Abstract
Ocular involvement by plasmablastic lymphoma is extremely rare with very few reports in the literature. Its morphological and immunological resemblance to plasma cell myeloma makes it a diagnostic challenge, while its clinical course, which is characterized by recurrence and death, makes therapy a challenge for clinicians. We present three cases of plasmablastic lymphoma, each of which has distinct clinicoradiological features, and we also review the literature on orbital plasmablastic lymphomas.
Keywords:
Introduction
Ocular adnexal lymphomas are rare, accounting for only 1% to 2% of all lymphomas.Citation1 The current World Health Organization classification recognizes plasmablastic lymphoma (PBL) as a distinct subtype of diffuse large B-cell lymphoma (DLBCL), characterized by the presence of neoplastic cells resembling B immunoblasts, but that have immunophenotypic features of plasma cells.Citation2 PBL was first described to involve the oral cavity of predominantly human immunodeficiency virus (HIV)-positive patients.Citation3 Recently, several reports and case series have published the occurrence of PBL in other anatomical locations and in HIV-negative patients.Citation4–Citation17 Ocular involvement by PBL is extremely rare with very few reports in the literature.Citation9–Citation15 Its morphological and immunological resemblance to plasma cell myeloma makes it a diagnostic challenge, while its clinical course, which is characterized by recurrence and death, makes therapy a challenge for clinicians. We present three cases of PBL, each of which has distinct clinicoradiological features, and we also review the literature on orbital PBLs.
Case reports
Case 1
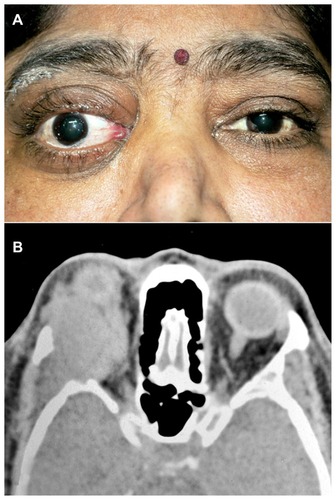
A 45-year-old previously healthy woman was referred to the ocular oncology clinic with a history of proptosis of the right eye for 6 months and diminished vision in the right eye for 2 months. At presentation, her best corrected visual acuity (BCVA) was limited to finger counting in the right eye. Severe proptosis of the right eye was noted with restriction of ocular motility in all directions (). Fundus examination of the right eye showed macular folds, a hyperemic disc, and dilated tortuous vessels, suggesting globe indentation and compressive optic neuropathy. A firm, hard, nontender mass was palpable in the superolateral aspect of the orbit on the right side with erythematous skin. Resistance was observed on retropulsion. Computerized tomography of the orbit showed a well-defined, large soft tissue mass, 4.0 cm × 3.0 cm × 2.6 cm in size, confined to the superolateral aspect of the right orbit with significant bony erosion involving the lateral wall with contiguous extension into the temporal fossa ().
Figure 1 External photograph and axial CT scan of the same patient. External photograph of the patient showing right eye proptosis with periocular swelling and mild conjunctival congestion (A). CT scan, axial cut, of the same patient showing a large mass occupying the lateral quadrant of the orbit with erosion of the lateral wall and extension into the temporal fossa (B).
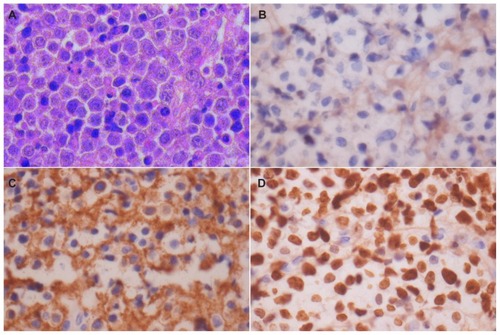
Microscopic examination showed a diffuse tumor composed of discohesive, large, round lymphoid cells with moderate amounts of amphophilic cytoplasm, eccentric to the central nucleus, and a prominent nucleolus in many of the cells (). A significant population of tumor cells had a plasmacytoid appearance, and both normal and abnormal mitotic figures were seen. Immunostaining revealed no immunoreactivity for CD20 () and CD45, but a strong, membranous expression of CD138 was noted (). The Ki-67 labeling index was 98% (). A diagnosis of PBL was confirmed. Serum protein electrophoresis was within normal limits. Chest X-ray and systemic examination were normal. A bone marrow aspiration and trephine biopsy were performed, which was normal. Our patient was staged as Ann Arbor 1A. An HIV screening test was performed and was found to be negative. The patient died within a week following diagnosis, even before further treatment could be initiated.
Figure 2 Microphotograph and immunohistochemical staining. Microphotograph showing atypical plasmacytoid tumors cells with abundant amphophilic cytoplasm and a large eccentric vesicular nucleus having a prominent nucleolus (HE × 400) (A). Immunohistochemical staining shows lack of immunoreactivity for CD20 (×400) (B). Strong membranous CD138 immunoreactivity (×400) (C). Strong Ki-67 immunoreactivity in almost all tumors cells (×400) (D).
Case 2
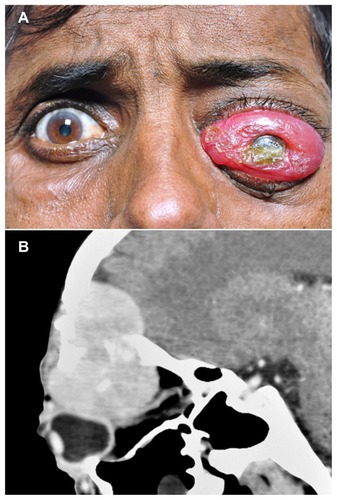
A 45-year-old Indian male was referred to our oncology clinic with protrusion of the left eye, as well as associated redness, watering, and pain. At presentation, his BCVA was 20/25 in the right eye and limited to light perception in the left eye. Conjunctival chemosis was observed with severe proptosis of the left eye (). Fundus examination could not be performed. Computed tomography showed a large hypodense mass in the anterior orbit on the superior and superolateral aspects of the globe. The mass indented the globe and was seen causing bony destruction with intracranial extension involving the frontal sinus and the ethmoid sinus (). Since the patient had a history of severe weight loss in the past 3 months, an HIV screening test was performed and was found to be positive.
Figure 3 External photograph and CT scan of the patient showing gross proptosis of the left eye with severe conjunctival chemosis, and a large mass lesion in the superior orbit. External photograph of the patient showing gross proptosis of the left eye with severe conjunctival chemosis (A). CT scan with sagittal reconstruction showing a large mass lesion in the superior orbit with extension into the frontal sinus and intracranial space (B).
Microscopic examination of the incision biopsy showed a diffuse tumor composed of a monotonous population of plasmacytoid cells with eccentric nuclei and a conspicuous to prominent nucleolus. Mitotic activity was brisk. Immunohistochemically, CD3, CD20, and CD5 were found to be negative. The tumor cells expressed strong membranous immunoreactivity for CD138 and weak reactivity for levocyte common antigen (LCA) (CD45). The Ki-67 index was close to 100%. No light chain restriction was seen. Morphology and immunostaining patterns were confirmative of a PBL.
Bone marrow aspiration done as part of the staging protocol revealed involvement by PBL. Ultrasonography of the abdomen revealed multiple deposits in the liver. Serum lactate dehydrogenase was normal. Our patient was thus staged as Ann Arbor 4.
He was commenced on highly active antiretroviral therapy (HAART) as well as on the cyclophosphamide, hydroxydanorubicin, oncovin, and prednisone (CHOP) regimen. The patient refused chemotherapy and died 6 months following presentation.
Case 3
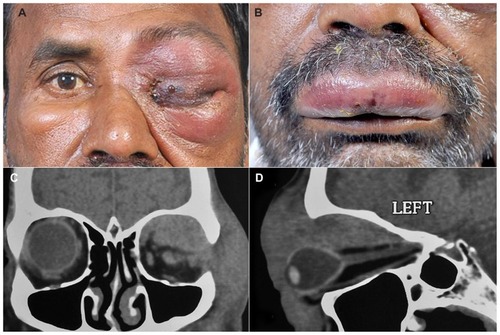
A 48-year-old Indian male presented with complaints of pain, redness, and watering in the left eye for the previous 3 days. At presentation, his BCVA was limited to finger counting and light perception in the affected eye. Clinical examination revealed proptosis with edema, tenderness and edema of the left upper and lower lids, and complete mechanical ptosis with restricted motility of the globe in all gazes (). Conjunctival chemosis was observed, and the rest of the anterior segment details could not be assessed. Severe periorbital, nonpitting edema with severe tenderness and bluish discoloration of the overlying skin accompanied by skin blistering was seen (). Upper lip edema with blisters were also noted (). A computerized tomography performed at this consultation showed a diffuse mass occupying the entire superior orbit with extension into the temporal fossa and orbital apex with globe compression and stretching of the optic nerve (). An incision biopsy was performed.
Figure 4 External photograph of the patient showing periocular edema with total ptosis, skin induration, and blisters, as well as lip edema and blisters. External photograph of the patient showing left periocular edema with total ptosis, skin induration, and blisters (A). External photograph showing upper lip edema with blisters (B). CT scan coronal cut of the same patient shows a diffuse ill-defined mass involving the entire superior orbit with superolateral bony erosion and extension into the temporal fossa (C). CT scan with sagittal reconstruction showing diffuse mass involving the entire superior quadrant up to the apex with indentation of the globe and optic nerve stretch (D).
Microscopic examination showed a diffuse tumor composed of large plasmablastic cells with eosinophilic to amphophilic cytoplasms and an eccentric vesicular nucleus having prominent nucleoli. Mitotic activity was brisk. Few apoptotic bodies and occasional binucleate cells were also seen. Immunohistochemistry revealed negative staining for CD45 and CD20. Strong membranous positivity for CD138 was observed. The Ki-67 index was 97%–98%. This confirmed the diagnosis of a PBL. An HIV screening test was performed and was found to be positive. Bone marrow aspiration did not reveal involvement by PBL. HAART therapy was commenced. Following two cycles of chemotherapy that included adriamycin, vincristine, and cyclophosphamide, the patient is still alive at 3 months postdiagnosis with improvement.
Discussion
PBL is a rare subtype of DLBCL. Ocular involvement by PBL is even rarer. Of all lymphomas diagnosed in our institute, PBL accounted for 0.62%. A large study showed that PBL has a mean age of 39 years at presentation in HIV-positive patients and 54 years in HIV-negative patients.Citation16 In patients with ocular involvement, including those in this case series; the mean age at presentation in HIV-positive patients was 44.8 years. PBL presents more commonly in men than women.Citation18 Of all the cases of PBL published in the literature with orbital involvement, 71.4% were males; in our series, 66.6% were males.Citation9–Citation13 PBL very rarely occurs in children.Citation19–Citation21 summarizes the clinical, radiological, and histopathological features of ocular PBL reported in the literature.
Table 1 Summary of clinical, radiological, and histopathologic features of ocular plasmablastic lymphomas reported in the literature
PBL accounts for 2.6% of all AIDS-related lymphomas.Citation15 In a literature review of 228 cases of PBL, 69% were HIV-positive while 39% were HIV-negative.Citation14 Moreover, six of seven (85.7%) PBL affected patients with orbital presentation described in the literature were HIV-positive. In the present study, two of three patients (66.6%) were HIV-positive. The association with other immunosuppressive states is conflicting. Some studies have reported some form of immunosuppression in HIV-negative patients with PBL,Citation17 while others found no such immunosuppression.Citation21 The HIV-negative patient reported by us had no other forms of immunosuppression. Despite the growing literature on PBL, its pathogenesis is still not clear. A large number of molecular and immunohistochemical studies have tried to shed light on this, but no definitive pathogenesis mechanisms have been established. PBL is characterized by immunoblastic morphology and plasma cell phenotype. In other words, plasmablasts are lymphoid cells that morphologically resemble B-cell immunoblasts, but they have acquired a plasma cell immunophenotype. Thus PBL probably develops from postgerminal center, terminally differentiated, active B-cells in transition from immunoblasts to plasma cells.Citation18
Recent studies have shown MYC gene rearrangements in PBL.Citation19–Citation22 MYC gene rearrangements have not yet been studied on PBL involving the orbit. Most PBL patients are positive for Epstein–Barr virus, thus confirming its role in the pathogenesis of PBL. The role of human herpes virus (HHV)-8 in the pathogenesis of PBL remains controversial. Some studies have detected HHV-8 ribonucleic acid in PBL,Citation23,Citation24 while others have found negative results.Citation25–Citation27 Some studies have documented the simultaneous occurrence of Kaposi sarcoma, Castleman disease, and PBL, thus suggesting further associations with PBL.Citation28 None of the cases of PBL involving the orbit have shown a positive association with HHV-8 to date.
A majority of patients with PBL present with diminution of vision and/or proptosis of the affected eye. Conjunctival chemosis,Citation9,Citation10 lid swelling,Citation9 ptosis,Citation10 and a loss of sensation along the trigeminal nerveCitation12 are other findings described upon clinical examination. Vision is either reduced or lost in the affected eye. Mild to severe globe motility restriction is a consistent finding on examination. The three patients in the present study showed all of the clinical findings mentioned above in various combinations (). Computed tomography revealed a soft tissue mass that is usually associated with bony destruction. Involvement of the paranasal sinuses,Citation9,Citation12,Citation13 intracranial tissues,Citation11,Citation12 nasopharynx,Citation13 and eyelidsCitation10 in contiguity may be seen. Involvement of the ethmoid sinus, lid, and temporal fossa was seen in the present series. At presentation, involvement of the liver, bone marrow, or lymph nodes is not uncommon given the highly aggressive nature of PBL.Citation10,Citation13 Two patients in the present study had an Ann Arbor stage 1 disease, while one patient showed involvement of bone marrow and liver at presentation (). Orbital inflammation was the most common differential diagnosis made clinically at presentation. This reflects the challenge posed to the diagnosing physician.
Table 2 Clinicopathological features of cases of plasmablastic lymphoma included in the present study
Three categories of PBL have been described in the literature.Citation13,Citation29,Citation30 PBL of the oral mucosa type has a monomorphic population of plasmablasts with minimal or no plasmacytic differentiation. They are found largely in the oral mucosa, but may also occur in other nodal or extranodal sites. PBL with plasmacytic differentiation is composed predominantly of plasmablasts, but exhibits a greater differentiation to mature plasma cells. These cells are round to oval with abundant eosinophilic to amphophilic cytoplasms, an eccentric nucleus, and a prominent nucleolus. Sometimes, a perinuclear Hof may be seen (this is the focal perinuclear clearing seen at the nuclear concavity in plasma cells representing the Golgi zones).Citation18 The third type of PBL is associated with Castleman disease and is typically nodal or splenic in its location.Citation31,Citation32 In the present study, all of the tumors were PBLs with plasmacytic differentiation.
Syndecan-1 (CD138), CD38, VS38c, and multiple myeloma oncogene, MUM1, are consistently expressed by PBL. Staining for CD45 and other B-cell markers, CD20 or CD79a, varies from absent to weak immunoreactivity. Reports on CD10, CD56, and Bcl-6 are conflicting.Citation5,Citation10,Citation12,Citation25 The Ki-67 index is usually around the 100% mark, thus explaining the highly aggressive nature of PBL. Positive regulatory domain 1 (PRDM1/BL IMP1) protein and activated transcription factor X-box binding protein 1 are proteins that have been recently described to reliably identify PBL,Citation33 as they are involved in terminal B-cell differentiation. Plasma cell myeloma, Burkitt’s lymphoma, DLBCL, anaplastic lymphoma kinase cell lymphoma with plasmacytoid features, and primary effusion lymphoma are other lymphoproliferative lesions that may exhibit a plasmablastic morphology. Detection of paraproteinemia in the blood and/or excess light chains (Bence–Jones proteins) in the urine, lytic bone lesions, and hypercalcemia or anemia favors the diagnosis of plasma cell myeloma over PBL. Negative or weak staining for PAX5 and CD20 coupled with positive staining for PAX5, as well as CD20 coupled with positive staining for PRDM1/BLIMP1 and X-box binding protein 1 help differentiate PBL from DLBCL; as such, a staining pattern is seen in <5% of DLBCL cases.Citation34 The strong expression of CD20 and CD79a help to differentiate Burkitt lymphoma from PBL. ALK expression and/or ALK-rearrangement confirm an ALK-positive large cell lymphoma with plasmacytoid features, while HHV-8 immunoreactivity helps to differentiate primary effusion lymphomas. Epstein–Barr virus encoded ribonucleic acid (EBER) in situ hybridization has been positive in a majority of PBL cases involving the orbit, although it can be negative in a minor population of HIV-positive and a large proportion of HIV-negative patients with PBL.Citation14 We suggest a strong CD138, VS38c immunoreactivity coupled with a negative/weak CD20, CD79a reactivity, and detection of EBER to be confirmative of PBL.
PBL is a fatal disease with a rapid clinical course characterized by relapse, or early death, despite treatment. Of all the cases of PBL with orbital presentation described in the literature, only two survived beyond a period of 10 months past diagnosis. No long term follow-up data are available. Two patients in the present study died within 6 months postdiagnosis, while one is on remission at 3 months postdiagnosis.
No validated guidelines are available for treating PBL. Most clinicians end up treating PBL like other lymphomas. CHOP and CHOP-like regimens have been tried with varying intensities; however, intensifying the CHOP regimen has not been shown to improve overall survival.Citation20 Rituximab added to the CHOP regimen does not play a role in the treatment of PBL given the CD20-negative nature of these tumors, although it could be added in those with a weak CD20 expression. Chemotherapy has been shown to yield an overall response rate of 77%. HIV-positive patients who discontinue or do not initiate HAART show a higher rate of relapse.Citation20 A combination of HAART and chemotherapy increases the response rate.Citation35,Citation36 etoposide, doxorubicin, vincristine, prednisone, and cycolophosphamide (EPOCH), cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide and cytarabine (CODOX-M/IVAC), dexamethasone, cytarabine, and cisplatin (DHAP), prednisolone, mitoxantrone, cyclophosphamide, etoposide, bleomycin, and vincristine (PmitCEBO), and bis-chloroethylnitrosourea, etoposide, cytarabine, melphalan (BEAM) have been tried in PBL with little or no success.Citation9,Citation20 Autologous stem cell transplantation has been experimented in PBL, but the follow-up data has not encompassed a long enough time-frame to conclude its effectiveness.Citation37 Responses to bortezomib have been encouraging, but the data available is limited.Citation38 Overall, the treatment of PBL is quite puzzling; it thus poses a therapeutic challenge to clinicians. The recent advancement has been the identification of a loss of p16 and MDR-1 in PBL.Citation39
Conclusion
Orbital involvement by PBL, although rare, is seen with a higher prevalence in HIV-positive individuals. Recent studies on MYC translocation and the positive identification of EBER have tried to explain the pathogenesis of PBL and its aggressive nature, yet the exact pathogenic mechanisms remain elusive. Morphological and immunohistochemical characteristics overlap with other lymphoproliferative lesions, which pose a diagnostic challenge to the pathologist. Though the treatment of PBL has revolved around CHOP and CHOP-like regimens, no validated treatments are currently available. Features at presentation simulate an inflammatory process, thus making PBL a clinical challenge. Further research is recommended to develop an effective treatment. Lastly, given the aggressive nature of PBL and its propensity to early death despite treatment, early clinical diagnosis may increase the overall survival of such patients.
Acknowledgments
This study has been reviewed by the ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Informed consent was obtained from the patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- BaireyOKremerIRakowskyEHadarHShaklaiMOrbital and adnexal involvement in systemic non-Hodgkin’s lymphomaCancer1994739239523998168043
- SwerdlowSHTumour of haematopoietic and lymphoid tissuesSwerdlowSHCampoEHarrisNLWorld Health Organization Classification of Tumors4th edLyon, FranceInternational Agency for Research on Cancer Press2008
- DelecluseHJAnagnostopoulosIDallenbachFPlasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infectionBlood1997894141314209028965
- ChabayPDe MatteoELorenzettiMVulvar plasmablastic lymphoma in a HIV-positive child: a novel extraoral localisationJ Clin Pathol200962764464619561233
- ThakralCThomasLGajraAHutchisonRERavizziniGCVajpayneeNPlasmablastic lymphoma in an immunocompetent patientJ Clin Oncol20092725e78e8119581534
- KhuranaAJalpotaYPlasmablastic lymphoma in a human immunodeficiency virus negative patientIndian J Pathol Microbiol201053236836920551562
- BrahmaniaMSylwesterowicTLeitchHPlasmablastic lymphoma in the ano-rectal junction presenting in an immunocompetent man: a case reportJ Med Case Rep2011516821539737
- KimJEKimYAKimWYHuman immunodeficiency virus-negative plasmablastic lymphoma in KoreaLeuk Lymphoma200950458258719373656
- MorleyAMVerityDHMeligonisGRoseGEOrbital plasmablastic lymphoma – comparison of a newly reported entity with diffuse large B-cell lymphoma of the orbitOrbit200928642542919929677
- ValenzuelaAAWalkerNJSullivanTJPlasmablastic lymphoma in the orbit: case reportOrbit200827322722918569836
- DegnanAJLevyLMOrbital plasmablastic lymphoma with remission following chemotherapyJ Radiology Case Rep20115217
- BarkhuysenRMerkxMAWeijsWLGerlachNLBergéSJPlasmablastic lymphoma mimicking orbital cellulitisOral Maxillofac Surg200812312512818597126
- ColomoLLoongFRivesSDiffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entitiesAm J Surg Pathol200428673674715166665
- CastilloJJWinerESStachurskiDClinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphomaLeuk Lymphoma201051112047205320919850
- CarboneAGloghiniAPlasmablastic lymphoma: one or more entities?Am J Hematol2008831076376418756546
- CastilloJPantanowitzLDezubeBJHIV-associated plasmablastic lymphoma: lessons learned from 112 published casesAm J Hematol2008831080480918756521
- Rafaniello RavielePPruneriGMaioranoEPlasmablastic lymphoma: a reviewOral Dis2009151384518939960
- CastilloJJReaganJLPlasmablastic lymphoma: a systematic reviewScientific World Journal20111168769621442146
- DawsonMASchwarerAPMcLeanCAIDS-related plasmablastic lymphoma of the oral cavity associated with an IGH/MYC translocation – treatment with autologous stem-cell transplantation in a patient with severe haemophilia-AHaematologica2007921e11e1217405744
- CastilloJJWinerESStachurskiDPrognostic factors in chemotherapy-treated patients with HIV-associated Plasmablastic lymphomaOncologist201015329329920167839
- McGlaughlinKLBajelAMowCDA case of plasmablastic lymphoma harbouring an IgH/MYC translocation in a HIV negative individualPathology201042769769921080889
- BoguszAMSeegmillerACGarciaRShangPAshfaqRChenWPlasmablastic lymphomas with MYC/IgH rearrangement: report of three cases and review of the literatureAm J Clin Pathol2009132459760519762538
- DongHYScaddenDTde LevalLTangZIsaacsonPGHarrisNLPlasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasmAm J Surg Pathol200529121633164116327436
- CarboneAGloghiniAGaidanoGIs plasmablastic lymphoma of the oral cavity an HHV-8-associated disease?Am J Surg Pathol2004281115381540 author reply 154015489661
- VegaFChangCCMedeirosLJPlasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profilesMod Pathol200518680681515578069
- BrownRSPowerDASpittleHFLankesterKJAbsence of immunohistochemical evidence for human herpesvirus 8 (HHV8) in oral cavity plasmablastic lymphoma in an HIV-positive manClin Oncol (R Coll Radiol)200012319410942339
- CarboneAGloghiniACanzonieriVTirelliUGaidanoGAIDS-related extranodal non-Hodgkin’s lymphomas with plasma cell differentiationBlood1997903133713389242574
- CiocAMAllenCKalmarJRSusterSBaiocchiRNuovoGOral plasmablastic lymphomas in AIDS patients are associated with human herpesvirus 8Am J Surg Pathol2004281414614707862
- Teruya-FeldsteinJDiffuse large B-cell lymphomas with plasmablastic differentiationCurr Oncol Rep20057535736316091196
- TavoraFGonzalez-CuyarLFSunCCBurkeAZhaoXFExtra-oral plasmablastic lymphoma: report of a case and review of literatureHum Pathol20063791233123616938530
- DupinNDissTLKellamPHHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphomaBlood20009541406141210666218
- OksenhendlerEBoulangerEGalicierLHigh incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman diseaseBlood20029972331233611895764
- SchebestaMHarveyBBusslingerMTranscriptional control of B-cell developmentCurr Opin Immunol200214221622311869895
- Montes-MorenoSGonzalez-MedinaARRodriguez-PinillaSMAggressive large B-cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotypeHaematologica20109581342134920418245
- Teruya-FeldsteinJChiaoEFilippaDACD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogeneous spectrum in both HIV-positive and -negative patientsAnn Oncol200415111673167915520070
- LesterRLiCPhillipsPImproved outcome of human immunodeficiency virus-associated plasmablastic lymphoma of the oral cavity in the era of highly active antiretroviral therapy: a report of two casesLeuk Lymphoma20044591881188515223650
- GotoHHagiwaraSHiraiRCase of relapsed AIDS-related plasmablastic lymphoma treated with autologous stem cell transplantation and highly active antiretroviral therapyRare Tumors201131e1121464873
- DunleavyKPittalugaSCzuczmanMSDifferential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphomaBlood2009113246069607619380866
- MatsukiEMiyakawaYAsakawaSIdentification of loss of p16 expression and upregulation of MDR-1 as genetic events resulting from two novel chromosomal translocations found in plasmablastic lymphoma of the uterusClin Cancer Res20111782101210921325069