Abstract
Background
The purpose of this study was to evaluate the visual outcome and self-reported vision-targeted health status in patients treated with intravitreal ranibizumab for wet age-related macular degeneration (AMD).
Methods
A total of 51 eyes from 50 patients aged 76 ± 7 years, with wet AMD not previously treated, were included in this prospective study. Best corrected visual acuity was examined using Early Treatment Diabetic Research Study charts and near vision reading. All patients underwent an ophthalmological examination, including fluorescein and indocyanine green angiography (occult cases) and optical coherence tomography. The Visual Function Questionnaire test was completed before and 37 ± 7 months after the start of intravitreal injections.
Results
The patients received a mean number of 7.8 ± 5.0 (range 2–22) injections. One month after the third intravitreal injection, significant improvement was seen in both visual acuity (53 ± 14 to 61 ± 14 letter, P = 0.001) and near vision (17 ± 9 to 11 ± 8 points, P = 0.001). During follow-up, mean visual acuity decreased from 53 ± 14 to 44 ± 24 letters (P = 0.011), and near vision decreased from 17 ± 9 to 20 ± 11 points (P = 0.048). Despite visual impairment, the quality of life test revealed no significant decrease in mental health (P = 0.529) or ability to read a newspaper (P = 0.21), but a decrease in distance activities (reading street signs, steps, going to the theater) from 57 ± 27 to 46 ± 31 points (P = 0.007) was documented.
Conclusion
Decreased visual acuity was related to a decrease in self-reported visual function for distance activities, while mental health items, such as worrying, were not influenced.
Introduction
Age-related macular degeneration (AMD) is one of the leading causes of vision loss in older people in the Western world.Citation1,Citation2 With an aging population, the figures are likely to rise. The most common variant of AMD is the untreatable dry type (85%), while the wet variant with new choroidal neovascularizations (CNV) has attracted most attention recently because of new treatment options. Two controlled studies, MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab In the treatment of Neovascular AMD) in occult CNVCitation3 and the ANCHOR (Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD) study in predominantly classic CNVCitation4,Citation5 have shown that ranibizumab (Lucentis®, Novartis, Basel, Switzerland) is effective in improving visual acuity after 2 years of monthly treatment.
In view of the large number of people with wet AMD, there has been a practical need to reduce the frequency of injections, and treatment in clinical practice has been based on clinical indications rather than monthly monitoring. Under these circumstances, it cannot be presumed that the same results will be achieved as in the controlled studies.
Visual outcome does not fully capture visual function, and visual functioningCitation6 and vision-related well-being have attracted attention recently.Citation7,Citation8 Furthermore, near vision and the ability to read a book have been addressed in a cohort treated with ranibizumab in a recent paper by Frennesson et al.Citation9
The National Eye Institute Visual Function Questionnaire (NEI VFQ-25) is an accepted tool for measuring the dimensions of self-reported vision-targeted health statusCitation6 and has been evaluated in patients with AMDCitation7,Citation8 and in patients treated with ranibizumab for AMD.Citation10
In the present prospective follow-up study of patients treated under usual care, visual acuity and near vision were measured at baseline and after 3 and 37 months of treatment with ranibizumab, and related to changes in quality of life using the NEI VFQ-25.
Materials and methods
Sixty-six consecutive patients diagnosed with wet AMD and attending the outpatient clinic at the Department of Ophthalmology in Helsingborg were included in this prospective study. Only one eye per participant was studied, and no patient had been treated previously for AMD or any other retinal disease.
Patient characteristics
Sixty-six patients were monitored and completed the NEI VFQ-25 questionnaire at 3 months and, of these, 51 patients were monitored further and completed the questionnaire again at 37 months. Of the patients who did not attend the last visit, six had died, two had moved, and seven did not want to participate. The cause of death in the six patients who died was not related to treatment with anti-vascular endothelial growth factor.
In patients monitored for only 3 months, near vision at baseline and at 3-month follow-up, as well as time from first symptoms to treatment, did not differ from the patient group monitored for 37 months, but patients who died (n = 6) were older at baseline (82 ± 11 years versus 76 ± 7 years, respectively). For the entire group, age at baseline was 76 ± 7 years and the mean time from first symptoms to treatment was 14 (range 2–72) weeks. The patients received, on average, 7.8 ± 5.0 injections during the 37-month follow-up period.
Examination included best corrected visual acuity (BCVA) using the Early Treatment Diabetic Research Study (ETDRS) chart and near vision using the Swedish Tomteboda chart (Tomteboda Resource Centre for Visually Handicapped Children, Stockholm, Sweden) which is graded in typographical points, the smallest text being 4 points and the largest 24 points. A thorough clinical examination was performed, including slit-lamp and fundus biomicroscopy, intraocular pressure measurement, fundus photography (Topcon TRC-50 IX, Tokyo, Japan), fluorescein and indocyanine green angiography, and optical coherence tomography (Zeiss Humphrey Instruments, Dublin, CA, USA).
Treatment
All patients were initially given three injections at monthly intervals with ranibizumab. A decision on reinjection was then made, based on visual acuity and the findings of optical coherence tomography.
NEI VFQ-25 questionnaire
The National Eye Institute 25-item Visual Function Questionnaire version 2000Citation11 was used as an interview instrument to evaluate self-reported vision-targeted health status. The test includes a 25-item base set of questions, and the results were calculated according to published guidelines for the NEI VFQ-25. All items are scored such that a high score represents better functioning. The questionnaire was completed during the examination visits, and an authorized Swedish version, the VFQ-25-Swedish/Sweden (vfq, S001_1), was used. Mean changes in NEI VFQ-25 subscale scores from baseline to the follow-up interviews at 3 and 37 months were registered and related to different visual outcomes. Scores for the 25 questions were averaged for each item to generate the prespecified VFQ-25 subscales. The averaged item scores at baseline and at the 37-month follow-up are presented in . Calculations are based on the averaged subscale scores.
Table 1 Responses to questions in the NEI VFQ-25 subscales at baseline and at 37-month follow-up (n = 51)
Statistics
Calculations were done using the Statistical Package for Social Sciences for Windows version 18.0 (SPSS Inc, Chicago, IL, USA). Values are given as the mean ± standard deviation or median and range. The Student’s t-test was used for normally distributed data and the Mann-Whitney U test for skewed data. Spearman’s rho was used to calculate correlations between the parameters.
Results
Visual acuity
BCVA increased one month after the third intravitreal injection, from 53 ± 14 letters to 61 ± 14 letters on the ETDRS charts (P = 0.001), and near vision improved from 17 ± 9 points to 11 ± 8 points (P = 0.001). At the final follow-up at 37 months, mean BCVA had decreased from 53 ± 14 letters at baseline to 44 ± 24 letters (P = 0.011), and near vision had decreased from 17 ± 9 points to 20 ± 11 points (P = 0.048). There was a correlation between impairment in BCVA and near vision (Spearman’s rho = 0.57, P = 0.001). Visual outcome at the 37-month follow-up correlated with visual acuity at baseline (P = 0.001). There was a positive correlation between both the number of injections given and BCVA at baseline (Spearman’s rho = 0.56, P = 0.001) and the number of injections given and BCVA at the 3-year follow-up (Spearman’s rho = 0.67, P = 0.001).
In 16 of 51 patients (31%), ETDRS letters and near vision increased over the 37-month observation period from 55 ± 14 to 69 ± 11 letters (P = 0.004), and 17 ± 9 to 7 ± 6 (P = 0.0001), respectively. These patients were the same age and had the same delay between onset of symptoms and the first visit as did patients whose vision decreased. BCVA was also the same at baseline in patients with a better outcome compared with those having a worse outcome, ie, 58 ± 14 letters versus 51 ± 14 letters (P = 0.10) and 15 ± 10 points versus 18 ± 9 points (P = 0.35), respectively. There was a trend toward an increased number of injections given in eyes with an improved visual outcome, ie, 9.5 ± 5.1 versus 6.9 ± 4.5 in eyes with a worse visual acuity outcome (P = 0.079).
Treatment of the better or worse eye
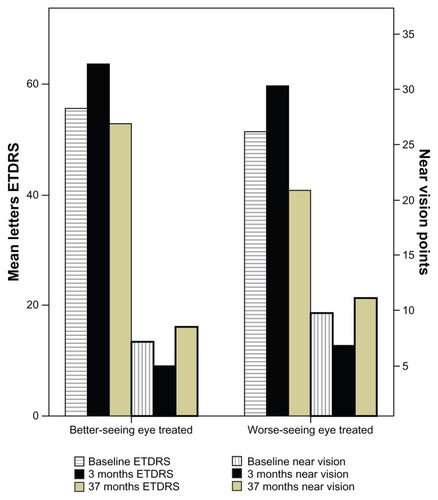
Twenty-six of 66 (39%) patients had poor vision, with a near vision score over 24 points in the second nontreated eye at baseline. Near vision, visual acuity (ETDRS), and duration before treatment were the same compared with people with better vision in the second eye, but the number of injections differed, ie, 9.6 ± 5.5 in patients with low vision in the second eye compared with 6.8 ± 4.6 (P = 0.038) in the other patients. The same proportion of people with or without low vision in the second eye were monitored for 37 months (17/26 and 34/40, respectively, not statistically significant). In addition, fewer people with affection in the second eye achieved stable visual acuity in the treated eye (4/17 [25%] versus 21/34 [62%], respectively, P = 0.016). In patients treated in the better eye, visual acuity and near vision were the same at 3-year follow-up as at baseline, while patients treated in the worse-seeing eye showed a drop in visual acuity (ETDRS) from 51 ± 14 letters to 41 ± 12 letters (P = 0.014) after 3 years.
Visual Function Questionnaire
At 3-month follow-up (all patients included, n = 66)
The quality of life test revealed an improvement in mental health (less worrying, better control) from 57 ± 30 points to 63 ± 29 points (P = 0.014) and in near vision-related items (reading newspaper, finding things on a crowded shelf, seeing well at close distance) from 48 ± 24 points to 56 ± 28 points (P = 0.002).
At 37-month follow-up (n = 51)
The quality of life test at the 37-month follow-up did not indicate improvement in any questionnaire items. There was a decrease in scores for general health from 56 ± 26 points to 46 ± 24 points (P = 0.004), ocular pain from 83 ± 22 points to 74 ± 29 points (P = 0.028), color vision (matching clothes) from 81 ± 30 points to 65 ± 30 points (P = 0.001), and in distance activities (reading street signs, steps, theatre visit) from 57 ± 27 points to 46 ± 31 points (P = 0.007). There was also a decrease in vision-specific items, such as social functioning (seeing people’s reactions, company) from 74 ± 27 points to 65 ± 30 points (P = 0.021) and role difficulties (limitations in performance) from 67 ± 28 points to 56 ± 32 points (P = 0.026).
Patients with better visual acuity at 37-month follow-up (n = 16)
In the 16 patients with an improvement in visual acuity at 37-month follow-up (change in ETDRS letters from 57 ± 14 letters to 69 ± 11 letters and in near vision from 15 ± 10 points to 7 ± 6 points), there was also an improvement in general vision items from 57 ± 18 to 70 ± 25 (P = 0.027).
Patients with visual impairment at 37-month follow-up (n = 35)
Patients with visual impairment at 37-month follow-up (ETDRS letters 51 ± 14 letters to 34 ± 20 letters and near vision 18 ± 9 points to 25 ± 8 points) demonstrated a decrease in general health (55 ± 28 points to 42 ± 24 points P = 0.003), worse distance activities (51 ± 27 to 38 ± 29, P = 0.006), and impaired color vision (matching clothes) (79 ± 33 to 63 ± 33, P = 0.009). In addition, there was a decline in response to social functioning questions (70 ± 29 to 57 ± 31, P = 0.019).
Patients with AMD in the other eye (n = 18): better-seeing eye treated
There was no change from baseline in visual outcome at 37-month follow-up in the treated eye (ETDRS 56 ± 14 letters to 53 ± 18 letters and near vision 13 ± 8 points to 16 ± 10 points) in patients with AMD in the second eye (). Quality of life testing demonstrated a decrease in general health from 61 ± 27 points to 47 ± 24 points (P = 0.007), and increased dependency from 69 ± 28 points to 54 ± 32 points (P = 0.026). However, near activities improved from 34 ± 16 points to 40 ± 24 points (P = 0.001).
Figure 1 Mean best corrected visual acuity (ETDRS letters) and near vision (points) at baseline, and at 3-month and 37-month follow-up in the better-seeing eye treated and the worse-seeing eye treated.
Abbreviation: ETDRS, Early Treatment Diabetic Research Study.
Patients with no AMD in the other eye (n = 34): worse-seeing eye treated
There was a decrease in BCVA from 52 ± 14 letters to 41 ± 25 letters (P = 0.014) in the worse-seeing eye treated, but near vision was similar to baseline (19 ± 9 points to 21 ± 11 points) at 3-year follow-up (). Quality of life testing showed a decrease in color vision (matching clothes) from 87 ± 29 points to 69 ± 29 points (P = 0.002), and in distance activities from 64 ± 27 points to 51 ± 31 points (P = 0.018) at 37-month follow-up.
Comparing patient groups with different outcomes
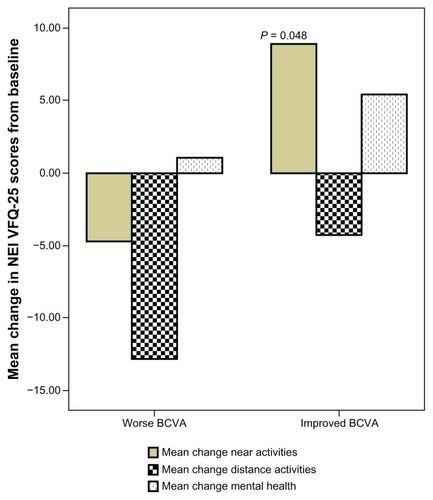
No difference was observed in mean change from baseline scores over 37 months in pooled near activities, distance activities, or mental health-related items when comparing patients with the better-seeing eye treated versus worse-seeing eye treated (). However, when the group with improved BCVA was compared with the group with a worse BCVA outcome at follow-up, we demonstrated a difference in pooled near activities of 9 ± 21 points versus −4.7 ± 18 points (P = 0.048, see ).
Figure 2 Pooled data for near activities (reading newspaper, seeing well at close distance, finding an item on crowded shelf), distance activities (street signs, steps, stairs in dim light), and mental health (worrying, “less control over what I am doing”) items in patients with better-seeing eye treated compared with worse-seeing eye treated.
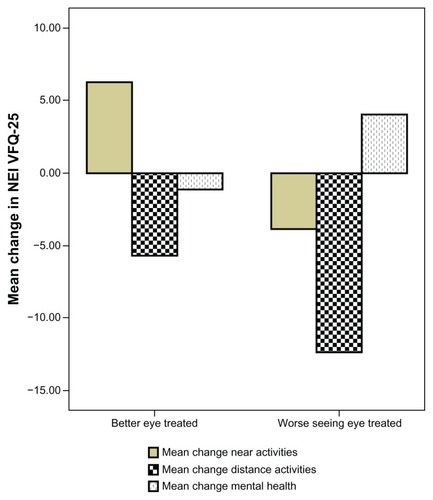
Figure 3 Pooled data for near activities (reading newspaper, seeing well at close distance, finding an item on crowded shelf), distance activities (street signs, steps, stairs in dim light), and mental health (worrying, “less control over what I am doing”) items in patients with worse visual outcome compared to patients with improved visual outcome.
Discussion
This study demonstrated an improvement in BCVA by an average of eight letters on the ETDRS chart at 3-month follow-up. This is comparable with the findings of the MARINA study in occult CNVCitation3 which showed an improvement after one year of 7.2 letters, and is slightly worse than those of the ANCHOR study in classic CNVCitation4 which demonstrated an improvement of 11.3 letters after one year. At the 3-year follow-up in our study, BCVA decreased by an average of nine letters and we demonstrated worse BCVA than before treatment. This is in contrast with the 2-year follow-up results for MARINA, which showed an increase of 6.6 letters, and for the ANCHOR study, which showed an increase of 10.7 letters. However, the results are better than for the sham-treated eyes in MARINA, which lost an average of two lines by one year and three lines by 2 years,Citation6 and in the PIER study, in which sham-treated eyes lost an average of three lines by one year.Citation12 However, we demonstrated a result after 3 years but one that was not distinguished between different sorts of CNV. Furthermore, most importantly, the mean number of injections over the 3 years was only eight compared with monthly injections in the treatment arms in the other studies. Consequently, it seems most likely that the patients were under-treated in the present study, given that even the uncontrolled PRONTO study,Citation13 which used three monthly injections initially followed by variable dosing, showed an increase of 11 letters after 2 years.
After the initiation phase, the strongest evidence is for continued monthly treatment, but this is often not feasible in clinical practice, so an individualized approach is the standard in many countries today. However, the risk is obvious, ie, there may be a delay in reinitiating treatment. It is reported that delayed initiation of treatment in patients with newly diagnosed AMD is associated with substantial BCVA loss.Citation14 In the present study, the interval between diagnosis and treatment was the same, irrespective of the visual outcome.
Near vision, which is important for reading, has not been or is rarely studied. In a previous paper, Frennesson et alCitation9 demonstrated an improvement in near reading from 9 to 6 points after three monthly injections with ranibizumab in patients with wet AMD. This is less than in our study, in which we showed an improvement from 17 to 11 points after the first three injections. However, patients in the present study started at a lower visual acuity level, and worse baseline BCVA has been reported to result in better improvement.Citation15 In contrast with the paper by Frennesson et al, we found a good correlation between changes in BCVA over time and near vision. This suggests that it may not be necessary to register the near vision parameter in order to assess the patient’s vision status.
The NEI VFQ-25 has recently been demonstrated to respond well to changes in visual acuity.Citation10 In the present study, mean changes in NEI VFQ-25 scores at 3 months for near activities are in agreement with the subanalyses from the MARINA and ANCHOR studies,Citation16 and also with a recent paper by Frennesson et al who found a greater improvement for near vision-related activities than for distance-related items after 3 months of follow-up.Citation9 Interestingly, we could also demonstrate an improvement in mental health-related items, like less worrying, at 3 months. The less favorable outcome of the NEI VFQ-25 after 37 months reflects the fact that the entire group had an impaired BCVA outcome, probably because of undertreatment. The positive correlation between number of injections given and visual outcome indicates that a more frequent treatment regime would have been favorable.
NEI VFQ-25 scores decreased on many of the questions but, interestingly, not in near activities items such as reading the newspaper and finding things on a crowded shelf. Mental health items like worrying and “less control over what I am doing” were also unchanged. The fact that the patients have received support and care while frequently visiting the outpatient clinic may have helped them to cope better with their visual disability.Citation17
The NEI VFQ-25 analysis in the ANCHOR and MARINA studies demonstrated an association between improving visual acuity and decreased dependency and improved distance activities.Citation10 We could not confirm that distance activities improved with a better visual outcome, but dependency increased with worse visual outcome.
Patients treated in the better-seeing eye had similar BCVA and near vision at follow-up as at baseline, which probably reflects the greater number of injections given, ie, 9.5 compared with 6.9. In spite of this, the NEI VFQ-25 test demonstrated worse general health and increased dependency, but there was an improvement in items correlated with near vision, such as the ability to read a newspaper.
Interestingly, no difference was observed when comparing the changes between baseline and at 37-month follow-up for pooled NEI VFQ-25 data on near and distance activities and mental health items between patients treated in the better-seeing or worse-seeing eye. Only near activities items proved to be significantly different between eyes with improved or decreased visual acuity outcome. The decrease in scores for general health in patients with two eyes affected contradicts a study that reported more depression among people with only one eye affected, on the basis that they would feel a greater uncertainty about the future.Citation18
To our knowledge, this is the first quality of life study that has monitored patients with wet AMD treated using ranibizumab in routine care for 3 years. Although the number of patients in the study was not very high, the strength is that as many as 77% of the patients in the study completed the last NEI VFQ-25 interview at the 37-month follow-up.
In conclusion, at 3-year follow-up, we demonstrated a decrease in visual acuity in the majority of patients treated, probably due to undertreatment, which emphasizes the importance of treating AMD patients frequently and without delay. Decreased visual acuity was related to a decrease in self-reported visual function for distance activities, while mental health items like worrying were not influenced.
Acknowledgments
This study was supported by grants from the Medical Faculty, Lund University, the Skåne County Council Foundation for Research and Development, and Crown Princess Margareta’s Committee for the Blind.
Disclosure
The authors report no conflicts of interest in this work.
References
- KlaverCCWolfsRCVingerlingJRHofmanAde JongPTAge specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam StudyArch Ophthalmol199811656536589596502
- BresslerNMAge-related macular degeneration is the leading cause of blindnessJAMA2004291151900190115108691
- RosenfeldPJBrownDMHeierJSfor the MARINA Study GroupRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- BrownDMMichelsMKaiserPKRanibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR studyOphthalmology20091161576519118696
- BrownDMKaiserPKMichelsMRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med2006355141432144417021319
- MargolisMKCoyneKKennedy-MartinTBakerTScheinORevickiDAVision-specific instruments for the assessment of health-related quality of life and visual functioning: a literature reviewPharmacoeconomics20022079181212236802
- MiskalaPHHawkinsBSMangioneCMResponsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization. SST report no 1Arch Ophthalmol2003121453153912695250
- MiskalaPHBresslerNMMeinertCLRelative contributions of reduced vision and general health to NEI-VFQ scores in patients with neovascular age-related macular degenerationArch Ophthalmol2004122575876615136325
- FrennessonCNilssonUPeeboBNilssonSSignificant improvement in near vision, reading speed, central visual field and related quality of life after ranibizumab treatment of wet age-related macular degenerationActa Ophthalmol201088442042519678811
- SuñerIJGreggTKokameYuEWardJDolanBresslerNMResponsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trialsInvest Ophthalmol Vis Sci20095083629363519255158
- MangioneCMBerrySSpritzerKIdentifying the content area for the National Eye Institute Vision Function Questionnaire (NEI-VFQ): results from focus groups with visually impaired personsArch Ophthalmol199811622272339488276
- RegilloCDBrownDMAbrahamPRandomized, double-masked, sham controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1Am J Ophthalmol2008145223924818222192
- FungAELalwaniGARosenfeldPJAn optical coherence tomography guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degenerationAm J Ophthalmol2007143456658317386270
- AriasLArmadaFDonateJDelay in treating age-related macular degeneration in Spain is associated with progressive vision lossEye200923232633318202712
- BoyerDSAntoszykANAwhCCBhisitkulRBShapiroHAcharyaNRMARINA Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degenerationOphthalmology2007114224625217270674
- BresslerNMChangTSSuñerIJVision-related function after ranibizumab treatment by better- or worse-seeing eyeOphthalmology2010117474775620189654
- ScottIUSmiddyWESchiffmanJFeuerWJPappasCJQuality of life of low-vision patients and the impact of low-vision servicesAm J Ophthalmol19991281546210482094
- SlakterJSSturMQuality of life in patients with age-related macular degeneration: impact of the condition and benefits of treatmentSurv Ophthalmol200550326327215850815