Abstract
Background
In cases of nonarteritic anterior ischemic optic neuropathy (NAION), retinal nerve fiber layer (RNFL) thickness changes have been described during the first 12 months following the acute event. The purpose of this study was to report on the long-term RNFL changes in these eyes beyond the first year following onset of NAION.
Methods
Fourteen eyes of 13 patients with NAION were analyzed in this retrospective observational case series study. Uninvolved eyes served as controls. All patients underwent a complete neuro-ophthalmological examination and repeat measurements of peripapillary RNFL thickness using Stratus optical coherence tomography.
Results
On optical coherence tomography scan performed on average 6 months following onset of NAION, the mean global RNFL thickness (59.8 ± 11.8 μm) was significantly thinner (P < 0.001) compared with uninvolved eyes (95.1 ± 13.9 μm). In a second optical coherence tomography scan performed on average 13 (range 12–23) months later, the mean global RNFL thickness (58.9 ± 6.5 μm) was not significantly different (P = 0.702) from the first scan.
Conclusion
There appears to be no further RNFL loss beyond the first 6 months following an acute event of NAION.
Introduction
Nonarteritic anterior ischemic optic neuropathy (NAION) is presumed to be caused by hypoperfusion and infarction of the anterior optic nerve, secondary to occlusion of the short posterior ciliary arteries.Citation1 It typically affects individuals between the ages of 50 and 70 years, and the reported annual incidence is 2.3–10.2 cases per 100,000 persons 50 years and older.Citation2 In acute NAION, the typical appearance is of a segmental disc edema that is often accompanied by flame-shaped nerve fiber layer hemorrhages and arteriolar narrowing at or near the swollen section of the disc.Citation3 Four to 6 weeks following the occurrence of visual loss, optic disc pallor, often in a sectorial pattern, replaces the previous disc edema.
Optical coherence tomography is an imaging technique that has been shown to be useful in quantifying peripapillary retinal nerve fiber layer (RNFL) thickness in the acute and resolving phases of NAION.Citation4 However, in previous studies, analysis of RNFL changes following NAION has been limited to the first year following the acute event. The aim of this study was to analyze the long-term effect of NAION on the RNFL layer beyond that period of time.
Materials and methods
This retrospective study of 13 patients seen at two medical centers between March 2004 and July 2009 was approved by both institutional review boards and was fully compliant with the Health Insurance Portability and Accountability Act policies and the principles of the Declaration of Helsinki.
All patients included in this study had a diagnosis of NAION based on an acute onset of painless visual loss associated with optic disc edema with or without peripapillary hemorrhage. At the time of study entry, all patients were all at least 3 months following NAION onset and were able to perform a reliable Humphrey visual field test and cooperate with optical coherence tomography imaging. None of them had symptoms suggestive of giant cell arteritis, including headache, jaw claudication, weight loss, malaise, anorexia, or scalp tenderness, or any other ocular pathology, such as glaucoma, diabetic retinopathy, age-related macular degeneration, or congenital optic disc anomalies that can affect the results of visual field tests or optical coherence tomography scans.
All patients included were examined by a neuro-ophthalmologist (BS or GD) during the acute and resolving phases of the disease. All underwent a complete ophthalmologic evaluation, including medical and ophthalmologic history and assessment of visual acuity, intraocular pressure measurement, pupillary reactions, slit-lamp biomicroscopy, and dilated funduscopy. Special attention was given to the appearance of the optic nerve head, nerve fiber layer, and macula.
Visual acuity was recorded using the Snellen chart and converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis. All patients with visual acuity of 20/100 or better underwent visual field testing on a Humphrey visual field analyzer (Carl Zeiss Meditec, Dublin, CA, USA) using the Central 24–2 program and the standard strategy of the Swedish interactive threshold algorithm. Humphrey visual field mean deviation and pattern standard deviation were recorded for statistical analysis.
All patients underwent optical coherence tomography imaging of the optic nerve through dilated pupils using a Stratus OCT (Carl Zeiss Meditec Inc, software version 3.0). Peripapillary RNFL thickness was measured at 256 points around a circle 3.4 in diameter surrounding the optic disc. Satisfactory image quality was defined when good centration of the optic disc and a signal strength of 6 or more (maximum = 10) were present. The scans were reviewed for adequate delineation of retinal layers and there were no major artifacts. Data were processed using the software provided by the manufacturer (RNFL Thickness Average Analysis Report version 4.0.1). For each eye, RNFL thickness was measured in 12 clock-hour positions, four quadrants, and globally for the entire 360° circle. All measurements of the left eye were mirror-imaged to those from the right eye.
The data were analyzed using Minitab release 14 software (Minitab Inc, State College, PA, USA). Statistical significance was defined at a level of P < 0.05. A paired two-sample t-test was used to compare sequential optical coherence tomography measurements of eyes with NAION, and the Student’s t-test was used to assess differences between optical coherence tomography measurements in eyes with NAION and the uninvolved eyes.
Results
Fourteen eyes of 13 patients (seven men, six women, mean age 60.54 ± 9.05 years) with NAION were recruited into the study. NAION was unilateral in 12 patients (nine left, three right) and bilateral in one patient. The 12 uninvolved eyes from patients with unilateral disease served as controls. The mean time from onset of NAION to recruitment in the study was 6 (range 4–12) months.
Visual acuity was 20/40 or better in seven eyes (50%), between 20/50 and 20/100 in six eyes (43%), and worse than 20/100 in one eye (7%). Visual acuity, mean Humphrey visual field deviation, and pattern standard deviation values obtained at the time of study entry were significantly different in eyes with NAION compared with uninvolved eyes (). For both eyes with NAION and uninvolved eyes, there was no statistically significant change between any of these initial measures and those obtained at the time of final assessment.
Table 1 Visual acuity, mean deviation, and pattern standard deviation of the Humphrey visual field in eyes with nonarteritic anterior ischemic optic neuropathy and fellow uninvolved eyes at the time of initial and final evaluations
Initial mean global RNFL thickness in eyes with NAION (59.8 ± 11.3 μm) was significantly thinner (P < 0.001) compared with uninvolved eyes (95.1 ± 13.9 μm). The RNFL thicknesses in each of the 12 clock-hour (30°) sections and in each of the four quadrants of the optic nerve head were also significantly thinner in NAION eyes than in uninvolved eyes ( and ). The most common visual field defect in NAION eyes was an inferior altitudinal field defect, which was present in ten eyes. The mean RNFL thickness in the superior quadrant of these eyes (56.0 ± 14.6 μm), which corresponds to hemifield defect, was significantly thinner (P = 0.001) compared with the inferior quadrant of these eyes (100.3 ± 24.7 μm) and the superior quadrant of uninvolved eyes (115.2 ± 18.8 μm). However, further analysis of the eyes with the inferior field defect demonstrated that the mean RNFL thickness in all other quadrants of the optic nerve head which did not correspond to the visual field defect, were also significantly thinner compared with uninvolved eyes ().
Figure 1 Retinal nerve fiber layer thickness in eyes with nonarteritic anterior ischemic optic neuropathy compared with uninvolved eyes at the time of initial evaluation.
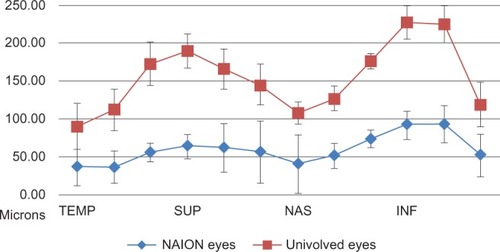
Table 2 Retinal nerve fiber layer thickness in eyes with nonarteritic anterior ischemic optic neuropathy compared with uninvolved eyes, by clock-hour sections, quadrants, and global average thickness
Table 3 Quadrantic and global retinal nerve fiber layer thickness in eyes with nonarteritic anterior ischemic optic neuropathy with an inferior altitudinal field defect compared with uninvolved eyes
The mean global RNFL thickness in a second optical coherence tomography scan (58.9 ± 6.5 μm) performed on average, 13.36 ± 5.41 (range 12–23) months following the initial scan was not significantly different (P = 0.603) from the first scan (59.8 ± 11.3 μm). Paired t-test analysis comparing initial and final measures revealed no significant change in RNFL thickness in any of the 12 clock-hour sections or in any of the four quadrants of the optic nerve head between these two sets of measurements ( and ). Four repeated RNFL thickness measurements performed annually in one of the study patients are presented in .
Figure 2 Retinal nerve fiber layer thickness in eyes with nonarteritic anterior ischemic optic neuropathy at the time of initial and final evaluation.
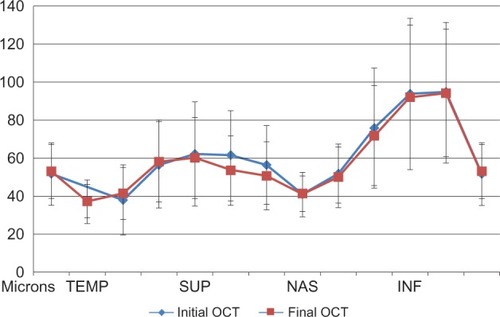
Figure 3 A 52-year-old woman developed an acute event of nonarteritic anterior ischemic optic neuropathy in her left eye. Her visual acuity in that eye was 20/20, color vision was decreased and there was a positive left relative afferent pupillary defect. The right eye was uninvolved. (A) Retinal nerve fiber layer thickness analysis report approximately 6 months after the acute event. In the right eye, the retinal nerve fiber layer is within normal limits in all sections of the optic nerve head and in the left eye there is severe retinal nerve fiber layer loss save for the temporal quadrant. This patient was followed annually with an optical coherence tomography scan performed on each visit. (B) Serial analysis report demonstrating stability of the retinal nerve fiber layer thickness in the involved eye over time.
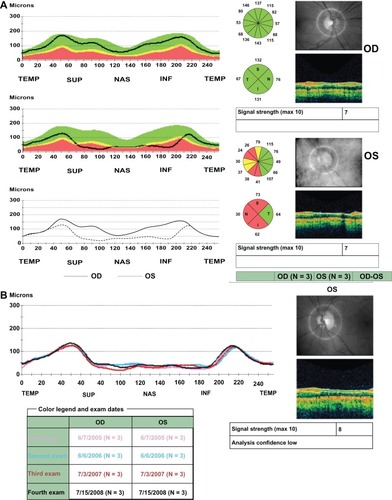
Table 4 Retinal nerve fiber layer thickness in eyes with nonarteritic anterior ischemic optic neuropathy at study entry and one year later, by clock-hour sections, quadrants, and global average thickness
Discussion
In this study, we performed repeated optical coherence tomography scans in eyes with optic atrophy secondary to NAION and were able to demonstrate long-term stability in RNFL loss following the acute event.
Optical coherence tomography has been shown to be useful for monitoring progression of RNFL thinning in various optic neuropathies.Citation5–Citation8 Its use in NAION has been described in several studies that analyzed RNFL changes following NAION. Savini et al repeated Stratus OCT scans to measure RNFL thickness in six patients with NAION from the time of acute onset up to 30 weeks later, and described progressive RNFL thinning as the disease progressed toward optic atrophy.Citation4 Rebolleda et al found a mean RNFL loss of 44.53 ± 20.09 μm compared with fellow uninvolved eyes at 6 months from onset of NAION.Citation9 Bellusci et al described a decrease of global RNFL thickness in eyes with NAION from a mean of 188.9 ± 56. μm within 2 weeks of onset of visual loss to 63.1 ± 14.2 μm 6 months later.Citation10 Contreas et al reported a mean RNFL thickness of 56.94 ± 11.94 μm 3 months following NAION, which was significantly lower compared with uninvolved fellow eyes (94.62 ± 21.28 μm).Citation11 Our results concur with previous findings of significant RNFL loss in eyes with NAION. We found that the mean RNFL thickness in involved eyes (59.8 ± 11.3 μm) was significantly thinner compared with uninvolved eyes (95.1 ± 13.9 μm) 6 months following the acute NAION event.
Two previous studies reported on RNFL changes up to a year from onset of NAION. One study was performed by Papchenko et al who measured a mean RNFL thickness of 72.8 ± 23.5 μm approximately one year after the documented optic disc swelling, compared with a mean RNFL thickness of 98.9 ± 9.7 μm in uninvolved fellow eyes;Citation12 however, this study did not report on sequential changes over time. In the other study, Contreas et al performed four repeated RNFL measurements during the first year starting at disease onset, and found that RNFL loss was almost complete by 3 months following onset of NAION; however, during the following 9 months, there was a further 6% loss of RNFL.Citation13 In our study, the RNFL thickness in NAION eyes measured approximately 6 months after disease onset was not significantly different compared with a repeat measurement performed approximately one year later. Therefore, we presume that no further loss of optic nerve axons occurred in the time elapsed between the repeat measurements. Long-term stability of RNFL thickness has been described in other types of optic neuropathies. Costello et al followed 78 patients with acute optic neuritis using repeat optical coherence tomography scans and demonstrated that RNFL loss stabilized at 7–12 months after onset and remained unchanged more than 2 years later.Citation14
Previous studies of RNFL thickness in NAION eyes suggested that temporal quadrant thickness is a good indicator of visual function. Deleón-Ortega et al demonstrated that in eyes with relatively good visual acuity following NAION, RNFL thickness in the temporal quadrant was similar to that of control eyes.Citation15 Horowitz et al showed that in eyes following NAION with reduced vision to a mean logMAR visual acuity of 0.43 ± 0.34 the RNFL thickness in the temporal quadrant was lower than in control eyes.Citation16 These findings support a possible correlation between mean RNFL thickness in the temporal quadrant and final visual acuity. The loss of fibers arriving from the macula may primarily account for the significant loss in visual acuity following NAION. In our study, the mean logMAR visual acuity in NAION eyes (0.46 ± 0.65) was significantly lower than in control eyes (0.05 ± 0.09) and the RNFL layer in the temporal quadrant (42.0 ± 11.3 μm) was significantly thinner compared with uninvolved eyes (65.2 ± 14.6 μm).
In our study, the most common type of visual field defect was an inferior altitudinal field defect that was associated with greater thinning of the RNFL in the superior quadrant of the optic nerve head. However, RNFL thinning was present, even in sections of the optic disc that did not correlate with the areas of visual field damage, suggesting that loss of RNFL in NAION eyes may be more extensive than anticipated by the visual field result. A similar finding of widespread damage to the peripapillary RNFL following NAION with a hemifield defect was reported by Deleón-Ortega et al.Citation15
The major limitation of this study is its relatively small sample size; however, we were still able to demonstrate that, in NAION eyes, there is no progressive loss of RNFL beyond the initial 6 months following the event.
The combination of visual loss, an afferent pupillary defect, and optic atrophy is nonspecific and may represent the chronic phase of various optic neuropathies. Trobe et al noted that it is impossible to determine with certainty the etiology of optic atrophy based on the appearance of the optic disc alone.Citation17 When the history and clinical features do not suggest a specific cause for the development of optic atrophy, a screening workup is usually undertaken. Baseline studies to determine visual function and fundus photography to detect subtle changes in contour over time are usually performed as well. Often when the investigative workup is negative, a presumptive diagnosis of a prior NAION event is made and observation is recommended. Some authors recommend performing optical coherence tomography scans of the optic nerve in this situation for quantitative evaluation of ganglion cell loss.Citation15 Our study indicates that there is another added benefit to the assessment of RNFL loss in these patients. Based on our findings, when the presumptive diagnosis of NAION is correct, then one should not expect any further loss of RNFL to occur later than the first 6 months following the event. However, if repeated optical coherence tomography scans demonstrate progressive RNFL thinning over time, then the diagnosis of NAION is unlikely and further workup is advised.
In conclusion, optical coherence tomography is a useful tool for documenting RNFL loss in eyes with longstanding NAION, and can aid in the management of patients presenting with optic atrophy.
Disclosure
None of the authors have any conflict of interest or financial interest in the data presented in this study.
References
- HoSFDhar-MunshiSNonarteritic anterior ischaemic optic neuropathyCurr Opin Ophthalmol200819646146718854690
- [No authors listed]Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the Ischemic Optic Neuropathy Decompression TrialArch Ophthalmol199611411136613748906027
- Ischemic Optic Neuropathy Decompression Trial Research GroupOptic nerve decompression surgery for non arteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmfulJAMA199527386256327844872
- SaviniGBellusciCCarbonelliMDetection and quantification of retinal nerve fiber layer thickness in optic disc edema using stratus OCTArch Ophthalmol200612481111111716908813
- WollsteinGSchumanJSPriceLLOptical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucomaArch Ophthalmol2005123446447015824218
- BarboniPSaviniGValentinoMLRetinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathyOphthalmology2005112112012615629831
- KanamoriANakamuraMMatsuiNOptical coherence tomography detects characteristic retinal nerve fiber layer thickness corresponding to band atrophy of the optic discsOphthalmology2004111122278228315582087
- MedeirosFAMouraFCVessaniRMSusannaRJrAxonal loss after traumatic optic neuropathy documented by optical coherence tomographyAm J Ophthalmol2003135340640812614771
- RebolledaGPérez-LópezMCasas-LleraPContrerasIMuñoz-NegreteFJVisual and anatomical outcomes of non-arteritic anterior ischemic optic neuropathy with high-dose systemic corticosteroidsGraefes Arch Clin Exp Ophthalmol2013251125526022441810
- BellusciCSaviniGCarbonelliMCarelliVSadunAABarboniPRetinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phasesGraefes Arch Clin Exp Ophthalmol2008246564164718305953
- ContrerasIRebolledaGNovalSMuñoz-NegreteFJOptic disc evaluation by optical coherence tomography in nonarteritic anterior ischemic optic neuropathyInvest Ophthalmol Vis Sci20074894087409217724191
- PapchenkoTGraingerBTSavinoPJGambleGDDanesh-MeyerHVMacular thickness predictive of visual field sensitivity in ischaemic optic neuropathyActa Ophthalmol2012906e463e46922690753
- ContreasINovalSRebolledaGMuñoz-NegreteJFollow-up of nonarteritic anterior ischemic optic neuropathy with optical coherence tomographyOphthalmology2007114122338234417719640
- CostelloFHodgeWPanYIEggenbergerECouplandSKardonRHTracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomographyMult Scler200814789390518573837
- Deleón-OrtegaJCarrollKEArthurSNGirkinCACorrelations between retinal nerve fiber layer and visual field in eyes with nonarteritic anterior ischemic optic neuropathyAm J Ophthalmol2007143228829417157797
- HorowitzJFishelzon-ArevTRathEZSegevEGeyerOComparison of optic nerve head topography findings in eyes with non-arteritic anterior ischemic optic neuropathy and eyes with glaucomaGraefes Arch Clin Exp Ophthalmol2010248684585120213479
- TrobeJDGlaserJSCassadyJCOptic atrophy. Differential diagnosis by fundus observation aloneArch Ophthalmol1980986104010457387506