Video abstract

Purpose
To evaluate programmed versus achieved laser-assisted in situ keratomileusis (LASIK) flap central thickness and investigate topographic flap thickness variability, as well as the effect of potential epithelial remodeling interference on flap thickness variability.
Patients and methods
Flap thickness was investigated in 110 eyes that had had bilateral myopic LASIK several years ago (average 4.5 ± 2.7 years; range 2–7 years). Three age-matched study groups were formed, based on the method of primary flap creation: Group A (flaps made by the Moria Surgical M2 microkeratome [Antony, France]), Group B (flaps made by the Abbott Medical Optics IntraLase™ FS60 femtosecond laser [Santa Ana, CA, USA]), and Group C (flaps made by the Alcon WaveLight® FS200 femtosecond laser [Fort Worth, TX, USA]). Whole-cornea topographic maps of flap and epithelial thickness were obtained by scanning high-frequency ultrasound biomicroscopy. On each eye, topographic flap and epithelial thickness variability was computed by the standard deviation of thickness corresponding to 21 equally spaced points over the entire corneal area imaged.
Results
The average central flap thickness for each group was 138.33 ± 12.38 μm (mean ± standard deviation) in Group A, 128.46 ± 5.72 μm in Group B, and 122.00 ± 5.64 μm in Group C. Topographic flap thickness variability was 9.73 ± 4.93 μm for Group A, 8.48 ± 4.23 μm for Group B, and 4.84 ± 1.88 μm for Group C. The smaller topographic flap thickness variability of Group C (FS200) was statistically significant compared with that of Group A (M2) (P = 0.004), indicating improved topographic flap thickness consistency – that is, improved precision – over the entire flap area affected.
Conclusions
The two femtosecond lasers produced a smaller flap thickness and reduced variability than the mechanical microkeratome. In addition, our study suggests that there may be a significant difference in topographic flap thickness variability between the results achieved by the two femtosecond lasers examined.
Introduction
We have previously reported, in agreement with many others, on the safety and accuracy of flap making with mechanical keratomes for correction of myopia and myopic astigmatismCitation1 as well as hyperopia.Citation2
The femtosecond laser – named for the ultrashort pulses it produces that last a few femtoseconds (ie, 10−15 of a second) – has provided an alternative option for flap creation since the introduction of the IntraLase™ (Abbott Medical Optics, Santa Ana, CA, USA) in 2001.Citation3 With wavelength around 1050 nm – at which the cornea tissue is transparent – the laser energy can be tightly focused within the corneal stroma. Typically, each laser pulse consists of very low energy (eg, of the order of 30 nJ/pulse) that is repeated at a very high frequency (pulse duration of a few 100 femtoseconds). Inside the stroma, the tightly controlled focused laser pulse breaks down, giving rise to a series of effects, such as plasma, a shockwave, and creation of a gas (CO2 and H2O) cavitation bubble.Citation4 A rasterized pattern of such successive, computer driven, tightly spaced (a few micrometers apart) cavitation bubbles forms a resection plane for a lamellar cut, enabling the separation of the overlying flap.Citation5
Flap thickness measurement in laser-assisted in situ keratomileusis (LASIK)-treated eyes
Measurement of flap thickness in LASIK-treated eyes postoperatively is inherently challenging. In addition, measurements of thickness variability require a modality that offers not just a single-point flap thickness measurement (usually at, or close to, the cornea center), but also the simultaneous measurement of a large number of flap thicknesses over the wider corneal area affected. Most reported studies use subtraction manual intraoperative ultrasound pachymetry measurements at or near the cornea.Citation6
There are two commercially available modalities for measuring flap thickness postoperatively for a more sophisticated and precise flap assessment: anterior-segment optical coherence tomography (AS-OCT)Citation7,Citation8 and arc-scanning high-frequency ultrasound biomicroscopy (HF-UBM),Citation9 which is capable of reporting total corneal, epithelial, residual stroma, flap, and flap-stromal composition maps.Citation10,Citation11 Even though AS-OCT can distinguish the epithelium and flap interface, to the best of our knowledge, there is only one recently commercially available OCT system that can offer a whole-cornea epithelial map visualization.Citation12 To the best of our knowledge, no such option exists for mapping the flap thickness of the entire corneal area by AS-OCT.
Epithelial average thickness and variability following LASIK
Postoperative epithelial thickness may be an indicator of continuing epithelial activity. Reinstein et al has introduced a new benchmark in evaluating post refractive surgery corneas by utilizing HF-UBM and evaluating the epithelial versus topographic corneal thickness distribution.Citation10
In the case of epithelial hypertrophy, particularly if it is associated with topographic epithelial thickness irregularity, we have suggested that this may be a sign indicative of a reactive process;Citation9 the epithelium may grow thicker in less rigid corneas that are inherently or iatrogenically weakened biomechanically and oscillate more to intraocular pressure variations and/or blinking and eye rubbing, resulting in epithelial thickening.
Precise knowledge of the epithelial central and average thickness, as well as topographic flap thickness variability over the entire cornea, may be a useful indication in the differential diagnosis of ectasia of possible myopic regression.Citation13
Purpose
The purpose of this study was to investigate flap thickness precision and accuracy over the entire flap area affected in the form of central flap thickness as well as paracentral and peripheral topographic flap thickness variability in conjunction with epithelial thickness on LASIK flaps created by a mechanical microkeratome and two different femtosecond lasers.
Patients and methods
This retrospective interventional case series study received approval by the ethics committee of our Institution, adherent to the tenets of the Declaration of Helsinki. Informed consent was obtained from each subject prior to the surgical intervention and prior to the HF-UBM measurements.
Patient inclusion and exclusion criteria
Patients included in the study had uneventful primary LASIK for myopia by the same surgeon (AJK) between 2004 and 2010, followed by a complication-free postoperative recovery. Treatments were between −2.00 and −10.00 diopters (D) and up to −3.00 D of astigmatism. None of the patients included in the study had a LASIK re-treatment. In all cases, the excimer laser ablation for the myopic correction was performed using an Allegretto Wave® Eye-Q 400 Hz excimer laser (WaveLight, Erlangen, Germany).Citation14
Patients considered for the original LASIK operation were selected on the basis of no presence and/or history of: corneal dystrophy or herpetic eye disease, keratoconus (as evidenced by Placido topography and/or Scheimpflug tomography), warpage from contact lens wear, corneal scaring, glaucoma, severe dry eye, and collagen vascular diseases.
HF-UBM corneal, flap, and epithelial thickness measurements were taken during patients’ scheduled, postoperative visits ranging from 2 to 10 years postoperatively. The same examiner (GA) performed all corneal imaging and data analysis. The HF-UBM imaging was preceded by a complete ocular evaluation to screen for corneal abnormalities or postoperative complications. For example, no case with flap scarring and/or epithelial ingrowth was measured by HF-UBM.
Study groups
An equal number of left eyes (OS) and right eyes (OD) of 55 patients (ie, 110 eyes) were included in the study. Three groups were formed on the basis of original flap creation technique/instrumentation. Preoperative refractive error was similar in all groups.
Patients enrolled in Group A (n = 21, of which seven were male and 14 female) had their flap created by the M2 microkeratome (Moria Surgical, Antony, France).Citation1,Citation2 The microkeratome cut was programmed to a flap thickness of 130 μm. Patients in Groups B and C had femtosecond laser-assisted flap creation. Specifically, in Group B (n = 14, six male, eight female), the flap was created by the IntraLase™ FS60 femtosecond laser (Abbott Medical Optics). The FS60 laser was programmed to a flap thickness of 120 μm and to a flap diameter of 8.50 mm, with a 70° angled side cut and a 55° hinge angle. The settings were: bed energy 0.65 MJ, side cut energy 0.8 MJ, and repetition frequency 60 kHz. Spot and line separations were, respectively, 9.0 and 9.0 μm for the bed cut and 5.0 and 3.0 μm for the side cut. Patients in Group C (n = 20, twelve male, eight female) had had their flap created by the WaveLight® FS200 femtosecond laser (Alcon, Fort Worth, TX, USA).Citation15 The FS200 laser was programmed to a flap thickness of 120 μm and to a flap diameter of 8.50 mm, with a 70° angled side cut and a 45° hinge angle. The settings were: bed energy 0.90 MJ, side cut energy 0.88 MJ, and repetition frequency 200 kHz. Spot and line separations were, respectively, 8.0 and 8.0 μm for the bed cut and 5.0 and 3.0 μm for the side cut.
Mean postoperative time (time span from the operation to the examination) was 66.5 ± 29.7 months (range 33–158 months) for Group A, 52.3 ± 14.7 months (range 26–59 months) for Group B, and 26.7 ± 15.3 months (range 28–44 months) for Group C.
Imaging technique
The Artemis II + superior HF-UBM system (Artemis Medical Technologies, Vancouver, BC, Canada) was employed in the study. This system produces layered corneal flap and epithelial thickness maps.Citation9–Citation11 Data were stored and processed using Zeus (v 1.0, build 11.780) software, licensed from Artemis Medical Technologies.
Data collection and analysis
For each eye, corneal flap and epithelial thickness measurements were obtained from the corneal report, a typical example of which is shown in . Mean epithelial thickness overall, mean flap thickness overall (0–6 mm), central flap thickness (0–3 mm), and peripheral flap thickness (3–6 mm) were recorded from the table on the lower-left corner of the report (). Note that thickness is referred to as “depth” in the report produced by the software.
Figure 1 Standard corneal analysis report used in our investigation.
Abbreviation: LASIK, laser-assisted in situ keratomileusis.
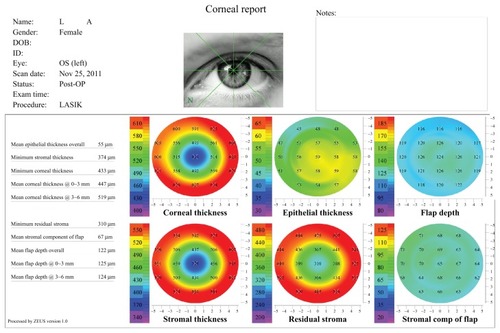
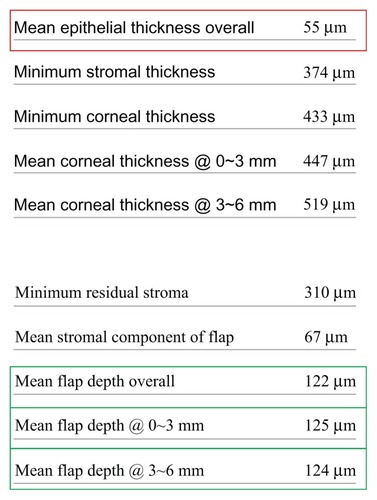
Figure 2 Detail from the lower-left table of the corneal analysis report depicted in , showing data recorded for mean epithelial thickness, mean flap depth (0–6 mm), central flap depth (0–3 mm), and peripheral flap depth (3–6 mm).
For each eye, topographic flap and epithelial thickness variability were computed as the standard deviation of 21 different point thickness values over the entire corneal area (such as those shown in and ). These points were spaced by 2 mm on the horizontal plane (0°–180° axis) and by 1.6 mm on the coronal plane (90°–270° axis).
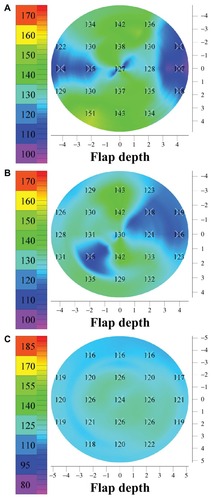
Figure 3 Three representative flap thickness maps (8 mm diameter) from flaps created with the modalities studied in this paper: (A) M2 microkeratome (Moria Surgical, Antony, France), (B) Intralase™ FS60 femtosecond laser (Abbott Medical Optics, Santa Ana, CA, USA), (C) WaveLight® FS200 femtosecond laser (Alcon, Fort Worth, TX, USA).
Descriptive statistics (average, minimum, maximum, standard deviation, bias, and range), and comparative statistics were compiled and determined and linear regression performed using Microsoft Excel 2010 (Microsoft, Redmond, WA, USA) and Origin Lab (v 8; OriginLab, Northampton, MA, USA). Analysis of variance between groups was performed using the OriginLab statistics tool.
Results
Mean age, as reported at the time of LASIK operations, was 33.0 ± 12.9 years (range 20–56 years) for Group A, 32.6 ± 10.7 years (range 24–54 years) for Group B, and 29.8 ± 12.1 years (range 16–48 years) for Group C.
Flap thickness and topographic variability
Flap thickness measurements and statistics for the three groups (all units in micrometers [μm]) are presented in . As shown in this table, Group A (members of which received LASIK treatment with the Moria M2) had an average postoperatively measured flap thickness of 138.83 ± 12.38 μm (average ± standard deviation) (range 114–159 μm). The intended (programmed) thickness was 130 μm. Group B (IntraLase FS60) had an average flap thickness of 128.46 ± 5.72 μm (range 119–137 μm), with an intended (programmed) thickness of 120 μm. Finally, Group C (WaveLight FS200) had an average flap thickness of 122.00 ± 5.64 μm (range 94–135 μm), with an intended (programmed) thickness of 120 μm. Representative flap thickness maps from each group are shown in .
Table 1 Flap thickness measurements, range, and topographic flap thickness variability statistics for the three groups examined
Column 5 in shows the grouped topographic flap thickness values, their range, and standard deviation.
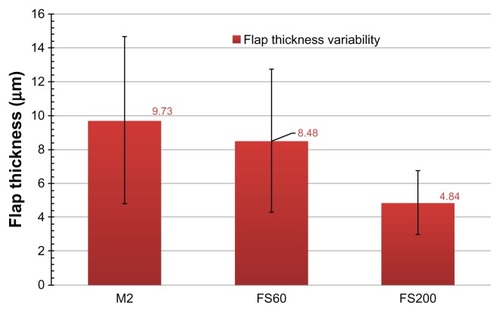
As presented in the tabulated data and illustrated in , the mean topographic flap thickness variability was 9.73 ± 4.93 μm for Group A, 8.48 ± 4.23 μm for Group B, and 4.84 ± 1.88 μm for Group C.
Figure 4 Postoperative topographic flap thickness variability for the three groups examined.
Paired comparisons between the three modalities () show that there is a statistically significant flap thickness difference between the FS200 and M2 microkeratome groups (P = 0.004), while the other two pairs (FS200 and FS60; FS60 and M2) were not statistically different (paired sample t-test, P = 0.078 and 0.095, respectively).
Table 2 Paired sample t-tests (P) between the three pairs of flap-creation modalities examined
Epithelial thickness and topographic variability
To determine any potential bias in these flap thickness and/or thickness variability measurements from epithelial masking, we investigated epithelial thickness. Results per group are reported in and illustrated in . The mean epithelial thickness was 51.50 ± 4.19 μm in Group A, 51.54 ± 4.16 μm in Group B, and 49.53 ± 4.28 μm in Group C. Topographic epithelial thickness variability for the three groups was 4.15 ± 1.53 μm in Group A, 5.11 ± 1.15 μm in Group B, and 3.97 ± 1.58 μm in Group C.
Figure 5 Postoperative epithelial thickness and topographic epithelial thickness variability for the three groups examined.
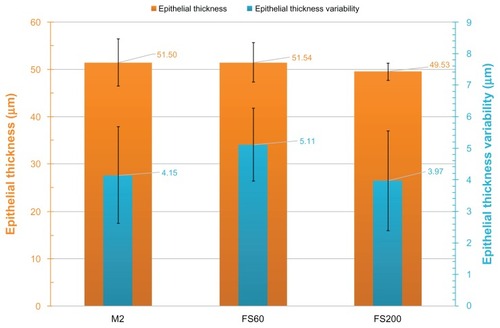
Table 3 Epithelial thickness measurements and statistics for the three groups examined
In our study, none of the cases showed a significant epithelial thickness deviation that suggested early ectasia, nor did the epithelium contribute to the flap thickness homogeneity differences found between the three groups.
Discussion
The importance of flap thickness
Flap parameter accuracy and homogeneity have been studied and debated at length by refractive surgeons globally over the last 10 years. There appear to be variable differences reported in the basic surgical outcomes when comparing procedures with flaps created either with a mechanical microkeratome or a femtosecond laser.Citation16 For example, a study in hyperopic patients showed significantly better refractive results with femtosecond laser flaps than with microkeratome flaps.Citation17 Another study showed that clinically significant epithelial ingrowth after femtosecond LASIK is an infrequent complication, the incidence being less than reported for microkeratome LASIK.Citation18
Despite the fact that multiple generations of femtosecond lasers for refractive surgery have been introduced so far, and while the “perfect LASIK flap” is becoming increasingly tangible, the field continues to welcome research on the comparative characteristics of the femtosecond laser versus mechanical microkeratome flap, including that on morphology, cut accuracy, flap thickness reproducibility, flap-edge quality, stromal-bed surface roughness, and histopathology.Citation19–Citation25
The femtosecond laser continues to be preferred for flap creation over the bladed mechanical microkeratome due to the increased safety, precision, and regularity this modality offers.Citation26,Citation27
Flap thickness is considered an important indicator of LASIK safety due to the critical importance of adequate residual stromal preservation, not only at the center of the cornea, but also for the overall area of the cornea affected. To ensure a thicker residual stroma, a thin flap is preferable in myopic treatments. A further benefit of a thin flap (in addition to a smaller diameter) is reduced interference of the superficial “running” nerves within the corneal stroma, which can lessen postoperative dry-eye syndrome.Citation28 However, the risk in opting for a thin flap is that the flap may end up too thin – that is, a flap < 90 μm. Such a flap may be associated with flap slippage, striae, irregularity, astigmatism, buttonholes, free caps, and corneal haze.Citation29,Citation30
However, thicker flaps (for myopic treatment, a flap > 140 μm is acknowledged as being too thick) may lead to a dangerously thin residual stroma (after the excimer ablation), possibly compromising the biomechanical corneal strength and leading to iatrogenic corneal ectasia.Citation31
However, the 140 μm flap has been considered by our team optimal for hyperopic ablation and its accompanying (large-diameter) blend zone, as a means to reduce epithelial ingrowth.Citation14
Thus, to ensure safety of the procedure and enable borderline decisions to be made – such as in operations with relatively thin residual stroma – it is of ultimate importance that both a higher precision (intended vs achieved thickness) and increased accuracy (improved homogeneity, or else reduced thickness variability) of the lamellar flap cut or stromal tissue separation be sought when selecting a femtosecond laser.
Our results indicate that the postoperative flap thickness, as measured by the HF-UBM method, is larger than the programmed flap thickness and that there are differences between the peripheral and the central thickness. In Group A, overall flap thickness was thicker than planned by +8.83 μm (minimum, 114 μm – ie, a −6 μm average difference; maximum, 159 μm – ie, a +39 μm difference) with an average thickness standard deviation of 12.38 μm. In addition, we observe that this group had the largest topographic thickness variability (9.73 ± 4.93 μm), which is an indication of the inhomogeneity of the flap thickness produced by the microkeratome. We also observe that in this group, on average, the flaps were thicker in the periphery (average 140.58 μm in the 3–6 mm zone vs an average of 138.33 μm in the central 0–3 mm zone), owing to the so-called meniscus shape.Citation23
In Group B, we also observe that the overall flap thickness was thicker than planned, by +8.46 μm. However, the range is smaller (minimum, 119 μm, maximum, 137 μm), and so is the standard deviation (6.80 μm). The flap thickness variability is smaller than that of Group A (8.48 ± 4.23 μm). In Group B, we observe that, on average, the flaps were thinner in the peripheral zone (average peripheral thickness, 128.15 μm) compared with in the central zone (average central thickness, 130.31 μm).
In Group C, we observe that the average postoperative flap thickness was just 2.00 μm thicker than programmed and that flaps in this group had the smallest topographic thickness variability (4.84 μm ± 1.88 μm). This group also had nonstatistically different peripheral and central flap thicknesses (central flap thickness, 122.20 ± 6.11; peripheral flap thickness, 122.53 ± 6.11 μm).
It is worth comparing our results to a similar recent study,Citation32 in which a handheld AS-OCT unit was used to measure postoperative flap thickness. In that study, the standard deviation for paracentral flap thickness and peripheral flap thickness was reported to be ±3.16 μm and ±3.26 μm, respectively, for the FS200 group and ±10.27 μm and ±10.35 μm for the Hansatome microkeratome, respectively.
Differences between the two femtosecond lasers in terms of flap thickness variability
An interesting finding of our study is that the measured topographic flap thickness variability was smaller for the FS200 group than for the FS60 and M2 microkeratome groups. The FS200 flaps appeared to be more uniform, with an average topographic thickness variability of 4.84 ± 1.88 μm, whereas this was 8.48 ± 4.23 μm for the FS60 group and 9.73 ± 4.93 μm for the M2 microkeratome group.
In addition, the FS200 flaps were associated with a statistically significant smaller epithelial average thickness (49.53 ± 4.28 μm, range 42–56 μm) over the other groups: the FS60 group had an average epithelial thickness of 51.54 ± 4.16 μm (range 44–58 μm) and the microkeratome group had an average epithelial thickness of 51.50 ± 4.19 μm (range 43–57 μm). The FS60 and M2 microkeratome were not statistically different in terms of epithelial thickness variability.
The difference between the flap thickness variability of the FS200 and the FS60 may stem from their different intraoperative gas-venting techniques and/or their different – active versus passive – intraoperative suction methods. Intraoperative gas buildup during creation of the lamellar part of the flap (opaque bubble layer)Citation33 may interfere with the precision of the femtosecond laser tissue separation. In contrast, variation in the stabilizing force to the cornea during this process, through the applanation pressure applied, may also result in tissue separation bias. The FS60 uses a passive syringe chamber-induced suction that is achieved prior to cornea applanation and maintained passively during the procedure, while the FS200 uses a tubing system that connects the suction ring to an active vacuum pump within the unit that monitors and maintains stable suction during the lamellar cut procedure.
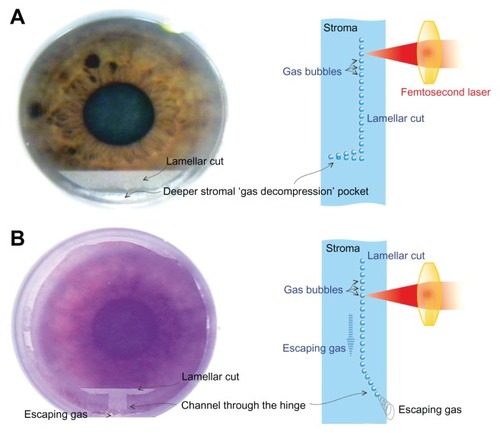
The first step in creating the flap is the creation of an externalizing channel peripheral to the hinge of the flap, permitting the generated gas to diffuse outside of the cornea. The different initial steps in creating femtosecond laser-assisted flaps are illustrated in – the channel is clearly shown in 6B (FS200), whereas there is no such channel in 6A (FS60).
Figure 6 Schematic of the architectural differences between the (A) Intralase™ FS60 (Abbott Medical Optics, Santa Ana, CA, USA) and (B) WaveLight® FS200 (Alcon, Fort Worth, TX, USA) femtosecond lasers.
We conclude that all three devices are very safe and offer great efficacy in flap making. Both femtosecond lasers appear to be more accurate in generating the desired central corneal flap thickness, as expected. However, the dramatic difference in overall flap thickness between the FS200 and the other two modalities studied herein may suggest that the FS200 has a better aberrations profile and better mesopic and scotopic visual functions. As our momentum in corneal imaging expands, we may come to explain and understand visual function parameters beyond acuity and refraction that may be significant in assessing modern refractive surgery.
Conclusion
Our study suggests that the WaveLight FS200 femtosecond laser has a statistically higher precision in planar flap thickness creation as flaps created with this laser have a statistically smaller flap thickness area variation when compared with the flaps produced by the IntraLase FS60 and M2 microkeratome. The difference between the FS200 and the FS60 may stem from their different intraoperative gas-venting techniques and/or their different – active versus passive – intraoperative suction methods.
Disclosure
AJK consults for Alcon. The authors declare no other conflicts of interest in this work.
References
- KanellopoulosAJPeLHKleimanLMoria M2 single use microkeratome head in 100 consecutive LASIK proceduresJ Refract Surg200521547647916209445
- KanellopoulosAJConwayJPeLHLASIK for hyperopia with the WaveLight excimer laserJ Refract Surg2006221434716447935
- Reggiani-MelloGKruegerRRComparison of commercially available femtosecond lasers in refractive surgeryExpert Rev Opthalmol2011615556
- JuhaszTKastisGASuárezCBorZBronWETime-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and waterLasers Surg Med199619123318836993
- SalomãoMQWilsonSEFemtosecond laser in laser in situ keratomileusisJ Cataract Refract Surg20103661024103220494777
- MurakamiYMancheEEComparison of intraoperative subtraction pachymetry and postoperative anterior segment optical coherence tomography of laser in situ keratomileusis flapsJ Cataract Refract Surg201137101879188321840682
- von JagowBKohnenTCorneal architecture of femtosecond laser and microkeratome flaps imaged by anterior segment optical coherence tomographyJ Cataract Refract Surg2009351354119101422
- XuYZhouXWangLXuHA morphological study of corneal flap after thin-flap laser-assisted in situ keratomileusis by anterior segment optical coherence tomographyJ Int Med Res20103861952196021226998
- KanellopoulosAJAslanidesIMAsimellisGCorrelation between epithelial thickness in normal corneas, untreated ectatic corneas, and ectatic corneas previously treated with CXL; is overall epithelial thickness a very early ectasia prognostic factor?Clin Ophthalmol2012678980022701079
- ReinsteinDZSilvermanRHRaevskyTArc-scanning very high-frequency digital ultrasound for 3D pachymetric mapping of the corneal epithelium and stroma in laser in situ keratomileusisJ Refract Surg200016441443010939721
- ReinsteinDZArcherTJGobbeMLASIK flap thickness profile and reproducibility of the standard vs zero compression Hansatome microkeratomes: three-dimensional display with Artemis VHF digital ultrasoundJ Refract Surg201127641742621410084
- KanellopoulosAJAsimellisGAnterior segment optical coherence tomography – assisted topographic corneal epithelial thickness distribution imaging of a keratoconus patientCase Rep Ophthalmol 10.1159/000350630.In press
- SpadeaLFascianiRNecozioneSBalestrazziERole of the corneal epithelium in refractive changes following laser in situ keratomileusis for high myopiaJ Refract Surg200016213313910766381
- KanellopoulosAJTopography-guided hyperopic and hyperopic astigmatism femtosecond laser-assisted LASIK: long-term experience with the 400 Hz eye-Q excimer platformClin Ophthalmol2012689590122791969
- MrochenMWüllnerCKrauseJKlafkeMDonitzkyCSeilerTTechnical aspects of the WaveLight FS200 femtosecond laserJ Refract Surg20102610S833S84020954680
- ZhangZHJinHYSuoYFemtosecond laser versus mechanical microkeratome laser in situ keratomileusis for myopia: Metaanalysis of randomized controlled trialsJ Cataract Refract Surg201137122151215922108110
- Gil-CazorlaRTeusMAde Benito-LlopisLMikropoulosDGFemtosecond laser vs mechanical microkeratome for hyperopic laser in situ keratomileusisAm J Ophthalmol20111521162121507378
- KamburoğluGErtanAEpithelial ingrowth after femtosecond laser-assisted in situ keratomileusisCornea200827101122112519034125
- KezirianGMStonecipherKGComparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusisJ Cataract Refract Surg200430480481115093642
- HolzerMPRabsilberTMAuffarthGUFemtosecond laser-assisted corneal flap cuts: morphology, accuracy, and histopathologyInvest Ophthalmol Vis Sci20064772828283116799021
- KimJYKimMJKimTIChoiHJPakJHTchahHA femtosecond laser creates a stronger flap than a mechanical microkeratomeInvest Ophthalmol Vis Sci200647259960416431956
- Montes-MicóRRodríguez-GalieteroAAlióJLFemtosecond laser versus mechanical keratome LASIK for myopiaOphthalmology20071141626817070593
- AhnHKimJKKimCKComparison of laser in situ keratomileusis flaps created by 3 femtosecond lasers and a microkeratomeJ Cataract Refract Surg201137234935721241920
- ChenSFengYStojanovicAJankovMR2ndWangQIntraLase femtosecond laser vs mechanical microkeratomes in LASIK for myopia: a systematic review and meta-analysisJ Refract Surg2012281152422233436
- KhoramniaRSalgadoJPLohmannCPKobuchKAvon MohrenfelsCWPrecision, morphology, and histology of corneal flap cuts using a 200-kHz femtosecond laserEur J Ophthalmol201222216116721623593
- KohnenTKlaprothOKDerhartunianVKookDResults of 308 consecutive femtosecond laser cuts for LASIKOphthalmologe20101075439445 [German]19777246
- HeichelJHammerTSietmannRDunckerGIWilhelmFScanning electron microscopic characteristics of lamellar keratotomies using the Femtec femtosecond laser and the Zyoptix XP microkeratome. A comparison of qualityOphthalmologe20091074333340 [German]19657659
- KanellopoulosAJPallikarisIGDonnenfeldEDDetorakisSKoufalaKPerryHDComparison of corneal sensation following photorefractive keratectomy and laser in situ keratomileusisJ Cataract Refract Surg199723134389100105
- ChoudhriSAFeigenbaumSKPeposeJSFactors predictive of LASIK flap thickness with the Hansatome zero compression microkeratomeJ Refract Surg200521325325915977882
- HatchBBMoshirfarMOllertonAJSikderSMifflinMDA prospective, contralateral comparison of photorefractive keratectomy (PRK) versus thin-flap LASIK: assessment of visual functionClin Ophthalmol2011545145721573091
- KochDDThe riddle of iatrogenic keratectasiaJ Cataract Refract Surg199925445345410198842
- ShettyRMalhotraCD’SouzaSWadiaKWaveLight FS200 vs Hansatome LASIK: Intraoperative Determination of Flap Characteristics and Predictability by Hand-held Bioptigen Spectral Domain Ophthalmic Imaging SystemJ Refract Surg20122811S815S82023447894
- KanellopoulosAJAsimellisGDigital analysis of flap parameter accuracy and objective assessment of opaque bubble layer in femtosecond laser-assisted LASIK: a novel techniqueClin Ophthalmol201371923277737