Abstract
Introduction:
Here, we describe a patient who presented with anterior ischemic optic neuropathy (AION) and subsequently developed uveitis.
Case:
A 69-year-old man was referred to our hospital and initially presented with best-corrected visual acuities (BCVA) of 20/40 (right eye) and 20/1000 (left eye) and relative afferent pupillary defect. Slit-lamp examination revealed no signs of ocular inflammation in either eye. Fundus examination revealed left-eye swelling and a pale superior optic disc, and Goldmann perimetry revealed left-eye inferior hemianopia. The patient was diagnosed with nonarteritic AION in the left eye. One week later, the patient returned to the hospital because of vision loss. The BCVA of the left eye was so poor that the patient could only count fingers. Slit-lamp examination revealed 1+ cells in the anterior chamber and the anterior vitreous in both eyes. Funduscopic examination revealed vasculitis and exudates in both eyes. The patient was diagnosed with bilateral panuveitis, and treatment with topical betamethasone was started. No other physical findings resulting from other autoimmune or infectious diseases were found. No additional treatments were administered, and optic disc edema in the left eye improved, and the retinal exudates disappeared in 3 months. The patient’s BCVA improved after cataract surgery was performed.
Conclusion:
Panuveitis most likely manifests after the development of AION.
Introduction
Anterior ischemic optic neuropathy (AION) is a vision-threatening disease that is caused by infarction of the optic nerve and primarily affects patients >55 years.Citation1 Sometimes, over a few days, optic disc edema will develop in association with flame hemorrhage of the swollen disc and/or nearby cotton wool exudates. Visual loss is usually permanent, though slight improvements will manifest within the first weeks or months. Optic disc edema is also followed by varying degrees of generalized or sectorial optic atrophy within the first few weeks.Citation2 AION presents in two forms: arteritic and nonarteritic (NA-AION). Arteritic AION is associated with giant cell arteritis, whereas hypertension, diabetes mellitus, ischemic heart disease, dyslipidemia, hypercoagulable states, and sleep apnea are associated with NA-AION.Citation2–Citation6 Although AION has rarely been reported in patients with uveitis,Citation7–Citation18 here we describe a patient who presented with AION and subsequently developed uveitis.
Case report
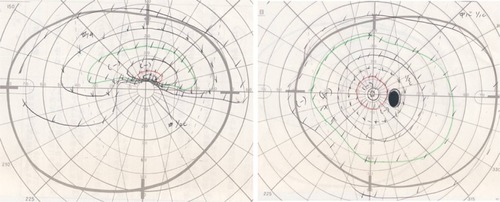
A 69-year-old man developed a low-grade fever around November 18, 2009. He noticed temporal visual field defects in his left eye on November 25 and visited an ophthalmologist 2 days later. Funduscopic examination revealed optic disc edema in the patient’s left eye, and he was referred to our hospital. Initially, his best-corrected visual acuity (BCVA) was 20/40 in the right eye and 20/1000 in the left eye, though the intraocular pressure was normal in both eyes. The critical flicker frequency ranged between 28–31 Hz (oculus dexter) and 21–24 Hz (oculus sinister), and relative afferent pupillary defect was diagnosed. Slit-lamp examination revealed moderate cataracts in both eyes but no signs of ocular inflammation. Fundus examination revealed small optic discs in both eyes, notably a swollen, pale left superior optic disc (). Fluorescein angiography revealed a filling delay in the superior optic disc and around the optic disc of the left eye during the early phase and optic disc hyperfluorescence in both eyes during the late phase, most notably intensive leakage in the left eye (). Goldmann perimetry revealed inferior hemianopia in the left eye, but no visual field abnormalities were noted in the right eye (). Laboratory examinations determined an erythrocyte sedimentation rate of 60 mm/hour during the first hour but no other abnormalities. Magnetic resonance imaging of the brain and optic nerves were normal. The patient was diagnosed with NA-AION in the left eye based on the lack of headache or tenderness. Accordingly, for 3 days, the patient was treated with 10 mg intravenous infusions of alprostadil (a vasodilator) and aspirin.
Figure 1 Color fundus photograph taken at onset.
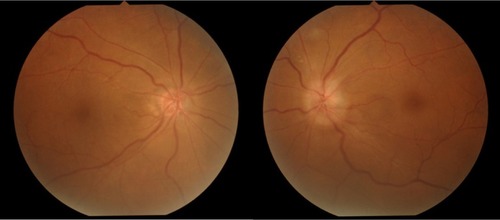
Figure 2 Fluorescein angiography performed at onset.
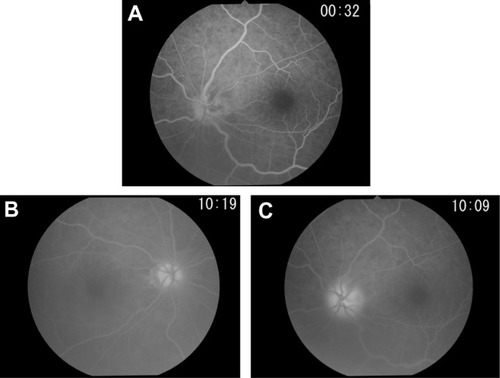
Figure 3 Goldmann perimetry at onset.
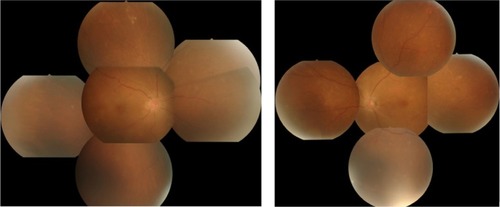
One week after the start of treatment, the patient returned to the hospital because of vision loss. The patient’s BCVA was so poor he was only able to count fingers using his left eye. Slit-lamp examination revealed 1+ cells in the anterior chamber and anterior vitreous in both eyes, and funduscopic examination revealed vasculitis and exudates in both eyes (). The patient was diagnosed with bilateral panuveitis, and treatment with topical betamethasone sodium phosphate was initiated. However, despite the administration of betamethasone, the number of inflammatory cells in the anterior chamber and anterior vitreous cavity did not decrease. Additionally, weight loss and fever developed, so the patient was referred to the Department of Autoimmune Neurology to investigate the cause of his weight loss.
Figure 4 Fundus examination performed 1 week after the start of treatment.
Laboratory analysis determined elevated C-reactive protein (2.19 U/mL) and soluble interleukin-2 receptor antibody (1057 U/mL) levels. Thoracoabdominal computed tomography was performed because malignant lymphoma was suspected, but no abnormalities were observed. No other physical findings were noted that were caused by other autoimmune or infectious diseases. No additional treatments were administered, and 1.5 months later the number of anterior chamber cells and anterior vitreous cells began to decrease. Three months later, optic disc edema in the left eye had improved, the retinal exudates had disappeared, and uveitis had not recurred.
Subsequently, both cataracts progressed and cataract surgery was performed to implant intraocular lenses in February 2011 (right eye) and January 2012 (left eye). No postoperative complications were found, and the patient’s BCVA improved to 20/16 in the right eye and 20/20 in the left eye. Inferior hemianopia still remains as a problem in the left eye, but the visual defects in the center of the visual field have slightly improved.
Discussion
Several types of AION-complicated uveitis have been reported, with Behçet’s disease being the most common.Citation7–Citation10 Optic nerve damage due to Behçet’s disease is believed to be caused by several mechanisms, including the spread of inflammation from the uveal tract to the optic nerve (uveopapillitis), occlusion of the small vessels in the optic nerve (vasculitis), and ischemia-induced demyelinization. Inflammation of the optic nerve may precede inflammation of the uvea.Citation7,Citation8 A common pathogenic mechanism – arterial obstruction due to inflammation – may result in giant cell arteritis.
On the other hand, Behçet’s disease may cause arterial obstruction via mechanisms other than arterial inflammation.Citation11 Indeed, how the pathology of AION is associated with Behçet’s disease remains unknown. The second most common type of AION-complicated uveitis is Vogt–Koyanagi–Harada (VKH) disease.Citation12,Citation13 Yokoyama et alCitation12 reported their experience of treating a patient who developed AION during the acute phase of VKH. However, how AION develops in association with VKH is unclear. According to histopathological studies on sympathetic ophthalmia, which is believed to be pathologically identical to VKH, the simultaneous presentation of severe uveitis at the juxtapapillary choroid, obliteration of the choriocapillaris, and inflammatory infiltration around the emissarial vessels and nerves via the scleral canal are associated with the severity of choroidal involvement.Citation14
Severe inflammation at the juxtapapillary choroid may interfere with how the posterior ciliary artery supplies the optic nerve head and the retrolaminar part of the optic nerve, thereby resulting in the development of AION. Nakao et alCitation13 reported that the characteristics of these patients suggest that AION may develop in association with VKH, particularly during the acute uveitic phase. AION can be bilateral or simultaneously affect both eyes. Swelling of the optic disc in VKH patients may, therefore, be a sign of AION complications, particularly in elderly patients with diabetes mellitus, small optic discs, and/or absent or small optic disc cups. AION may result in the development of optic discs with anatomical risk factors such as small size, complex circulatory impairment in the short posterior ciliary artery, and other risk factors associated with circulatory disorders such as hypertension and hyperlipidemia. In addition, patients with Posner–Schlossman syndrome, human leukocyte antigen B27-associated anterior uveitis, and birdshot chorioretinopathy can reportedly develop AION.Citation15–Citation18 Uveitis may also play a role in the onset of AION. Patients who develop NA-AION in the optic nerve head present with anatomical characteristics such as small disc area, zero to minimal physiological cupping, and an increase in the number of branches from the central retinal vessels within the disc (patients with these characteristics are often considered “disc at risk”). Small physiological cupping is the result of a relatively small scleral canal. In either case, the axons crowd as they traverse the restricted space of the lamina cribrosa of the scleral canal. If subclinical ischemia develops due to changes in the arteriolar sclerotic vasculature, axoplasmic swelling within the restricted space will limit the egress pathway, compress the nutrient capillary bed, and venous compromise will ensue, causing further ischemia, swelling, and the cessation of axoplasmic transport. Ischemia and axonal swelling result in disc hyperemia, hemorrhage of the nerve fiber layer and, ultimately, infarction.Citation19–Citation21
Our patient’s small optic nerve head and cup were considered at risk, and this condition may have developed in one of three ways: (1) panuveitis manifested after the development of AION; (2) AION and panuveitis developed separately; or (3) AION and panuveitis developed simultaneously in association with systemic disease. Because both presented around the same time, the separate development of AION and panuveitis is unlikely in this case. Although the exact causes of uveitis remain unclear, the development of fever and weight loss prior to the onset of ocular symptoms, as well as increased C-reactive protein and high soluble interleukin-2 receptor antibody levels, suggest that AION and panuveitis are associated with various systemic diseases such as malignant lymphoma. However, because CRP did normalize within 1.5 months after the start of betamethasone treatment, systemic symptoms such as fever resolved, and no significant findings were noted at the most recent follow-up examination, we believe panuveitis manifested after the development of AION.
Given the rarity of this condition, no optimal treatment has been established. Several cases have reportedly demonstrated improvement following treatment with systemic corticosteroids,Citation7,Citation8,Citation15 high-dose corticosteroid therapy,Citation7,Citation13 subtenon injection of triamcinolone acetonide,Citation9,Citation16 immunomodulatory therapy,Citation8 and topical betamethasone sodium phosphate (like the case presented here).Citation18 Ultimately, in order to determine optimal treatment modalities, additional studies on this rare condition are needed.
Disclosure
The authors report no conflicts of interest in this work.
References
- RepkaMXSavinoPJSchatzNJSergottRCClinical profile and long-term implications of anterior ischemic optic neuropathyAm J Ophthalmol19839644784836624829
- HayrehSSJoosKMPodhajskyPALongCRSystemic diseases associated with nonarteritic anterior ischemic optic neuropathyAm J Ophthalmol199411867667807977604
- TalksSJChongNHGibsonJMDodsonPMFibrinogen, cholesterol and smoking as risk factors for non-arteritic anterior ischaemic optic neuropathyEye (Lond)19959Pt 185887713255
- SalomonOHuna-BaronRKurtzSAnalysis of prothrombotic and vascular risk factors in patients with nonarteritic anterior ischemic optic neuropathyOphthalmology1999106473974210201595
- WegerMStangerODeutschmannHHyperhomocyst(e)inaemia, but not MTHFR C677T mutation, as a risk factor for non-arteritic ischaemic optic neuropathyBr J Ophthalmol200185780380611423453
- AchesonJFSandersMDCoagulation abnormalities in ischaemic optic neuropathyEye (Lond)19948Pt 189928013726
- VorosGMSandhuSSPanditRAcute optic neuropathy in patients with Behçet’s disease. Report of two casesOphthalmologica2006220640040517095888
- KansuTKirkaliPKansuEZileliTOptic neuropathy in Behçet’s diseaseJ Clin Neuroophthalmol1989942772802531168
- YamauchiYCruzJMKaplanHJGotoHSakaiJUsuiMSuspected simultaneous bilateral anterior ischemic optic neuropathy in a patient with Behçet’s diseaseOcul Immunol Inflamm200513431732516159724
- ScourasJKoutroumanosJIschemic optic neuropathy in Behçet’s syndromeOphthalmologica197617311118958672
- OzdemirHAtillaHAtillaSIşikSZilelioğluGDiagnosis of ocular involvement in Behçet’s disease: value of spectral and color Doppler sonographyAJR Am J Roentgenol19951645122312277717235
- YokoyamaAOhtaKKojimaHYoshimuraNVogt-Koyanagi-Harada disease masquerading anterior ischaemic optic neuropathyBr J Ophthalmol199983112310209450
- NakaoKMizushimaYAbematsuNGohNSakamotoTAnterior ischemic optic neuropathy associated with Vogt-Koyanagi-Harada diseaseGraefes Arch Clin Exp Ophthalmol2009247101417142519568763
- CroxattoJORaoNAMcLeanIWMarakGEAtypical histopathologic features in sympathetic ophthalmia. A study of a hundred casesInt Ophthalmol1982431291357068330
- Caballero-PresenciaADiaz-GuiaELopez-LopezJMAcute anterior ischemic optic neuropathy in birdshot retinochoroidopathyOphthalmologica1988196287913362511
- ThamVMCunninghamEJrAnterior ischemic optic neuropathy in a patient with HLA-B27 associated anterior uveitis and ankylosing spondylitisBr J Ophthalmol200185675611426425
- KimRVan StavernGJuzychMNonarteritic anterior ischemic optic neuropathy associated with acute glaucoma secondary to Posner-Schlossman syndromeArch Ophthalmol2003121112712812523901
- IrakIKatzBJZabriskieNAZimmermanPLPosner-Schlossman syndrome and nonarteritic anterior ischemic optic neuropathyJ Neuroophthalmol200323426426714663306
- SaitoHTomidokoroATomitaGAraieMWakakuraMOptic disc and peripapillary morphology in unilateral nonarteritic anterior ischemic optic neuropathy and age- and refraction-matched normalsOphthalmology200811591585159018342941
- SaitoHTomidokoroASugimotoEOptic disc topography and peripapillary retinal nerve fiber layer thickness in nonarteritic ischemic optic neuropathy and open-angle glaucomaOphthalmology200611381340134416797709
- BurdeRMOptic disk risk factors for nonarteritic anterior ischemic optic neuropathyAm J Ophthalmol199311667597648250081