Abstract
Objective
To assess ocular discomfort upon instillation and patient preference for brinzolamide/timolol relative to dorzolamide/timolol, in patients with open-angle glaucoma or ocular hypertension.
Methods
This was a multicenter, prospective, patient-masked, randomized, crossover study. On day 0, patients received one drop of brinzolamide/timolol in one eye and one drop of dorzolamide/timolol in the contralateral eye. On day 1, patients were randomly assigned to receive one drop of either brinzolamide/timolol or dorzolamide/timolol in both eyes; on day 2, patients received one drop of the alternate treatment in both eyes. Measures included a patient preference question on day 2 (primary) and mean ocular discomfort scale scores on days 1 and 2 (secondary). Safety assessments included adverse events, visual acuity, and slit-lamp examinations.
Results
Of 120 patients who enrolled, 115 completed the study. Of these, 112 patients instilled both medications and expressed a study medication preference on day 2. A significantly greater percentage preferred brinzolamide/timolol to dorzolamide/timolol (67.0% versus 30.4%; P < 0.001). The ocular discomfort (expressed as mean [standard deviation]) with brinzolamide/timolol was significantly lower than with dorzolamide/timolol (day 2:1.9 [2.3] versus 3.7 [2.8], respectively [P = 0.0003]; both days combined: 2.1 [2.5] versus 3.5 [2.9], respectively [P = 0.00014]). On day 1, five patients receiving brinzolamide/timolol reported five nonserious adverse events (AEs): flu (n = 1), bitter taste (n = 2), and headache (n = 2). Four events, bitter taste (two events) and headache (two events), were considered related to brinzolamide/timolol. Events were mild in intensity, except bitter taste of moderate intensity reported by one patient. No AEs were reported at day 2. All AEs resolved without additional treatment. No clinically relevant changes from baseline were observed in best-corrected visual acuity or slit-lamp examinations of ocular signs.
Conclusion
Patients had less discomfort with brinzolamide/timolol than with dorzolamide/timolol, and more expressed a preference for brinzolamide/timolol. Both treatments were generally safe and well tolerated.
Introduction
Glaucoma includes a diverse family of related, progressive, and irreversible optic neuropathies. Untreated glaucoma may progressively lead to retinal ganglion cell death, optic nerve damage, and vision loss. Several studies have shown that elevated intraocular pressure (IOP) is a major risk factor for the development of primary open-angle glaucoma.Citation1–Citation4 Further, elevated IOP is the only risk factor amenable to treatment; the use of topical ocular hypotensive medications to reduce IOP has been effective in delaying or preventing disease progression and is therefore the mainstay of glaucoma treatment.Citation5 Major classes of medications for IOP reduction include the beta-blockers (eg, timolol), alpha-adrenergic agonists (eg, brimonidine), carbonic anhydrase inhibitors (eg, brinzolamide or dorzolamide), and prostaglandin analogs (eg, travoprost).Citation6
While monotherapy with a single class of medication may be effective in lowering IOP, many patients require more than one medication for the adequate, long-term control of IOP.Citation5 Therefore, in clinical practice, a two-drug regimen, consisting of a topical beta-blocker in combination with a carbonic anhydrase inhibitor or prostaglandin analog, is commonly administered to patients with insufficient IOP control with monotherapy. Fixed combinations of a beta-blocker and a carbonic anhydrase inhibitor that are currently available include brinzolamide 1%/timolol 0.5% (Azarga®; Alcon Inc, Fort Worth, TX, USA) and dorzolamide 2%/timolol 0.5% (Cosopt®; Merck and Co, Inc, Whitehouse Station, NJ, USA). In a previous clinical study, the IOP-lowering efficacy of brinzolamide/timolol was noninferior to dorzolamide/timolol in patients with open-angle glaucoma or ocular hypertension.Citation7
While the IOP-lowering efficacy of any glaucoma therapy is critical, selection of a suitable topical ocular medication for glaucoma also depends on other factors that may influence patient adherence to therapy, such as drop comfort upon instillation and overall tolerability.Citation8 Therefore, the objective of this crossover study was to assess ocular discomfort upon instillation and patient preference for brinzolamide/timolol relative to dorzolamide/timolol, in patients with open-angle glaucoma or ocular hypertension.
Methods
Study design
This was a multicenter, prospective, patient-masked, randomized, interventional, crossover 3-day study. At screening (day 0), patients were assessed for study eligibility. Demographic information, medical histories, and information regarding contact lens wear (if applicable) were collected. A urine pregnancy test was conducted for women of childbearing potential. Additional assessments included best-corrected visual acuity (BCVA), a slit-lamp examination, and IOP measured bilaterally with Goldmann applanation tonometry. On day 0, patients were randomly assigned by eye (left or right) in a 1:1 ratio to receive one drop of brinzolamide 1%/timolol 0.5% (Azarga) in one eye and one drop of dorzolamide 2%/timolol 0.5% (Cosopt) in the contralateral eye, followed by day one treatment including one drop of either brinzolamide/timolol or dorzolamide/timolol, bilaterally. On day 2, patients received one drop of the alternate treatment assigned at day 1, bilaterally. Patients were unaware of their assigned treatment throughout the study.
Patients
Eligible patients included men and women of any race or ethnicity who were 18 years of age or older and diagnosed with glaucoma (primary open-angle or pigment dispersion) or ocular hypertension in both eyes. Patients must have been on a stable IOP-lowering regimen for at least 30 days before screening. Specifically, patients must have been using any form of monotherapy for glaucoma (ie, any single therapeutic agent in a bottle) with the exception of a topical carbonic anhydrase inhibitor, which must not have been used (as a monotherapy or in a fixed combination product) within a year of study screening. Patients were not eligible if they had ocular conditions that could have prevented the safe administration of study medications or could have interfered with planned study assessments. Patients were not eligible if they had histories of ocular surgery, inflammatory eye disease, corneal dystrophies, opacities, and ocular infections; or signs and symptoms that were associated with these or other ocular conditions.
Upon entry and prior to participating in any study-related assessments, all potential patients provided their written informed consent. An independent ethics committee in each country approved the protocol, the participating investigator, and the informed consent document. The study was conducted in accordance with Good Clinical Practices and the ethical principles described within the Declaration of Helsinki; this study was registered at ClinicalTrials.gov as NCT01471158.
Assessments
Assessment of ocular discomfort and patient preference
On both days 1 and day 2, within 1 minute following drop instillation, the patients rated their level of ocular discomfort with the study drug, using a 10-point Ocular Discomfort Scale (ODS). The ODS specifically ranged in whole-unit increments from 0 (no discomfort) to 9 (substantial discomfort). Separately, on day 2, after assessing their ocular discomfort, patients completed the patient preference question, reporting that they either preferred the first medication (instilled on day 1) or the second medication (instilled on day 2).
Safety
Safety parameters included adverse events (AEs), evaluations of ocular signs (cornea, lens, eyelids/conjunctiva, and iris/anterior chamber) as assessed through slit-lamp biomicroscopy, and determinations of BCVA. Specifically, AEs were collected after the initial study drug instillation on day 0 through the end of the study at day 2. The assessments of ocular signs and BCVA were conducted at screening and prior to exiting the study at day 2.
Statistics
All eligible patients who received study drugs and completed the ODS and the patient preference question on days 1 and 2 were included in the per-protocol (PP) population. All patients who had at least one instillation of study drug were included in the safety population.
The primary efficacy endpoint was the percentage of patients with a stated preference for either study medication, as assessed with the patient preference question. The difference in the percentage of patients (PP population) who had a preference for either brinzolamide/timolol or dorzolamide/timolol was determined using a one-sample Chi-square test.
The secondary efficacy endpoint was the difference in the mean ODS score for the two study medications. The difference in the mean ODS score reported for brinzolamide/timolol and dorzolamide/timolol (PP population) was determined using a Chi-square test.
Safety was evaluated by reviewing reported AEs and changes in ocular signs and BCVA assessments from day 0 to day 2 (safety population).
Results
Patient characteristics and disposition
A total of 120 patients were enrolled at six investigational centers in South America (three in Argentina, one in Brazil, and two in Chile). All enrolled patients (n = 120) were included in the safety population and 115 were included in the PP population.
Patient demographics and baseline characteristics were analyzed for the PP population. The demographic and baseline characteristics of patients in each treatment group were similar (). Nearly two-thirds of enrolled patients (64.3%) were women, and most (93.9%) had primary open-angle glaucoma.
Table 1 Demographic and baseline characteristics
Patient preference
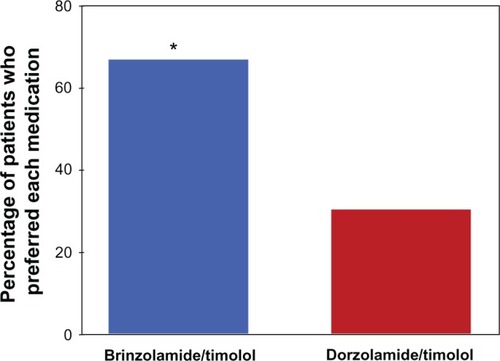
On day 2, of the 115 patients in the PP population, 112 expressed a preference for one study drug, while three patients expressed no preference. Of the 112 patients with a stated preference, a significantly greater percentage preferred brinzolamide/timolol compared with dorzolamide/timolol (67.0% versus 30.4%; P < 0.001) ().
Figure 1 Patient preference for study medication.
Ocular discomfort
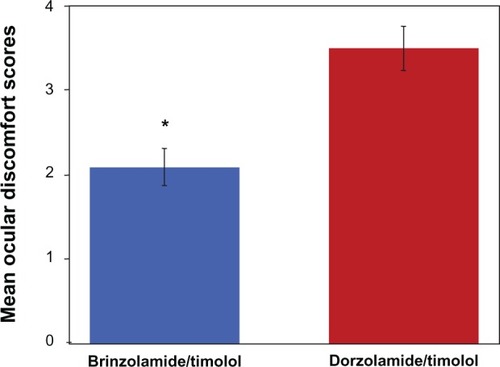
On day 1, no significant differences were observed between treatment groups in the ODS score, expressed as mean (standard deviation [SD]), after study-drug instillation (brinzolamide/timolol = 2.4 [2.6]; dorzolamide/timolol = 3.3 [2.9]; P = 0.0770). However, on day 2, patients who received brinzolamide/timolol had a significantly lower mean (SD) ODS score after drop instillation than did patients who received dorzolamide/timolol (1.9 [2.3] versus 3.7 [2.8]; P = 0.0003). Further, when data from both days were combined, the mean (SD) ODS score for all patients after instillation of brinzolamide/timolol was significantly lower than that for all patients after instillation of dorzolamide/timolol (2.1 [2.5] versus 3.5 [2.9]; P = 0.00014) ().
Figure 2 Mean ocular discomfort scores with standard error of the mean error bars after instillation of brinzolamide/timolol or dorzolamide/timolol.
Safety
No serious AEs occurred. On day 0, three patients had one AE each, including dry throat (mild intensity), headache (mild intensity), and bitter taste (mild intensity). Day 0 events could not be assessed for relatedness to a specific study treatment because patients received one drop each of both study drugs. On day 1, five patients receiving brinzolamide/timolol reported five nonserious AEs: flu (n = 1), bitter taste (n = 2), and headache (n = 2). Four of these events, bitter taste (two events) and headache (two events), were considered by the investigators to be related to brinzolamide/timolol. All day 1 AEs were mild in intensity, with the exception of bitter taste of moderate intensity reported by one patient. No AEs were reported at day 2. All AEs resolved without additional treatment, and none resulted in patient discontinuation from the study. No clinically relevant changes from baseline were observed in BCVA or slit-lamp examinations of ocular signs.
Discussion
Fixed combinations of different classes of IOP-lowering agents in the same bottle offer advantages over the use of the same individual drugs in separate bottles, such as a simplified dosing regimen and the elimination of the washout effect that occurs when multiple drugs are instilled without an adequate waiting period between instillations.Citation9,Citation10 Patient adherence to therapy may be influenced not only by efficacy and dosing regimen, but also by drop comfort upon instillation and overall tolerability.Citation8 Ocular comfort upon instillation, in particular, is a critical aspect of the overall tolerability of any topical ocular medication, and low tolerability has been identified as a barrier to treatment adherence.Citation11
In the present study, ocular discomfort, as quantified by the mean ODS score, was significantly lower after instillation of brinzolamide/timolol than after instillation of dorzolamide/timolol (P = 0.00014). This observation is consistent with a previous study that used the same scaleCitation12 and also with other studies that used different scales to evaluate the comfort of the same two fixed-combination medications.Citation13,Citation14 Further, in a recent open-label, multicenter, observational study of more than 14,000 patients in Germany,Citation15 nearly two-thirds more of the patients who transitioned from dorzolamide/timolol to brinzolamide/timolol (n = 2937) rated the latter drug as the more tolerable treatment (brinzolamide/timolol: 88.9%; dorzolamide/timolol: 28.9%). Patients in this study also preferred brinzolamide/timolol to previous therapy by a ratio of almost 9:1. A possible reason for the superior ocular comfort of brinzolamide/timolol relative to dorzolamide/timolol is that the brinzolamide/timolol formulation has a pH that is closer to the physiological pH of human eyes than that of the dorzolamide/timolol formulation.Citation16,Citation17 In addition, the brinzolamide/timolol product is formulated without additional buffers, while the dorzolamide/timolol product is buffered using sodium citrate,Citation16,Citation17 which could irritate the ocular surface upon drop instillation.
A greater percentage of patients in the present study preferred brinzolamide/timolol to dorzolamide/timolol (67% versus 30%). These results were consistent with a previously reported direct-comparison study conducted in the United States, in which the majority of patients expressing a preference preferred brinzolamide/timolol to dorzolamide/timolol (79% versus 21%; P < 0.0001).Citation12 When the observed patient preference for brinzolamide/timolol relative to dorzolamide/timolol is considered in combination with the observed lower ODS score associated with brinzolamide/timolol, the study results support the relationship that has been proposed in the literature between drop comfort and patient preference.Citation18
A positive correlation between patient preference and likelihood of adherence to therapy was suggested in a study of 447 glaucoma patients who transitioned from dorzolamide to brinzolamide.Citation19 Further, a recent study found that patients with open-angle glaucoma who adhered to their treatment (as measured using an electronic dosing aid) experienced diminished progression or worsening of visual field function.Citation20 Thus, high therapeutic adherence rates may be associated with better clinical outcomes in glaucoma. If this is true, the results of this study suggest that patients who are treated with brinzolamide/timolol have the potential for good treatment adherence.
Four AEs were assessed as related to brinzolamide/timolol, both of which occurred on day 1: bitter taste (two reports: one mild and one moderate in intensity) and headache (two reports: both mild). In a previous clinical study wherein patients received brinzolamide/timolol or dorzolamide/timolol twice daily for 12 months, the incidence of dysgeusia (bad taste) was low and was reported at a similar frequency in both treatment groups (2.8%–3.2%).Citation7 Dysgeusia is a known adverse effect of carbonic anhydrase inhibitors.Citation21 Overall, based on AEs and changes in ocular signs and visual acuity, no safety issues or trends were observed during the present study.
These study results were limited due to a short-term exposure to each study drug. Long-term exposure to either drug may influence patient perceptions of comfort. Evaluation of the effects on ocular signs, as assessed by biomicroscopy, was limited by the brief exposure to study drug and short study duration. There was no washout period before study initiation, and patients with previous exposure to the individual component agents of the study drugs were not excluded. While these limitations had the potential to introduce bias, this effect was minimized by the randomized crossover study design that implemented within-patient comparisons.
Conclusion
Patients in this study experienced less discomfort upon instillation of brinzolamide/timolol compared with dorzolamide/timolol, and more patients expressed a preference for brinzolamide/timolol than for dorzolamide/timolol. Both brinzolamide/timolol and dorzolamide/timolol were generally safe and well tolerated.
Disclosure
Alcon Research, Ltd sponsored this study. Medical writing support was funded by Alcon Research, Ltd and provided by Cullen T Vogelson, PhD and Usha Sivaprasad, PhD. Joao Franca Lopes is a consultant for Alcon Laboratories, Inc. Apart from this, the authors report no conflicts of interest in this work.
References
- LeskeMCHeijlAHusseinMBengtssonBHymanLKomaroffEEarly Manifest Glaucoma Trial GroupFactors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma TrialArch Ophthalmol20031211485612523884
- The AGIS InvestigatorsThe Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deteriorationAm J Ophthalmol2000130442944011024415
- GordonMOBeiserJABrandtJDThe Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucomaArch Ophthalmol20021206714720 discussion 829–83012049575
- MigliorSTorriVZeyenTPfeifferNVazJCAdamsonsIthe EGPS GroupIntercurrent factors associated with the development of open-angle glaucoma in the European Glaucoma Prevention StudyAm J Ophthalmol2007144226627517543874
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol20021206701713 discussion 829–83012049574
- European Glaucoma SocietyTerminology and Guidelines for Glaucoma3rd edSavona, ItalyDogma2008
- ManniGDenisPChewPThe safety and efficacy of brinzolamide 1%/timolol/0.5% fixed combination versus dorzolamide 2%/timolol/0.5% in patients with open-angle glaucoma or ocular hypertensionJ Glaucoma200918429330019365194
- AptelFDenisPBalancing efficacy and tolerability of prostaglandin analogues and prostaglandin-timolol fixed combinations in primary open-angle glaucomaCurr Med Res Opin201127101949195821878000
- FechtnerRDRealiniTFixed combinations of topical glaucoma medicationsCurr Opin Ophthalmol200415213213515021225
- KhouriASRealiniTFechtnerRDUse of fixed-dose combination drugs for the treatment of glaucomaDrugs Aging200724121007101618020533
- TsaiJCMcClureCARamosSESchlundtDGPichertJWCompliance barriers in glaucoma: a systematic classificationJ Glaucoma200312539339814520147
- MundorfTKRauchmanSHWilliamsRDNotivolRthe Brinzolamide/Timolol Preference Study GroupA patient preference comparison of Azarga (brinzolamide/timolol fixed combination) vs Cosopt (dorzolamide/timolol fixed combination) in patients with open-angle glaucoma or ocular hypertensionClin Ophthalmol20082362362819668763
- VoldSDEvansRMStewartRHWaltersTMallickSA one-week comfort study of BID-dosed brinzolamide 1%/timolol 0.5% ophthalmic suspension fixed combination compared to BID-dosed dorzolamide 2%/timolol 0.5% ophthalmic solution in patients with open-angle glaucoma or ocular hypertensionJ Ocul Pharmacol Ther200824660160519049301
- RossiGCPasinettiGMSandoloFBordinMBianchiPEFrom dorzolamide 2%/timolol 0.5% to brinzolamide 1%/timolol 0.5% fixed combination: a 6-month, multicenter, open-label tolerability switch studyExpert Opin Pharmacother201112162425243121679090
- LanzlIRaberTEfficacy and tolerability of the fixed combination of brinzolamide 1% and timolol 0.5% in daily practiceClin Ophthalmol2011529129821468336
- AZARGA® Eye Drops Suspension [summary of product characteristics]Hünenberg, SwitzerlandAlcon Laboratories, Inc2008 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000960/WC500029827.pdfAccessed. April 30, 2012
- COSOPT® Sterile Ophthalmic Solution [package insert]Whitehouse Station, NJMerck and Co, Inc2011
- SyedMFLoucksEKUpdate and optimal use of a brinzolamide-timolol fixed combination in open-angle glaucoma and ocular hypertensionClin Ophthalmol201151291129621966204
- BarnebeyHKwokSYPatients’ acceptance of a switch from dorzolamide to brinzolamide for the treatment of glaucoma in a clinical practice settingClin Ther200022101204121211110231
- RossiGCPasinettiGMScudellerLRadaelliRBianchiPEDo adherence rates and glaucomatous visual field progression correlate?Eur J Ophthalmol201121441041421140373
- DohertyMFraserSPhelanPBrinzolamide-timolol suspension: acceptability and side effect profileClin Ophthalmol2011541942321499567