremia occurs secondary to a mutation in the CHM gene leading to progressive RPE and photoreceptor cell death.Citation44 There are some challenges for gene therapy in choroideremia, including insufficient resemblance of animal models to functional and morphological manifestations of the disease, and uncertainty of which retinal layer is affected most.Citation45,Citation46 Gene therapy outcomes for choroideremia have been less successful than RPE65-related Leber congenital amaurosis (LCA).Citation47 There are currently nine clinical trials registered on ClinicalTrials.gov evaluating interventional gene therapies for these patients – all of which use AAV vectors. Only one of them uses the intravitreal route, and the remaining eight deliver the drug subretinally.
Retinitis Pigmentosa and Usher Syndrome
Retinitis Pigmentosa (RP) is a heterogeneous disease, and it occurs in an autosomal recessive (AR) pattern in 50–60% of the cases.Citation48 The most common genes include RPGR (retinitis pigmentosa GTPase regulator) which accounts for ~70% of X-linked RP; rhodopsin (RHO) which causes ~25% of AD RP, and the Usherin2A (USH2A) gene that is linked to approximately 20% of AR RP.Citation49 Currently, there are multiple clinical trials assessing different viral and non-viral vectors, through intravitreal and subretinal injections, for different types of RP.
A novel approach, which is described as optogenetic therapy, is also under investigation for retinitis pigmentosa. It is based on delivering genetic information that codes for light sensitive proteins to non-photoreceptor retinal neurons such as ganglion cells, rendering them sensitive to light stimulation and hence, bypassing the photoreceptors. This approach has the potential to improve visual perception in cases of RP and other inherited retinal diseases where the photoreceptors are severely damaged.Citation50
Usher syndrome displays AR inheritance with different large-sized causative genes, including MYO7A, USH2A and GPR98. Similar to Stargardt disease, lentiviruses are required to deliver the large-sized genetic material.Citation51,Citation52 A single clinical trial was conducted for Usher syndrome with a mutation in MYO7A gene using a lentivirus vector through a subretinal injection, but it was terminated.Citation53 Recently, a promising phase I/II clinical trial was initiated to evaluate the safety and tolerability of an intravitreal RNA antisense oligonucleotide for Usher syndrome.Citation54
Achromatopsia
For achromatopsia, there are six different gene mutations (CNGA3, CNGB3, GNAT2, PDE6C, PDE6H, and ATF6) of which CNGA3 and CNGB3 are the most common.Citation55 Clinical trials are being conducted targeting these two genes with an AAV vector through subretinal injection.
Leber Congenital Amaurosis
Leber congenital amaurosis (LCA) is an AR disease with mutations in numerous genes. LCA2 occurs specifically due to RPE65 gene mutations, a gene expressed highly in RPE cells. Despite the significant visual impairment in LCA, retinal cells and photoreceptors are relatively spared.Citation56 Therefore, RPE65-mediated LCA has shown to be a favorable candidate for ocular gene therapy as it requires a certain amount of viable cells to be effective. The FDA approved the first gene therapy for ocular disease following the Phase III clinical trial of AAV2-hRPE65v2 (voretigene neparvovec, Luxturna®) that evaluated the efficacy and safety of bilateral sequential subretinal injections to both eyes in patients with LCA.Citation4
The trial with voretigene neparvovec involved 29 pediatric patients older than 3 years with LCA and visual acuity of 20/60 or less. Participants were randomized (2:1) to intervention or control groups. The results of this trial have demonstrated that voretigene neparvovec ameliorated functional vision in RPE65-mediated LCA, and there were no treatment-related serious adverse events. Mild ocular inflammation was reported in only two patients (10%) which was resolved. The authors did not provide details regarding how the ocular inflammation was managed.Citation4
Age-Related Macular Degeneration (AMD)
Different vector therapy trials are being conducted for both non-neovascular and neovascular AMD. The safety of subretinally delivered lentiviral EIAV vector expressing endostatin and angiostatin for neovascular AMD has been established with well-tolerated doses, no dose-limiting toxicities, and little to no ocular inflammation.Citation30 Intravitreal injection of AAV carrying aflibercept gene has also shown very promising results in controlling neovascular AMD disease activity.Citation57 Additional studies are being conducted to confirm the potential role and utility of vector therapy, via various approaches, including subretinal, intravitreal, and suprachoroidal delivery, in the management of AMD.
Diabetic Retinopathy/Diabetic Macular Edema
Suprachoroidal delivery of an AAV vector carrying an anti-vascular endothelial growth factor (anti-VEGF) fab segment is being evaluated for diabetic retinopathy without central involving macular edema in a phase I clinical trial.Citation58 A clinical trial is also evaluating an intravitreally delivered AAV vector coding for aflibercept for diabetic macular edema.Citation59
Optic Nerve Diseases
Leber Hereditary Optic Neuropathy
Leber hereditary optic neuropathy (LHON), an inherited mitochondrial optic neuropathy related to retinal ganglion cell death, is most commonly linked to ND1 (G3460A), ND4 (G11778A), and ND6 (T14484C) gene mutations.Citation60 As delivering genes into the ganglion cell mitochondria is difficult, “allotopic expression” strategy (mitochondrial gene expression in nucleus) has been developed.Citation61 Different trials have demonstrated the safety and efficacy of gene therapy using an AAV vector, delivered through an intravitreal route, for LHON.Citation62–Citation64
Modes of Delivery of Vector Therapy and Associated Ocular Inflammation
Gene therapy can be administered into the eye via different ways such as intravitreal, subretinal, and suprachoroidal routes. Each mode of delivery has its own advantages and unique effects on how the immune system reacts to the vector, which in turn affects the phenotype of ocular inflammation. Different types of intraocular gene delivery are illustrated in .
Figure 1 Images showing different methods of intraocular gene delivery: (A) intravitreal, (B) suprachoroidal, and (C) subretinal.
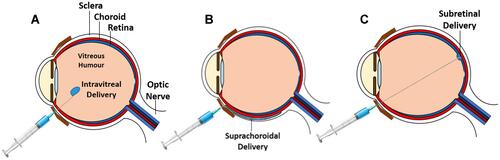
Intravitreal Delivery
The intravitreal route is the traditional route used by ophthalmologists to deliver most intraocular medications into the eye, such as anti-vascular endothelial growth factors (anti-VEGFs), antibiotics, and steroids. The intravitreal route is a convenient choice; it can be administered in office settings and requires fewer instruments and equipment than the subretinal route. In addition, it is surgically simpler, less invasive, and generally safe. Also, this method is more logistically plausible due to its relative ease in delivery. Another advantage of intravitreal gene delivery is its theoretic ability to transduce the whole retinal surface, in contrast to the localized transduction induced by subretinal delivery. Such approach may be beneficial in cases where the targeted cells are not limited only to the macula.Citation65,Citation66
There are two main limitations of the intravitreal route: the inability to transduce outer retina layers and possible immune and inflammatory reaction. The outer retina is the main target of gene therapy for most retinal diseases. Most of the retinal genetic diseases are due to defects in the RPE or photoreceptor cells.Citation67 Vectors delivered via the intravitreal route have limited ability to transduce the outer retina possibly due to the internal limiting membrane (ILM), evidenced by the improvement of outer retinal transduction after removing or degrading the ILM.Citation68,Citation69 However, other studies showed that some specially engineered vectors, via directed evolution, can transduce the outer retina after being injected intravitreally.Citation65,Citation70 If this result can be reproduced, it may revolutionize gene therapy.
Several reports showed that the vitreous cavity may be considered an immune-privileged space.Citation71–Citation73 Yet, the intravitreal vector approach has still shown a different and distinct immune response from subretinal vector delivery.Citation74 Studies on humans and NHPs have demonstrated consistently that intravitreal delivery of vectors induces a significant humoral immune response.Citation13,Citation21,Citation75,Citation76 The response is marked by the production of Abs, which may not lead to inflammation, but can significantly reduce the efficacy of treatment by attacking and eliminating transduced cells through the neutralizing antibodies. Intravitreal injection of one eye has also been shown to block vector expression in the contralateral eye when injected intravitreally with the same vector, due to the production of neutralizing Abs.Citation75
Reichel et al directly compared the degree of inflammation between intravitreal and subretinal gene therapy.Citation13 In their study, they found that the subretinal route caused more anterior and posterior segment inflammation than the intravitreal route, although the intravitreal route caused a stronger humoral response. The finding suggests that there might not be a clear association between intraocular inflammation and humoral response,Citation77,Citation78 which is further supported by the work of Bouquet et alCitation79 who showed that neither baseline Abs nor the degree of immune response, defined by increased Ab titers, correlated with the degree of intraocular inflammation.
However, Cukras et al showed that antibody titers were correlated with the degree of inflammation.Citation41 In this phase I/II clinical trial, patients were divided into low dose, intermediate dose, and high-dose groups. No patient in the low-dose group had a significant increase in their neutralizing Ab titers. Ab titers increased significantly in both intermediate and high-dose groups. In this study, anterior chamber inflammation correlated significantly with higher Ab levels. All patients of the high-dose group had high Abs titers for 18 months. It should be noted that, in this study, inflammation in all cases in this study was controlled by topical and oral steroids.
In the relatively recent INFINITY clinical trial that evaluated intravitreal AAV for diabetic macular edema (DME), late onset severe intraocular inflammation was observed. In this trial, patients were randomized to three groups: high virus dose (N=12), lower virus dose (N=13) and aflibercept (N=9) groups. Three patients in the high-dose group developed late onset significant inflammation including panuveitis, which resulted in hypotony. All three were treated with pars plana vitrectomy (PPV), silicone oil (SO) and Retisert implantation. None of the patients in the low dose or aflibercept groups developed hypotony. Most patients in both virus treatment groups developed some sort of intraocular inflammation that required difluprednate eye drops beyond a prophylactic post injection 10 weeks period. Most of the high-dose group required additional medications including steroids administered through multiple routes including oral, periocular, and intraocular. Two patients in the high-dose groups required mycophenolate therapy to further suppress intraocular inflammation. Around 50% of the low dose group required additional periocular or intravitreal steroid therapy.Citation80
Interestingly, the same viral concentrations were administered in the Optic trial for AMD, but no hypotony developed and the reported intraocular inflammation was less frequent and less severe, and all incidences were successfully treated with topical steroids only.Citation81 This clearly suggests a role of the underlying disease in the development of inflammation in addition to the route and dose. Inflammatory processes are known to be integral in the pathogenesis of diabetic retinopathy,Citation82 and this might explain the difference in rates of intraocular inflammation between eyes with diabetic retinopathy and those with AMD, despite being treated with the same vector and concentration.
Subretinal Delivery
Subretinal vector delivery requires pars plana vitrectomy. After completion of the vitrectomy, a macular retinotomy is done via a small gauge cannula. Detachment of the macula is achieved by injecting balanced saline solution followed by vector injection in the preformed bleb.Citation14,Citation83
Using the subretinal approach to deliver vector therapy is well established as it has many advantages. It ensures transduction of outer retinal layers through direct vector delivery. The efficacy of transducing the outer retina by injecting vectors through this route has been reported extensively and consistently.Citation4,Citation14,Citation29,Citation84–Citation86 The subretinal space itself has a special immunological response, which will be discussed below. As mentioned previously, voretigene neparvovec, the only FDA approved ocular gene therapy, is delivered through this approach.
The main disadvantage of this approach is the surgery itself. Traditional vitrectomy complications such as cataract, IOP rise and retinal tears, as well as subretinal injection-related complications such as a macular hole.Citation4,Citation87,Citation88 The technique itself requires significant surgical experience. Ochacovski et alCitation89 have shown that subretinal injection, in NHPs, can cause mild thinning of the outer layers of the fovea in comparison with an intravitreal injection of the same vector.Citation89,Citation90 However, the difference was not clinically significant. Photoreceptor damage was also reported in the phase I trial for the same gene therapy.
The subretinal space is immune privileged and has a deviant immune response. Immune responses to antigens in this space can be similar to anterior chamber associated immune deviation (ACAID); immune responses are suppressed by activating T helper 2 cells.Citation19,Citation91,Citation92 Many studies have shown minimal to absent humoral response to antigens delivered to this space.Citation13,Citation74,Citation75 Furthermore, Anand et al reported suppression of pre-existing cellular immune response to certain antigens after injecting them into the subretinal space.Citation19 Other studies also found that gene transduction was achievable if injected in the subretinal space even if the subjects have neutralizing antibodies.Citation74,Citation92 This beneficial immune response has raised the question of whether it is better not to use steroids with a subretinal injection, as this would theoretically negate the “good” suppressive nature of that deviant immune response.Citation13,Citation19
As mentioned above, few studies found that subretinal route can induce more inflammation compared to the intravitreal route.Citation13,Citation77,Citation78 One may consider this secondary to the surgical technique itself rather than the immune response to the vector; however, Reichel et al showed that subretinal delivery of a vector caused more inflammation than just performing the same surgical procedure without injecting a vector.Citation13 Nevertheless, to our knowledge, none of the studies that evaluated the subretinal gene delivery have reported devastating panuveitis with loss of vision similar to the above mentioned case that was associated with intravitreal delivery.
Another infrequent but potentially severe immune/inflammatory response to subretinal injection is the poorly understood phenomenon of intraretinal hyperreflective foci, found on optical coherence tomography, following subretinal injection which has been reported in several studies. It was reported at least in two human subjects in two different studies: one study had a subject who suffered from irreversible photoreceptor and vision loss despite steroid use, and another study demonstrated the adverse event in one subject but fortunately resolved following oral steroid treatment.Citation14,Citation93 It was also reported in NHPs, but resolved and the retina returned to baseline.Citation13 To our knowledge, it has not been reported with voretigene neparvovec. It is possible that this reaction represents a unique side effect of a special type of vector, but it was reported with the use of both AAV2 and AAV8 subtypes, suggesting it may not be vector specific.
Suprachoroidal Delivery
With both intravitreal and subretinal routes having their disadvantages, suprachoroidal route was studied to overcome these challenges. The suprachoroidal route is less invasive than the subretinal route. It also has the potential of a weaker immune response than the intravitreal route, leading to lower levels of produced antibodies.Citation78 It can also deliver the vectors to the outer retina, which is a main limitation of the intravitreal route.Citation78 This route is also being investigated for delivering non-viral vectors with promising results in animals.Citation94
Different techniques are being used for suprachoroidal drug delivery. It can be done via a microcatheter,Citation95–Citation97 hypodermic needle,Citation98 or microneedles.Citation99,Citation100 Microcatheters require a sclerotomy that requires visualization with a surgical microscope. Cautious dissection of the sclera is done until the scleral-choroidal junction has been reached. The blunt-tipped catheter is then inserted and directed toward the posterior pole, using a light pulp at the tip of the catheter to aid in visualizing its position. The hypodermic needle technique requires merely inserting the needle at an oblique angle through the sclera until a release sensation is just felt. Because it is a somewhat “blind” procedure, it may lead to choroidal or retinal penetration. Another technique utilizes the microneedle which is a very short needle that is applied perpendicularly into the sclera. A large clinical trial employed this method with no serious side effects.Citation99
Yiu et al have shown that humoral response to suprachoroidal gene therapy was weaker than the response to the intravitreal route, with levels of neutralizing Abs being significantly lower.Citation78 To our knowledge, this is the only published study that compared antibody production between the two routes. Although the sample size was small, and the study was on NHPs, it is very encouraging. This study has also shown that suprachoroidal delivery was associated with widespread transduction of RPE cells, in contrast to the subretinal delivery in which the transduction is localized to the macular bleb.
However, there are issues with the suprachoroidal route as well. There are inconsistent reports about its efficacy in infecting photoreceptors. Two studies involving NHPs showed conflicting results in the efficacy of photoreceptor cell uptake.Citation78,Citation101 This may be due to the difference in AAVs capsid or promoter sequence, but further studies are needed. Studies on animals other than NHPs showed that the suprachoroidal route can be used effectively to infect photoreceptors.Citation95,Citation101 Another issue is the non-sustainability of gene expression via this route, which can be gradually diminished over 3 months.Citation78 Such non-sustainability may be due to the high flow of the choroidal circulation, with a stronger immune response.
Safety of the suprachoroidal route is also an issue. Yiu et al had inadvertent subretinal injection twice by a microneedle when they changed the site of injection from 4 to 10 mm posterior to the limbus.Citation78 Other human clinical trials did not report this adverse event.Citation99,Citation100
Inflammation can also occur secondary to the suprachoroidal route. Different degrees of posterior segment inflammation following delivery of the same antigen via intravitreal, subretinal, and suprachoroidal routes have been reported. While the intravitreal route did not cause significant posterior segment inflammation in the form of chorioretinitis, both suprachoroidal and subretinal routes elicited it.Citation78 There has been no evidence of a deviant immune response in the suprachoroidal space.
The key differences between intravitreal, subretinal and suprachoroidal modes of delivery are listed in .
Table 2 Key Differences Between Different Types of Modes of Delivery of Gene Therapy
Electrotransfection
Electrotransfection is a non-viral gene therapy modality that uses high voltage short electrical pulses that increases cell membrane permeability to naked genetic material. To our knowledge, there are no published data regarding safety of electrotransfection in human eyes. In different studies performed on rabbits and rodents, there were no reported safety issues.Citation33,Citation102–Citation104 There were no histological or functional (electroretinogram) toxicities following electrotransfection. Postoperative inflammation could not be accurately assessed as the studies were done on animal models of intraocular inflammation. However, the studies demonstrated that electrotransfection was effective in decreasing ocular inflammation in these models.Citation33,Citation102–Citation104
Effect of Vector Dose and Constituents on the Type of Ocular Inflammation
Viral Capsid and Genome
Timmers et al have shown that the site of ocular inflammation following intravitreal injection varies according to the presence of the viral genome and/or capsid.Citation16 They demonstrated that inflammation in the anterior segment is dependent on the presence of the genome while viral particles with no genome (empty capsids) did not induce an anterior segment reaction. Contrarily, both full viral particles and empty capsids induced vitreal inflammation. Empty capsids induced vitreal inflammation at a lower level than the full particles, implying that capsids induced vitreal inflammation only, while the genetic material induced inflammation in both anterior and posterior segments. The investigators also found that Abs were generated regardless of the presence of the genome, meaning that it is mainly generated by the capsid.
Relationship Between Viral Dose and Ocular Inflammation
Pre-clinical and clinical studies have indicated that gene therapy with AAV can induce dose-dependent innate and adaptive immune responses. This was true in both subretinal and intravitreal deliveries, although the same viral dose induced lower immune responses when delivered via the subretinal route.Citation91,Citation105–Citation107 For example, Cukras et al found a dose-dependent response of ocular inflammation.Citation41 They evaluated the safety of AAV8 as the vector for delivery of RS1 gene in patients with XLRS caused by RS1 gene mutations in a phase I/II an open-label clinical trial. They administered three increasing doses of 1 × 109, 1 × 1010, and 1 × 1011 viral particle/eye of AAV8 to nine patients through intravitreal injections. The authors found that ocular inflammation was dose-dependent, but it resolved with administration of oral and topical corticosteroids. There was also a dose-dependent increase in the serum level of systemic antibodies against AAV8.
Another clinical trial on the safety and efficacy of subretinal AAV2 injection in patients with LCA with RPE65 gene deficiency showed comparable findings. Le Meur et al administered low (1.22–2 × 1010) or high (3.27–4.8 × 1010) viral genomes of AAV2 to nine patients.Citation108 Although no clinical abnormality was observed, a mild increase in anterior chamber protein flare was seen, indicating ocular inflammation, in only three patients who received the highest dose. The inflammation was minimal and resolved with no consequences after 14 days with local and systemic steroids. In the aforementioned INFINITY trial, rates and severity of ocular inflammation were higher in the high-dose group. Three patients developed severe hypotony in the high dose (6 × 1011) group while none of the low dose (2 × 1011) group showed such complication. The OPTIC trial, which used the same vector type in neovascular AMD patients, did also show a dose dependent inflammatory response but much less in severity when compared with INFINITY trial, implying that the underlying disease should also be considered a factor when assessing the risk of inflammation.Citation80,Citation81,Citation109
Few studies did not show this association between the inflammatory responses and the viral doses. In an open-label phase I/II randomized clinical trial, Bouquet et alCitation79 assessed the link between immune response and intraocular inflammation in 15 patients diagnosed with ND4 LHON undergoing ocular gene therapy with rAAV2 vectors. The authors introduced four increasing doses of 9 × 109, 3 × 1010, 9 × 1010, and 1.8 × 1011 viral genomes per eye through intravitreal injections. Following the injections, mild inflammatory reactions were noted in nearly all patients, regardless of the administered dose. Intensity of inflammation showed no correlation with dosage, and it was also not associated with baseline immune responses and antibody titers. Similarly, Guy et al evaluated the efficacy of AAV2 at two escalating doses of 5 × 109and 2.46 × 1010 vector genomes in LHON patients.Citation64 Their results indicated that only 14% of the eyes showed ocular inflammation over a one-year follow-up, which was not associated with vector dose. One can explain the absence of the dose dependent ocular inflammation in these studies by the small number of patients, which might not have been enough to detect the relationship.
As mentioned, Timmers et alCitation16 found that the relationship between the dose and the inflammation may be dependent on the site of intraocular inflammation.Citation16 No or weak correlation was found between the dose and degree of anterior segment inflammation (which was incited by the capsids only), while a strong correlation was noticed with vitreal inflammation (incited by both the genome and capsid).
Management of Intraocular Inflammation Following Gene Therapy
Steroid administration through different routes has been shown control ocular inflammation following viral vector delivery in most cases. Many studies reported that the resultant ocular inflammation was mild, transient, and controlled by systemic or even topical steroid therapy.Citation4,Citation13,Citation14,Citation29,Citation41,Citation79,Citation87 Such control was reported regardless of vector type/subtype or mode of delivery.
Russel et al used perioperative oral steroids at the dose of 1 mg/kg/day, up to 40 mg/day, as a prophylactic method against postoperative inflammation after subretinal delivery of AAV vector.Citation4 In their study, only 10% of the patients developed mild transient inflammation, which resolved in all patients. Bouquet et al have not used perioperative steroids in their trial which involved an intravitreal injection of AAV vector.Citation79 Most patients who developed postoperative vitreal inflammation were controlled by topical steroids. Oral steroids were used effectively in 2/11 patients who did not respond to topical therapy.
On the other hand, the results of the INFINITY trial clearly show that ocular inflammation following gene therapy does not always respond well to steroids. As mentioned before, some patients responded poorly to intravitreal steroids and required surgery as well as mycophenolate therapy to ameliorate the inflammation.
In such cases where ocular inflammation is significant and can lead to permanent visual loss, aggressive management with zero-tolerance to inflammation should be a rule of thumb to avoid vision threatening complications. It should be noted that the inflammation can develop lately up to 20 weeks following an initial inflammation-free period. Follow-up should be done with due diligence to avoid such late onset, devastating complication.
Conclusions
Gene therapy can be delivered via viral or non-viral vectors. The mode of delivery of gene therapy as well as the underlying ocular pathology influence the postoperative inflammatory and immune responses. While there is no clear evidence of weaker or less inflammatory reactions of the subretinal route when compared with the intravitreal one, immune responses with antibody formations are clearly stronger in the latter. The dose of delivered viral vectors plays a factor in the development of immune and inflammatory responses, and high dose viral vectors were associated with sight threatening ocular inflammation. In general, although the inflammation associated with gene therapy of all routes of delivery has proven to be usually mild and often controlled with steroid therapy, intravitreal route may be associated with late onset sight threatening inflammation.
Suprachoroidal delivery is a potentially simpler and safer approach to deliver gene therapy, but further studies need to determine how unique it is regarding the immune and inflammatory responses. Non-viral gene therapy is also potentially a safe alternative, but its use for inherited diseases can be limited by the short-term expression of transduced genetic materials.
Abbreviations
AAV, adeno-associated virus; Ab, antibody; ACAID, anterior chamber associated immune deviation; AMD, age-related macular degeneration; Anti-VEGF, anti-vascular endothelial growth factor; AR, autosomal recessive; AV, adenovirus; DME, diabetic macular edema; DNA, double-stranded deoxyribonucleic acid; EEC, ectrodactyly-ectodermal dysplasia-clefting; EIAV, equine infectious anemia virus; FDA, Food and Drug Administration; HIV, human immunodeficiency virus; IOP, intraocular pressure; LCA, Leber congenital amaurosis; LHON, Leber hereditary optic neuropathy; MPS, mucopolysaccharidosis; NHP, non-human primates; PPV, pars plana vitrectomy; RHO, rhodopsin; RNA, ribonucleic acid; RP, retinitis pigmentosa; RPE, retinal pigment epithelium; RPGR, retinitis pigmentosa GTPase regulator; siRNA, small interference RNA; SO, silicone oil; SS, Sjogren’s syndrome; USH2A, Usherin2A; XLRS, X-linked retinoschisis.
Disclosure
Diana Do reports grants from and consultancy for Regeneron during the conduct of the study and grants from and advisory board for Genentech outside the submitted work. Quan Dong Nguyen reports grants and personal fees from Genentech, Novartis, and Regeneron, and personal fees from Rezolute, outside the submitted work. The authors report no other potential conflicts of interest in relation to this work.
Additional information
Funding
References
- Ramamoorth M, Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. 2015;9(1):GE01–GE06. doi:10.7860/JCDR/2015/10443.5394
- Guimaraes TAC, Georgiou M, Bainbridge JWB, Michaelides M. Gene therapy for neovascular age-related macular degeneration: rationale, clinical trials and future directions. Br J Ophthalmol. 2020;105:151–157. doi:10.1136/bjophthalmol-2020-316195
- Bennett J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003;10(11):977–982. doi:10.1038/sj.gt.3302030
- Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849–860. doi:10.1016/S0140-6736(17)31868-8
- Li Z, Dullmann J, Schiedlmeier B, et al. Murine leukemia induced by retroviral gene marking. Science. 2002;296(5567):497. doi:10.1126/science.1068893
- Bordet T, Behar-Cohen F. Ocular gene therapies in clinical practice: viral vectors and nonviral alternatives. Drug Discov Today. 2019;24(8):1685–1693. doi:10.1016/j.drudis.2019.05.038
- Rodrigues GA, Shalaev E, Karami TK, Cunningham J, Slater NKH, Rivers HM. Pharmaceutical development of AAV-based gene therapy products for the eye. Pharm Res. 2018;36(2):29. doi:10.1007/s11095-018-2554-7
- Gregory SM, Nazir SA, Metcalf JP. Implications of the innate immune response to adenovirus and adenoviral vectors. Future Virol. 2011;6(3):357–374. doi:10.2217/fvl.11.6
- Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80(1–2):148–158. doi:10.1016/j.ymgme.2003.08.016
- Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89(1):21–39. doi:10.1093/jnci/89.1.21
- Bastola P, Song L, Gilger BC, Hirsch ML. Adeno-associated virus mediated gene therapy for corneal diseases. Pharmaceutics. 2020;12(8):767. doi:10.3390/pharmaceutics12080767
- Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. J Gene Med. 2008;10(4):375–382. doi:10.1002/jgm.1126
- Reichel FF, Dauletbekov DL, Klein R, et al. AAV8 can induce innate and adaptive immune response in the primate eye. Mol Ther. 2017;25(12):2648–2660. doi:10.1016/j.ymthe.2017.08.018
- Fischer MD, Michalakis S, Wilhelm B, et al. Safety and vision outcomes of subretinal gene therapy targeting cone photoreceptors in achromatopsia: a nonrandomized controlled trial. JAMA Ophthalmol. 2020;138(6):643–651. doi:10.1001/jamaophthalmol.2020.1032
- Liu MM, Tuo J, Chan CC. Gene therapy for ocular diseases. Br J Ophthalmol. 2011;95(5):604–612. doi:10.1136/bjo.2009.174912
- Timmers AM, Newmark JA, Turunen HT, et al. Ocular inflammatory response to intravitreal injection of adeno-associated virus vector: relative contribution of genome and capsid. Hum Gene Ther. 2020;31(1–2):80–89. doi:10.1089/hum.2019.144
- Pepose JS, Leib DA. Herpes simplex viral vectors for therapeutic gene delivery to ocular tissues. Recent breakthroughs in the molecular genetics of ocular diseases. Invest Ophthalmol Vis Sci. 1994;35(6):2662–2666.
- Buck TM, Wijnholds J. Recombinant Adeno-Associated Viral Vectors (rAAV)-vector elements in ocular gene therapy clinical trials and transgene expression and bioactivity assays. Int J Mol Sci. 2020;21(12):4197. doi:10.3390/ijms21124197
- Anand V, Duffy B, Yang Z, Dejneka NS, Maguire AM, Bennett J. A deviant immune response to viral proteins and transgene product is generated on subretinal administration of adenovirus and adeno-associated virus. Mol Ther. 2002;5(2):125–132. doi:10.1006/mthe.2002.0525
- Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–712. doi:10.1089/hum.2009.182
- Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22(2):116–126. doi:10.1038/gt.2014.115
- Wan C, Li F, Li H. Gene therapy for ocular diseases mediated by ultrasound and microbubbles (Review). Mol Med Rep. 2015;12(4):4803–4814. doi:10.3892/mmr.2015.4054
- Balaggan KS, Ali RR. Ocular gene delivery using lentiviral vectors. Gene Ther. 2012;19(2):145–153. doi:10.1038/gt.2011.153
- Philippe S, Sarkis C, Barkats M, et al. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc Natl Acad Sci U S A. 2006;103(47):17684–17689. doi:10.1073/pnas.0606197103
- Nuzbrokh Y, Kassotis AS, Ragi SD, Jauregui R, Tsang SH. Treatment-emergent adverse events in gene therapy trials for inherited retinal diseases: a narrative review. Ophthalmol Ther. 2020;9(4):709–724. doi:10.1007/s40123-020-00287-1
- Jiang J, Zhang X, Tang Y, Li S, Chen J. Progress on ocular siRNA gene-silencing therapy and drug delivery systems. Fundam Clin Pharmacol. 2020;35:4–24. doi:10.1111/fcp.12561
- Kalesnykas G, Kokki E, Alasaarela L, et al. Comparative study of adeno-associated virus, adenovirus, bacu lovirus and lentivirus vectors for gene therapy of the eyes. Curr Gene Ther. 2017;17(3):235–247. doi:10.2174/1566523217666171003170348
- Binley K, Widdowson P, Loader J, et al. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol Vis Sci. 2013;54(6):4061–4071. doi:10.1167/iovs.13-11871
- Binley K, Widdowson PS, Kelleher M, et al. Safety and biodistribution of an equine infectious anemia virus-based gene therapy, RetinoStat((R)), for age-related macular degeneration. Hum Gene Ther. 2012;23(9):980–991. doi:10.1089/hum.2012.008
- Campochiaro PA, Lauer AK, Sohn EH, et al. Lentiviral vector gene transfer of endostatin/angiostatin for Macular Degeneration (GEM) Study. Hum Gene Ther. 2017;28(1):99–111. doi:10.1089/hum.2016.117
- Bloquel C, Bourges JL, Touchard E, Berdugo M, BenEzra D, Behar-Cohen F. Non-viral ocular gene therapy: potential ocular therapeutic avenues. Adv Drug Deliv Rev. 2006;58(11):1224–1242. doi:10.1016/j.addr.2006.07.023
- Oliveira AV, Rosa da Costa AM, Silva GA. Non-viral strategies for ocular gene delivery. Mater Sci Eng C Mater Biol Appl. 2017;77:1275–1289. doi:10.1016/j.msec.2017.04.068
- Kowalczuk L, Touchard E, Camelo S, et al. Local ocular immunomodulation resulting from electrotransfer of plasmid encoding soluble TNF receptors in the ciliary muscle. Invest Ophthalmol Vis Sci. 2009;50(4):1761–1768. doi:10.1167/iovs.08-3027
- Solinis MA, Del Pozo-Rodriguez A, Apaolaza PS, Rodriguez-Gascon A. Treatment of ocular disorders by gene therapy. Eur J Pharm Biopharm. 2015;95(Pt B):331–342. doi:10.1016/j.ejpb.2014.12.022
- Di Iorio E, Barbaro V, Alvisi G, et al. New frontiers of corneal gene therapy. Hum Gene Ther. 2019;30(8):923–945. doi:10.1089/hum.2019.026
- Bettin P, Di Matteo F. Glaucoma: present challenges and future trends. Ophthalmic Res. 2013;50(4):197–208. doi:10.1159/000348736
- Bucolo C, Salomone S, Drago F, Reibaldi M, Longo A, Uva MG. Pharmacological management of ocular hypertension: current approaches and future prospective. Curr Opin Pharmacol. 2013;13(1):50–55. doi:10.1016/j.coph.2012.09.012
- Moreno-Montanes J, Sadaba B, Ruz V, et al. Phase I clinical trial of SYL040012, a small interfering RNA targeting beta-adrenergic receptor 2, for lowering intraocular pressure. Mol Ther. 2014;22(1):226–232. doi:10.1038/mt.2013.217
- Naik S, Shreya AB, Raychaudhuri R, et al. Small interfering RNAs (siRNAs) based gene silencing strategies for the treatment of glaucoma: recent advancements and future perspectives. Life Sci. 2021;264:118712. doi:10.1016/j.lfs.2020.118712
- Byrne LC, Ozturk BE, Lee T, et al. Retinoschisin gene therapy in photoreceptors, Muller glia or all retinal cells in the Rs1h-/- mouse. Gene Ther. 2014;21(6):585–592. doi:10.1038/gt.2014.31
- Cukras C, Wiley HE, Jeffrey BG, et al. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol Ther. 2018;26(9):2282–2294. doi:10.1016/j.ymthe.2018.05.025
- Han Z, Conley SM, Naash MI. Gene therapy for Stargardt disease associated with ABCA4 gene. Adv Exp Med Biol. 2014;801:719–724.
- Parker MA, Choi D, Erker LR, et al. Test-retest variability of functional and structural parameters in patients with Stargardt disease participating in the SAR422459 gene therapy trial. Transl Vis Sci Technol. 2016;5(5):10. doi:10.1167/tvst.5.5.10
- Seabra MC, Brown MS, Slaughter CA, Sudhof TC, Goldstein JL. Purification of component A of Rab geranylgeranyl transferase: possible identity with the choroideremia gene product. Cell. 1992;70(6):1049–1057. doi:10.1016/0092-8674(92)90253-9
- Dimopoulos IS, Chan S, MacLaren RE, MacDonald IM. Pathogenic mechanisms and the prospect of gene therapy for choroideremia. Expert Opin Orphan Drugs. 2015;3(7):787–798. doi:10.1517/21678707.2015.1046434
- Vasireddy V, Mills JA, Gaddameedi R, et al. Correction: AAV-mediated gene therapy for choroideremia: preclinical studies in personalized models. PLoS One. 2015;10(6):e0129982. doi:10.1371/journal.pone.0129982
- MacDonald IM, Moen C, Duncan JL, Tsang SH, Cehajic-Kapetanovic J, Aleman TS. Perspectives on gene therapy: choroideremia represents a challenging model for the treatment of other inherited retinal degenerations. Transl Vis Sci Technol. 2020;9(3):17. doi:10.1167/tvst.9.3.17
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. doi:10.1016/S0140-6736(06)69740-7
- Zhang Q. Retinitis pigmentosa: progress and perspective. Asia Pac J Ophthalmol. 2016;5(4):265–271. doi:10.1097/APO.0000000000000227
- Simunovic MP, Shen W, Lin JY, Protti DA, Lisowski L, Gillies MC. Optogenetic approaches to vision restoration. Exp Eye Res. 2019;178:15–26. doi:10.1016/j.exer.2018.09.003
- Mathur P, Yang J. Usher syndrome: hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852(3):406–420. doi:10.1016/j.bbadis.2014.11.020
- Hashimoto T, Gibbs D, Lillo C, et al. Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther. 2007;14(7):584–594. doi:10.1038/sj.gt.3302897
- Weleber R, Sahel J. Study of SAR421869 in participants with retinitis pigmentosa associated with usher syndrome type 1B. Identifier: NCT01505062; 2012. Available from: https://ClinicalTrials.gov/show/NCT01505062. Accessed May 26, 2022.
- National Library of Medicine (U.S.). Study to evaluate safety and tolerability of QR-421a in subjects with RP due to mutations in Exon 13 of the USH2A gene. Identifier NCT03780257; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03780257. Accessed May 26, 2022.
- Thiadens AA, Slingerland NW, Roosing S, et al. Genetic etiology and clinical consequences of complete and incomplete achromatopsia. Ophthalmology. 2009;116(10):1984–1989 e1981. doi:10.1016/j.ophtha.2009.03.053
- Chung DC, Traboulsi EI. Leber congenital amaurosis: clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS. 2009;13(6):587–592. doi:10.1016/j.jaapos.2009.10.004
- National Library of Medicine (U.S.). ADVM-022 intravitreal gene therapy for wet AMD. Identifier NCT03748784. 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03748784. Accessed May 26, 2022.
- National Library of Medicine (U.S.). RGX-314 gene therapy administered in the suprachoroidal space for participants with Diabetic Retinopathy (DR) without Center Involved-Diabetic Macular Edema (CI-DME) (ALTITUDE). Identifier NCT04567550; 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04567550. Accessed May 26, 2022.
- National Library of Medicine (U.S.). ADVM-022 Intravitreal Gene Therapy for DME (INFINITY). Identifier NCT04418427; 2020. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04418427. Accessed May 26, 2022.
- Cwerman-Thibault H, Augustin S, Ellouze S, Sahel JA, Corral-Debrinski M. Gene therapy for mitochondrial diseases: Leber Hereditary Optic Neuropathy as the first candidate for a clinical trial. C R Biol. 2014;337(3):193–206. doi:10.1016/j.crvi.2013.11.011
- Bonnet C, Augustin S, Ellouze S, et al. The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes. Biochim Biophys Acta. 2008;1783(10):1707–1717. doi:10.1016/j.bbamcr.2008.04.018
- Wan X, Pei H, Zhao MJ, et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy. Sci Rep. 2016;6:21587. doi:10.1038/srep21587
- Feuer WJ, Schiffman JC, Davis JL, et al. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123(3):558–570. doi:10.1016/j.ophtha.2015.10.025
- Guy J, Feuer WJ, Davis JL, et al. Gene therapy for Leber hereditary optic neuropathy: low- and medium-dose visual results. Ophthalmology. 2017;124(11):1621–1634. doi:10.1016/j.ophtha.2017.05.016
- Dalkara D, Byrne LC, Klimczak RR, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5(189):189ra176. doi:10.1126/scitranslmed.3005708
- Klimczak RR, Koerber JT, Dalkara D, Flannery JG, Schaffer DV. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Muller cells. PLoS One. 2009;4(10):e7467. doi:10.1371/journal.pone.0007467
- Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21(3):509–519. doi:10.1038/mt.2012.280
- Takahashi K, Igarashi T, Miyake K, et al. Improved intravitreal AAV-mediated inner retinal gene transduction after surgical internal limiting membrane peeling in cynomolgus monkeys. Mol Ther. 2017;25(1):296–302. doi:10.1016/j.ymthe.2016.10.008
- Dalkara D, Kolstad KD, Caporale N, et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther. 2009;17(12):2096–2102. doi:10.1038/mt.2009.181
- Byrne LC, Day TP, Visel M, et al. In vivo-directed evolution of adeno-associated virus in the primate retina. JCI Insight. 2020;5(10). doi:10.1172/jci.insight.135112
- Sonoda KH, Sakamoto T, Qiao H, et al. The analysis of systemic tolerance elicited by antigen inoculation into the vitreous cavity: vitreous cavity-associated immune deviation. Immunology. 2005;116(3):390–399. doi:10.1111/j.1365-2567.2005.02239.x
- Jiang LQ, Streilein JW. Immune privilege extended to allogeneic tumor cells in the vitreous cavity. Invest Ophthalmol Vis Sci. 1991;32(1):224–228.
- Atherton SS, Pesicka GA, Streilein JW. Retinitis and deviant immune responses following intravitreal inoculation of HSV-1. Invest Ophthalmol Vis Sci. 1987;28(5):859–866.
- Reichel FF, Peters T, Wilhelm B, et al. Humoral immune response after intravitreal but not after subretinal AAV8 in primates and patients. Invest Ophthalmol Vis Sci. 2018;59(5):1910–1915. doi:10.1167/iovs.17-22494
- Li Q, Miller R, Han PY, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008;14:1760–1769.
- Marangoni D, Wu Z, Wiley HE, et al. Preclinical safety evaluation of a recombinant AAV8 vector for X-linked retinoschisis after intravitreal administration in rabbits. Hum Gene Ther Clin Dev. 2014;25(4):202–211. doi:10.1089/humc.2014.067
- Ramachandran PS, Lee V, Wei Z, et al. Evaluation of dose and safety of AAV7m8 and AAV8BP2 in the non-human primate retina. Hum Gene Ther. 2017;28(2):154–167. doi:10.1089/hum.2016.111
- Yiu G, Chung SH, Mollhoff IN, et al. Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates. Mol Ther Methods Clin Dev. 2020;16:179–191. doi:10.1016/j.omtm.2020.01.002
- Bouquet C, Vignal Clermont C, Galy A, et al. Immune response and intraocular inflammation in patients with Leber hereditary optic neuropathy treated with intravitreal injection of recombinant adeno-associated virus 2 carrying the ND4 gene: a secondary analysis of a phase 1/2 clinical trial. JAMA Ophthalmol. 2019;137(4):399–406. doi:10.1001/jamaophthalmol.2018.6902
- Wykoff Obotii CC. Intravitreal gene therapy for diabetic macular edema with ADVM-022: first-time data presentation of prospective, randomized phase 2 INFINITY trial [Conference presentation]. American Society of Retina Specialists Annual Scientific Meeting; October 8-12, 2021; 2021; San Antonio, Texas, USA.
- Dante Pieramici O. ADVM-022 intravitreal gene therapy for neovascular AMD: phase 1 OPTIC study [Conference presentation]. American Society of Retina Specialists Annual Scientific Meeting; October 8-12, 2021; 2021; San Antonio, Texas, USA.
- Rubsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19(4):942. doi:10.3390/ijms19040942
- Xue K, Groppe M, Salvetti AP, MacLaren RE. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye. 2017;31(9):1308–1316. doi:10.1038/eye.2017.158
- Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi:10.1089/hum.2008.107
- Seitz IP, Michalakis S, Wilhelm B, et al. Superior retinal gene transfer and biodistribution profile of subretinal versus intravitreal delivery of AAV8 in nonhuman primates. Invest Ophthalmol Vis Sci. 2017;58(13):5792–5801. doi:10.1167/iovs.17-22473
- Manfredi A, Marrocco E, Puppo A, et al. Combined rod and cone transduction by adeno-associated virus 2/8. Hum Gene Ther. 2013;24(12):982–992. doi:10.1089/hum.2013.154
- Constable IJ, Pierce CM, Lai CM, et al. Phase 2a randomized clinical trial: safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168–175. doi:10.1016/j.ebiom.2016.11.016
- Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi:10.1056/NEJMoa0802315
- Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9–24. doi:10.1001/archophthalmol.2011.298
- Ochakovski GA, Peters T, Michalakis S, et al. Subretinal injection for gene therapy does not cause clinically significant outer nuclear layer thinning in normal primate foveae. Invest Ophthalmol Vis Sci. 2017;58(10):4155–4160. doi:10.1167/iovs.17-22402
- Bucher K, Rodriguez-Bocanegra E, Dauletbekov D, Fischer MD. Immune responses to retinal gene therapy using adeno-associated viral vectors - implications for treatment success and safety. Prog Retin Eye Res. 2020;83:100915. doi:10.1016/j.preteyeres.2020.100915
- Weed L, Ammar MJ, Zhou S, et al. Safety of same-eye subretinal sequential readministration of AAV2-hRPE65v2 in non-human primates. Mol Ther Methods Clin Dev. 2019;15:133–148. doi:10.1016/j.omtm.2019.08.011
- Dimopoulos IS, Hoang SC, Radziwon A, et al. Two-year results after AAV2-mediated gene therapy for choroideremia: the Alberta experience. Am J Ophthalmol. 2018;193:130–142. doi:10.1016/j.ajo.2018.06.011
- Shen J, Kim J, Tzeng SY, et al. Suprachoroidal gene transfer with nonviral nanoparticles. Sci Adv. 2020;6(27). doi:10.1126/sciadv.aba1606
- Peden MC, Min J, Meyers C, et al. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS One. 2011;6(2):e17140. doi:10.1371/journal.pone.0017140
- Olsen TW, Feng X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142(5):777–787. doi:10.1016/j.ajo.2006.05.045
- Rizzo S, Ebert FG, Bartolo ED, et al. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina. 2012;32(4):776–784. doi:10.1097/IAE.0b013e3182278b0e
- Chen M, Li X, Liu J, Han Y, Cheng L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release. 2015;203:109–117. doi:10.1016/j.jconrel.2015.02.021
- Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: phase 3 randomized trial. Ophthalmology. 2020;127(7):948–955. doi:10.1016/j.ophtha.2020.01.006
- Barakat MR, Wykoff CC, Gonzalez V, et al. Aflibercept with or without suprachoroidal CLS-TA for diabetic macular edema: a randomized, double-masked, parallel-design, controlled study. Ophthalmol Retina. 2020;5:60–70. doi:10.1016/j.oret.2020.08.007
- Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest. 2019;129(11):4901–4911. doi:10.1172/JCI129085
- Touchard E, Benard R, Bigot K, et al. Non-viral ocular gene therapy, pEYS606, for the treatment of non-infectious uveitis: preclinical evaluation of the medicinal product. J Control Release. 2018;285:244–251. doi:10.1016/j.jconrel.2018.07.013
- Touchard E, Berdugo M, Bigey P, et al. Suprachoroidal electrotransfer: a nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Mol Ther. 2012;20(8):1559–1570. doi:10.1038/mt.2011.304
- Chalberg TW, Vankov A, Molnar FE, et al. Gene transfer to rabbit retina with electron avalanche transfection. Invest Ophthalmol Vis Sci. 2006;47(9):4083–4090. doi:10.1167/iovs.06-0092
- Chan YK, Dick AD, Hall SM, et al. Inflammation in viral vector-mediated ocular gene therapy: a review and report from a workshop hosted by the foundation fighting blindness, 9/2020. Transl Vis Sci Technol. 2021;10(4):3. doi:10.1167/tvst.10.4.3
- Bucher K, Rodriguez-Bocanegra E, Dauletbekov D, Fischer MD. Immune responses to retinal gene therapy using adeno-associated viral vectors - implications for treatment success and safety. Prog Retin Eye Res. 2021;83:100915.
- Mehta N, Robbins DA, Yiu G. Ocular inflammation and treatment emergent adverse events in retinal gene therapy. Int Ophthalmol Clin. 2021;61(3):151–177. doi:10.1097/IIO.0000000000000366
- Le Meur G, Lebranchu P, Billaud F, et al. Safety and long-term efficacy of AAV4 gene therapy in patients with RPE65 Leber congenital amaurosis. Mol Ther. 2018;26(1):256–268. doi:10.1016/j.ymthe.2017.09.014
- Zack DJ. Ocular gene therapy. From fantasy to foreseeable reality. Arch Ophthalmol. 1993;111(11):1477–1479. doi:10.1001/archopht.1993.01090110043019
