Abstract
Purpose
To describe outcomes of intravitreal ranibizumab using a pro re nata regimen for treatment-naive exudative age-related macular degeneration (AMD), in Japanese patients over the first 2 years.
Methods
Clinical records were retrospectively reviewed of 48 eyes of 48 patients with treatment-naive exudative AMD who underwent intravitreal ranibizumab therapy. After three monthly injections (induction), patients were examined monthly, and subsequent injections were performed as needed (pro re nata) for any residual activity, by fundus biomicroscopy and imaging studies, regardless of severity.
Results
Twenty-nine (60%) of the patients were men, and 19 (40%) were women; the mean age was 76.1 years. Of the 48 eyes evaluated, 17 (35%) had findings consistent with polypoidal choroidal vasculopathy, and five (10%) with retinal angiomatous proliferation. A mean of 6.0 ranibizumab injections were given in the first year, 3.5 in the second year, and 9.5 over the 2-year period. The best-corrected visual acuity (logarithm of minimum angle of resolution) improved significantly, from 0.35 at baseline to 0.21 at 12 months (P < 0.01), and remained stable at 0.21 at 24 months (P < 0.01). The mean central macular thickness decreased significantly, from 355.4 μm at baseline to 237.9 μm at 12 months (P < 0.01) and 247.7 μm at 24 months (P < 0.01).
Conclusion
Improved visual acuity and decreased central macular thickness were observed and maintained over a 2-year period, in a Japanese population receiving 3 monthly induction injections followed by a pro re nata regimen of ranibizumab for exudative AMD.
Age-related macular degeneration (AMD) is the leading cause of blindness among the elderly in most developed countries and is the fourth common cause of legal blindness for all ages in Japan.Citation1 Ranibizumab, the recombinant, humanized antibody fragment against vascular endothelial growth factor (VEGF)-A was shown to improve mean visual acuity in eyes with exudative AMD in the MARINA and ANCHOR Phase III clinical trials, which used scheduled monthly doses.Citation2,Citation3 However, due to the high burden associated with monthly intravitreal injections, several regimens involving less frequent dosing have been investigated, including injections every 3 months (the PIER study),Citation4 dosing as needed or pro re nata (PRN) (the PrONTO and SUSTAIN studies),Citation5–Citation7 and “treat and extend” dosing.Citation8,Citation9 These studies were all conducted in the United States or Europe, and therefore, it is unclear whether their treatment outcomes apply to Japanese and other Asian patients with exudative AMD.
It has been reported that 23%–55% of exudative AMD in Japan is due to polypoidal choroidal vasculopathy (PCV),Citation10–Citation13 greatly exceeding those rates reported for patients in the United States.Citation14 Preliminary studies using anti-VEGF agents in Japanese patients have shown mixed results. One study of 47 eyes described a good response to intravitreous bevacizumab in eyes with classic choroidal neovascularization (CNV) but only limited efficacy in eyes with occult CNV.Citation15 Other studies using bevacizumab have suggested that anti-VEGF therapy was not very effective for PCV in Japanese eyes.Citation13,Citation16 A Phase I–II clinical trial comparing two doses of monthly administrations of ranibizumab for exudative AMD in Japan showed good clinical efficacy of both doses over 1 year.Citation17 However, a subsequent study found that the response to ranibizumab therapy over the first 3 months was shown to be better in PCV eyes than in non-PCV eyes.Citation18
In the current retrospective study, we describe 2-year outcomes of intravitreal ranibizumab for treatment-naive exudative AMD in Japanese patients who received three monthly injections (induction) followed by injections based on a PRN scheme.
Patients and methods
We retrospectively reviewed clinic charts of patients who underwent intravitreal ranibizumab injections (Lucentis®; Novartis Pharmaceuticals Corp, Basel, Switzerland; Genentech, Inc, South San Francisco, CA, USA) for the first time between March 2009 and October 2009 for the indication of exudative AMD. Patients had to be at least 50 years of age at the first ranibizumab injection and had to be treatment-naive for inclusion in the study. During the study period, 61 patients were found to have initiated ranibizumab therapy for exudative AMD, of which 48 eyes of 48 patients were followed for at least 24 months. Monthly examinations were performed on all patients, including measurement of best-corrected visual acuity (BCVA), dilated fundus biomicroscopy, color fundus photography, and optical coherence tomography (Cirrus™, Carl Zeiss Meditec, Jena, Germany). Optical coherence tomography (OCT) was performed using the macular cube mode (200 × 200 scans). The initial evaluation of the eyes included fluorescein angiography (VX-10, Kowa Company, Ltd, Nagoya, Japan) and indocyanine green angiography (Heidelberg Retina Angiograph 2; Heidelberg Engineering Inc, Heidelberg, Germany).
Injections of ranibizumab (0.5 mg/0.05 cc) were performed in the outpatient clinic, using a departmental protocol for intravitreal injections. After topical anesthesia and standard antisepsis preparation and draping, injections were performed using a 30 gauge needle, under a 5 × operating microscope. Preoperative and postoperative topical antibiotic drops were used. An induction of three ranibizumab injections was performed at roughly 4 week intervals, followed by monthly examinations at which time additional injections (reinjections) were performed for one or more of the following: (1) any decrease in BCVA associated with fluid in the macula, as assessed by OCT; (2) persistence of intraretinal or subretinal fluid, by OCT, that continued to diminish with additional injections; (3) any increase in central macular thickness or macular volume, by OCT; (4) new or worsening macular hemorrhage, by fundus biomicroscopy; and (5) new-onset classic CNV, as assessed by fundus biomicroscopy and/or fluorescein angiography. All reinjections were performed on the same day after confirming the necessity of reinjection.
BCVA was measured using metric charts at a distance of 5 meters and was converted to a logarithm of the minimum angle of resolution (logMAR) for statistical analysis. Improvement or worsening of BCVA was defined as a change of at least 0.3 logMAR. The comparison of two means was performed using the paired Student’s t-test, while the comparison of three means was performed using analysis of variance (ANOVA), as indicated. Multiple logistic regression analysis was performed to examine the possible association of baseline characteristics with visual improvement. All statistical analyses were performed using JMP® 10 software (version 10.0.2; SAS Institute Inc, Cary, NC, USA). P < 0.05 was considered statistically significant.
The study protocol followed the tenets set forth in the Declaration of Helsinki, and all patients provided written informed consent before beginning intravitreal injections of ranibizumab.
Results
Baseline clinical characteristics
Forty-eight eyes of 48 patients were included in this study. Twenty-nine (60%) of the patients were men, and 19 (40%) were women. Patient age ranged from 64 to 89 years, with a mean of 76.1 years. summarizes the baseline clinical characteristics of the 48 eyes. The mean visual acuity (logMAR) was 0.35, and the mean central macular thickness was 355.4 μm. Seventeen (35%) of the eyes had features of PCV, based on criteria previously published,Citation19 and five eyes (10%) had features consistent with retinal angiomatous proliferation (RAP) all with stage II lesions.Citation20 Fluorescein angiography revealed the mean greatest linear dimension (GLD) to be 3811.5 μm. Lesions were subfoveal (involving the center of the fovea) in 54% of the eyes. Predominantly classic CNV was found in 12 eyes (25%), minimally classic CNV in 12 eyes (25%), and occult with no classic CNV in 24 eyes (50%).
Table 1 Baseline clinical characteristics of the exudative age-related macular degeneration eyes
Number of injections, central macular thickness, and visual acuity outcomes
The mean number of intravitreal ranibizumab injections per eye was 6.0 ± 2.0 over the first 12 months, 3.5 ± 3.4 over the second 12 months, and 9.5 ± 4.9 over the 24-month study period. Overall, the mean central macular thickness decreased significantly, from 355.4 ± 97.6 μm (range 151 to 475 μm) at baseline to 237.9 ± 67.4 μm (range 134 to 434 μm) at 12 months (P < 0.01) and 247.7 ± 82.5 μm (range 110 to 430 μm) at 24 months (P < 0.01). The mean BCVA (logMAR) improved significantly, from 0.35 ± 0.35 (range −0.08 to 1.15) at baseline to 0.21 ± 0.28 (range −0.08 to 1.10) at 12 months (P < 0.01), and this was maintained at 0.21 ± 0.28 (range −0.08 to 1.00) at 24 months (P < 0.01).
Lesion size analysis
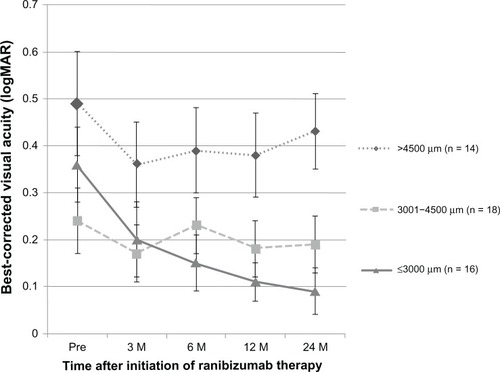
As shown in , when the eyes were analyzed according to lesion size, the mean BCVA improved best in the eyes with a lesion GLD of 3000 μm or less (n = 16), with statistically significant improvement over baseline at 3 months (P < 0.01), 6 months (P < 0.01), 12 months (P < 0.01), and 24 months (P < 0.01). The eyes with a lesion GLD of 3001 to 4500 μm showed a statistically significant improvement over baseline at 3 months (P = 0.04), but significance was lost at 6 months (P = 0.66), 12 months (P = 0.13), and 24 months (P = 0.36). The eyes with a lesion GLD greater than 4500 μm showed no improvement in BCVA. The mean number of ranibizumab injections over the 24-month period was 7.4 for the eyes with a lesion GLD ≤ 3000 μm, 10.3 for the eyes with a lesion GLD 3001 to 4500 μm, and 10.8 for the eyes with a lesion GLD > 4500 μm. Although there was a trend for fewer injections in the eyes with a lesion GLD ≤ 3000 μm, the differences were not statistically significant by ANOVA.
Lesion composition analysis
shows the change in BCVA according to the lesion composition. Among the eyes with predominantly classic CNV, the mean BCVA improved from 0.42 at baseline to 0.11 (P < 0.01) at 24 months. For the eyes with minimally classic CNV, the mean BCVA improved from 0.56 at baseline to 0.37 at 24 months (P = 0.02). For the eyes with occult with no classic CNV, there was no statistically significant improvement between the mean BCVA of 0.22 at baseline and the mean BCVA of 0.19 at 24 months (P = 0.50). Overall, more ranibizumab injections were administered in the eyes with a greater proportion of occult CNV. The mean number of ranibizumab injections over the 24-month period was 5.5 for the eyes with predominantly classic CNV, 9.6 for the eyes with minimally classic CNV, and 11.4 for the eyes with occult with no classic CNV; the difference was statistically significant only between the eyes with predominantly classic CNV and the eyes with occult with no classic CNV (P < 0.01).
Table 2 Change in best corrected visual acuity, according to lesion compositionTable Footnotea,Table Footnoteb
Analysis based on PCV and RAP features
shows the change in BCVA according to the type of exudative AMD, whether consistent with PCV or RAP, or lacking the features of either (termed typical AMD). Among the eyes with typical AMD, the mean BCVA improved from 0.43 at baseline to 0.23 at 24 months (P < 0.01). Among the eyes with PCV, the mean BCVA improved from 0.26 at baseline to 0.19 at 24 months; this improvement was statistically significant until month 16 (data not shown), but significance was lost thereafter. Examination of the data showed that only two of the 17 PCV eyes had a greater than 0.3 logMAR worsening of BCVA. Of these two eyes, one eye had undergone 13 injections over 18 months, with visual improvement to 1.0; however, 2 months after the 13th injection, the BCVA worsened to 0.2, and this was attributed to the onset of a hemorrhagic retinal pigment epithelium tear. The one other PCV eye with poor visual outcome had worsening of hemorrhage despite six injections over the first 6 months, with the BCVA dropping from 0.9 to 0.5.
Table 3 Change in best corrected visual acuity, according to type of exudative age-related macular degenerationTable Footnotea,Table Footnoteb
For the eyes with RAP, the mean BCVA was 0.27 at baseline and 0.17 at 24 months; this difference was not statistically significant compared with baseline. There was no difference in the mean number of injections administered over the 24 months in the eyes with typical AMD (9.1 injections) versus that in eyes with PCV (9.9 injections) or RAP (9.8 injections). Specifically among the 17 PCV eyes, the mean number of injections was 5.4 in the first year and 4.2 in the second year.
Baseline factors predictive of visual improvement
In order to examine whether baseline clinical characteristics were predictive of visual improvement (±0.3 logMAR), multiple logistic regression analysis was performed. As shown in , this confirmed that a smaller lesion GLD was, indeed, independently associated with visual improvement. Furthermore, younger patient age and better initial BCVA were identified to be independently predictive of visual improvement.
Table 4 Multiple logistic regression analysis of baseline characteristics possibly associated with visual improvementTable Footnotea
Complications
No injection-related complications, such as endophthalmitis or retinal detachment, were observed during the study period. In addition, there were no reports of stroke or other thromboembolic events.
Discussion
In summary, the present study of Japanese patients with exudative AMD treated by intravitreal ranibizumab monotherapy revealed that three monthly induction injections followed by monthly examinations and a PRN regimen for subsequent injections resulted in significantly improved visual acuity by the end of the first year that was maintained at the end of the second year. The improved visual acuity corresponded to a decrease in central macular thickness that also remained stable through the second year. As one would expect, the eyes with smaller lesions had the best visual acuity improvement and a trend towards the least number of injections; smaller-lesion GLD was confirmed to be an independent factor associated with visual improvement. Furthermore, the eyes with predominantly classic CNV and minimally classic CNV both obtained significant improvements in visual acuity, but this was not so for the eyes with occult with no classic CNV. The eyes with typical AMD showed improvement in mean visual acuity at 2 years, while the PCV and RAP eyes showed an initial visual improvement that was subsequently lost by the end of 2 years.
Although a direct comparison with the two major studies of induction followed by a PRN regimen (PrONTO and SUSTAIN) is difficult, due to differences in the measurement of visual acuity, the different criteria for reinjection, the different OCT equipment used (higher-resolution spectral domain OCT in the present study, as opposed to the lower-resolution time domain OCT in most previous studies), and the fact that a lower dose (0.3 mg) was used initially in the SUSTAIN study, our results in Japanese patients showed an overall similar improvement in visual acuity and decrease in central macular thickness.Citation5–Citation7 In our patients, a slightly lower mean number of injections were performed compared with patients in the PrONTO study (9.5 injections over 2 years in the present study versus 9.9 injections over 2 years in the PrONTO study), while a slightly greater mean number of injections were performed compared with those in the SUSTAIN study (6.0 injections over 1 year in the present study versus 5.7 injections over 1 year in the SUSTAIN study). Of note, we considered any worsening of the macula, whether by visual acuity (worsening of BCVA had to be accompanied by fluid, on OCT) or by findings on OCT, fundus biomicroscopy, and/or fluorescein angiography, to be an indication for reinjection. In contrast, the reinjection criteria for PrONTO and SUSTAIN included a visual acuity reduction of at least five letters (with OCT evidence of fluid in the macula, for PrONTO) or an increase in OCT central retinal thickness of at least 100 μm. Furthermore, our use of spectral domain OCT may have resulted, in our study, in the detection of smaller amounts of fluid in the macula, prompting reinjections that may not have been deemed necessary had time domain OCT been used.
Our study included a fairly high proportion of PCV eyes (35%), as one would expect for Japan. Although the percentages of eyes with PCV features were not reported for PrONTO and SUSTAIN, these studies were conducted in the US and Europe respectively, and therefore, one would expect a much lower proportion of PCV. One study by Tsujikawa et alCitation16 on bevacizumab injected using a PRN regimen, in 17 eyes of 16 Japanese patients with PCV, revealed that visual acuity was initially improved at 3 months but then declined subsequently, such that it was unchanged at 12 months compared with baseline. We were able to achieve statistically significant improvement in visual acuity of the PCV eyes through month 16, although this was lost at month 24. In our study, a greater mean number of injections were given to the PCV eyes (5.4 ranibizumab injections over the first year alone) than in the Tsujikawa study (4.0 bevacizumab injections over a follow-up period varying from 12–30 months), suggesting that more frequent injections lead to better visual outcomes in PCV eyes.
Eyes with PCV features may require more intensive anti-VEGF treatment over the long term compared with eyes without PCV. In PCV eyes, the administration of ranibizumab for 3 months followed by a PRN regimen of administration over 1 year led to improved visual acuity, with a mean of 4.2 injections in Japanese patientsCitation21 and 3.3 injections in Taiwanese patients. Other studies examined the use of bevacizumab or ranibizumab in Asian PCV eyes, using a PRN regimen after photodynamic therapy (PDT) treatment, and these showed no visual improvement but did achieve reduction in macular fluid, by OCT.Citation22,Citation23 One study from Korea, using anti-VEGF therapy given in three monthly injections followed by a PRN regimen, also showed only visual acuity stabilization after a mean of 4.72 bevacizumab injections or a mean of 5.52 ranibizumab injections over a 1-year period.Citation24 In another study, comparing ranibizumab monotherapy to ranibizumab/ PDT combination therapy for PCV, the addition of PDT did not result in additional improvement over ranibizumab therapy in 1-year outcomes.Citation25 More recently, the 6-month results from the EVEREST study, a multicenter, prospective, randomized trial of 61 PCV eyes, found significantly higher rates of polyp regression, by indocyanine green angiography assessment, at 6 months in the ranibizumab/PDT-combination and PDT-alone groups (77.8% and 71.4%, respectively) compared with the ranibizumab monotherapy group (28.6%).Citation26 However, the mean gain in visual acuity was not different in the three groups. Moreover, the proportion of eyes gaining at least 15 letters was better in the ranibizumab monotherapy group (33.3%) than in either the ranibizumab/PDT or the PDT-alone groups (21% and 19%, respectively).Citation26 The results of our 2-year study, taken together with results of other groups, suggest that best visual outcomes are obtained with ranibizumab monotherapy given frequently. We acknowledge that without periodic IA assessments to examine the effect of treatment on the polypoidal lesions and branching vascular networks in the PCV eyes, we cannot delineate the exact mechanism of efficacy with ranibizumab monotherapy. However, we speculate that, had we given more reinjections to the PCV eyes in the second year, perhaps a statistically significant improvement in vision could have been maintained longer than 16 months. Longer-acting drugs (such as aflibercept) may have the potential to maintain visual improvement in these PCV eyes.
The analysis of the eyes with typical AMD also revealed good visual improvement and reduction in central macular thickness, in our study. Due to the small number, we were unable to draw a meaningful conclusion for RAP eyes. A study from Spain pointed out that differences in clinical results at 1 year depended upon the stage of RAP, with stage II eyes without pigment epithelial detachment having better outcomes compared with stage II with pigment epithelial detachment or stage III eyes.Citation27 Interestingly, an analysis of RAP eyes in Japanese and Korean patients treated with a combination of ranibizumab and PDT suggests that, at least at the 1-year time point, mean visual acuity improved.Citation28,Citation29 Therefore, RAP may be one type of exudative AMD in which the addition of PDT to anti-VEGF therapy does improve visual outcomes.
The strengths of our study included the long follow-up period of 2 years and the fairly large number of eyes examined. In addition, our study analyzed treatment in a typical clinical setting using a uniform PRN regimen in all patients. The weaknesses of our study included its retrospective nature and the absence of a comparison treatment strategy. Furthermore, during the same time period, 13 eyes that had initiated ranibizumab treatment were not analyzed for a variety of reasons that included the return of some patients to the referring ophthalmologist, transition of the patient to a different treatment strategy (bevacizumab or combined anti-VEGF and PDT treatment), or loss of the patient to follow up. We believe that the lack of inclusion of the results from these eyes could have produced either positive or negative bias to our results.
In conclusion, in Japanese eyes with exudative AMD, ranibizumab monotherapy performed as three monthly induction injections followed by a PRN regimen resulted in an improvement in mean visual acuity and a decreased mean central macular thickness, using a mean of 9.5 injections performed over a 2-year period. Smaller lesion GLD size was independently associated with visual improvement.
Disclosure
Dr Okada serves as a consultant for GlaxoSmithKline, Novartis Pharmaceutical Corporation, and XOMA, and received lecture fees from Mitsubishi Tanabe Pharma, Novartis Pharma Japan, Pfzer Japan, and Santen Pharmaceuticals. The authors report no other conflicts of interest.
References
- Ministry of Health Labour Welfare of Japan2005 Nanchisei shikkan kokufuku kenkyu jigyou moumyakurakumaku/shishinkei ishukushou ni taisuru kenkyu Heisei 17nendo soukatsu/buntan kenkyu houkokusho [Report of the Research Committee on Chorioretinal Degenerations and Optic Atrophy]2006263267 Japanese
- RosenfieldPJBrownDMHeierJSMARINA Study GroupRanibizumab for neovascular age-related degenerationN Engl J Med2006355141419143117021318
- BrownDMKaiserPKMichelsMANCHOR Study GroupRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med2006355141432144417021319
- RegilloCDBrownDMAbrahamPRandomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1Am J Ophthalmol2008145223924818222192
- FungAELalwaniGARosenfieldPJAn optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degenerationAm J Ophthalmol2007143456658317386270
- LalwaniGARosenfeldPJFungAEA variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO StudyAm J Ophthalmol20091481435819376495
- HolzFGAmoakuWDonateJSUSTAIN Study GroupSafety and efficacy of a fexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN studyOphthalmology2011118466367121459217
- GuptaOPShienbaumGPatelAHFecarottaCKaiserRSRegilloCDA treat and extend regimen using ranibizumab for neovascular age-related macular degeneration: clinical and economic impactOphthalmology2010117112134214020591490
- OubrahamHCohenSYSamimiSInject and extend dosing versus dosing as needed: a comparative retrospective study of ranibizumab in exudative age-related macular degenerationRetina2011311263020890246
- ShoKTakahashiKYamadaHPolypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristicsArch Ophthalmol2003121101392139614557174
- ObataRYanagiYKamiJTakahashiHInoueYTamakiYPolypoidal choroidal vasculopathy and retinochoroidal anastomosis in Japanese patients eligible for photodynamic therapy for exudative age-related macular degenerationJpn J Ophthlmol2006504354360
- MarukoIIidaTSaitoMNagayamaDSaitoKClinical characteristics of exudative age-related macular degeneration in Japanese patientsAm J Ophthalmol20071441152217509509
- GomiFSawaMSakaguchiHEfficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathyBr J Ophthalmol2008921707317567661
- YannuzziLAWongDWSforzoliniBSPolypoidal choroidal vasculopathy and neovascularized age-related macular degenerationArch Ophthalmol1999117111503151010565519
- SuzukiMGomiFSawaMTsujikawaMSakaguchiHBevacizumab treatment for choroidal neovascularization due to age-related macular degeneration in Japanese patientsJpn J Ophthalmol201054212412820401560
- TsujikawaAOotoSYamashiroKTamuraHOtaniAYoshimuraNTreatment of polypoidal choroidal vasculopathy by intravitreal injection of bevacizumabJpn J Ophthalmol201054431031920700799
- TanoYOhjiMEXTEND-I Study GroupEXTEND-I: safety and efficacy of ranibizumab in Japanese patients with subfoveal choroidal neovascularization secondary to age-related macular degenerationActa Ophthalmol201088330931620163368
- MatsumiyaWHondaSBesshoHKusuharaSTsukaharaYNegiAEarly responses to intravitreal ranibizumab in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathyJ Ophthalmol2011742020
- Japanese Study Group of Polypoidal Choroidal VasculopathyCriteria for diagnosis of polypoidal choroidal vasculopathyNippon Ganka Gakkai Zasshi20051097417427 Japanese16050460
- YannuzziLANegrãoSIidaTRetinal angiomatous proliferation in age-related macular degenerationRetina200121541643411642370
- HikichiTHiguchiMMatsushitaTOne-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patientsAm J Ophthalmol2012154111712422465366
- KimKSLeeWKBevacizumab for serous changes originating from a persistent branching vascular network following photodynamic therapy for polypoidal choroidal vasculopathyJpn J Ophthalmol201155437037721647565
- WakabayashiTGomiFSawaMTsujikawaMNishidaKIntravitreal bevacizumab for exudative branching vascular networks in polypoidal choroidal vasculopathyBr J Ophthalmol201296339439921719568
- ChoHJKimJWLeeDWChoSWKimCGIntravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathyEye (Lond)201226342643322173075
- SongMHRyuHWRohYJOne-year results of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathyOphthalmologica2011226311912621757883
- KohALeeWKChenLJEVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathyRetina20123281453146422426346
- Reche-FrutosJCalvo-GonzalezCPérez-TrigoSFernandez-PerezCDonate-LopezJGarcia FeijooJRanibizumab in retinal angiomatous proliferation (RAP): influence of RAP stage on visual outcomeEur J Ophthalmol201121678378821484755
- LeeMYKimKSLeeWKCombination therapy of ranibizumab and photodynamic therapy for retinal angiomatous proliferation with serous pigment epithelial detachment in Korean patients: twelve-month resultsRetina2011311657321187732
- SaitoMIidaTKanoMCombined intravitreal ranibizumab and photodynamic therapy for retinal angiomatous proliferationAm J Ophthalmol2012153350451422078902