Abstract
Purpose
Optic neuritis (ON) observed during neuromyelitis optica (NMO) is in most cases very severe and with poor prognosis. This study’s objective was to analyze visual field (VF) abnormalities observed in the absence of ON and post-ON episode.
Methods
Twenty-seven cases of both NMO and multiple sclerosis (MS) were selected. Thorough ophthalmologic exam was performed at least 6 months post-ON attack. The VF was collected using the Humphrey 750 perimeter. We used the central threshold tests 24-2 with FASTPAC strategy. The abnormalities were categorized based on the Optic Neuritis Treatment Trial classification.
Results
After one ON, 40% of the NMO group’s eyes showed total VF loss (P = 0.01), 21% showed abnormalities of neurologic aspect, and 27% showed fascicular abnormalities of which 12% were altitudinal. Given the total VF loss, the positive predictive value in favor of an NMO was 92.8% and the negative predictive value was 47.3%.
Conclusion
Alterations of the VF during the NMO differ from those observed in the course of the MS. One ON, blinding from the first attack, must call to mind an NMO. The altitudinal deficits point to a vascular mechanism.
Introduction
Neuromyelitis optica (NMO), a rare inflammatory and demyelinating disease, impacts specifically the optic nerves and spinal cord.Citation1,Citation2 This pathology is predominantly found among women (80%), spread worldwide, and has poor prognosis. Many clinical, immunological, and histological characteristics separate NMO from multiple sclerosis (MS), including sparing of the brain at the onset of the disease, frequent association (10%–40%) with autoimmune diseases, diffuse damage of the white and grey matter of the spinal cord, as well as the involvement of functional and vital prognosis in the short term.Citation3
Of unknown etiology, NMO is now considered an autoimmune disease linked to humoral immunity pathology, as recently determined based on the finding of the antibody directed against aquaporin 4 (AQP4-Ab).Citation4 Lesions are characterized by perivascular immunoglobulin (IgM) and complement (C9) deposits, but also by necrosis zones.Citation5
Visual function is severely affected as confirmed by frequent ocular attacks and severity of its degradation.Citation6 A remote optic neuritis (ON), prior to NMO, is often considered idiopathic or due to MS; the most frequent treatment is intravenous corticotherapy with high dosage, which generally has poor results.Citation7 Recently, plasma exchanges have been shown to be very efficient treatments for ON due to NMO, increasing the need to differentiate ON due to NMO as soon as possible from ON due to MS.Citation8–Citation10
The study’s objective was to analyze visual field (VF) abnormalities observed in the absence of ON and post-ON episode as well as to specify their role in early NMO diagnosis.
Methods
Our study was conducted at the University Hospital Center of Fort de France in Martinique, where all patients have been regularly followed since 1985. All patients had no previous history of any of the following ON: familial, infectious, glaucoma related, vascular, compressive, due to general diseases, toxic, or due to deficiency. All patients were human immunodeficiency virus (HIV) and human T-lymphotropic virus 1 (HTLV-1) negative.
Population studied
Twenty-seven NMO cases were selected. The NMO diagnosis was based on Wingerchuk et al’s diagnostic criteria established in 1999 and revised in 2006.Citation11,Citation12 The revised diagnostic criteria require the presence of two absolute criteria as well as of two out of three major criteria. The absolute criteria are: the existence of unified or bilateral ON, and of acute myelitis. The major criteria are: normal magnetic resonance imaging (MRI) at the onset of the disease, hypersignal extended to at least three vertebral segments on the medullar MRI, and positive search results for AQP4-Ab. The acute myelitis diagnosis was based on the presence of medullar damage including sphincter, sensitive, or motor disorders, for which maximum deficit is reached in less than 4 weeks. Ocular or medullar attack was defined as the apparition or worsening of signs or symptoms for at least 24 hours. A second attack was a new event that happened at least 1 month after the initial attack. The final index attack confirmed the diagnosis. We revealed that the optic-medullary period matched the time frame that separated the first and final index attack. The disease’s global handicap was ranked using the Kürtzke scale from 0 to 10 (expanded disability status scale [EDSS]).Citation13 We calculated the handicap progression rapport as followed: EDSS/length of the disease (years).
We compared the results of the NMO patients with those obtained in the MS patients’ group matched by age and sex. The MS patients (n = 27) met McDonald et al’s diagnosis criteria.Citation14
We used our Martinique NMO and MS patients’ database to obtain characteristics of the disease’s history. Data was collected prospectively over a 20-year period. All patients were tested for AQP4-Ab.
Analysis
For each patient, a thorough eye exam was conducted at least 6 months post-ON onset, including a precise measure of refraction. Visual acuity was collected using the Snellen and Early Treatment Diabetic Retinopathy Study (ETDRS) scales. Sensibility to spatial contrast was measured with the Pelli-Robson and Sloan charts.Citation15 On the Pelli-Robson test, letters are organized by groups of triplets of different contrast.Citation11 An identified triplet consists of two out of the three letters being correctly recognized. A score of 15 or higher (converted in logarithmic units for statistical analysis) is considered normal. According to Sloan, weak contrast cards depend on the identification of grey letters, whose size progressively decreases. The format is similar to the ETDRS scale, and has five letters per line. These letters appear on a backlit white base (Precision Vision, La Salle, IL, USA) and are tested at 2 m. We used two levels of contrast (1.25% and 2.5%). Each letter read correctly contributed one point to the total score. The total score was the addition of all correctly read letters (0–70). We assessed colors vision with the 100-hue test of Farnsworth. The square root of the score was used for statistical analysis. The score’s normal value by age was obtained using Verriest’s tables. We also used frequency doubling technology perimetry (FDTP) (Carl Zeiss Meditec AG, Jena, Germany). We used the N-30 threshold program. The thickness of retinal peripapillary nerve fibers was measured with a Stratus OCT (Carl Zeiss Meditec AG) equipped with the 4.0 version of the software. We followed the fast retinal nerve fiber layer (RFNL) thickness protocol to collect data. The equipment produced three circular scans (resolution: 256) of 3.4 mm of diameter centered on the head of the optic nerve in 1.92 s. We measured the average thickness of the layer of retinal nerve fiber, as well as the average thickness in the temporal, superior, nasal, and inferior quadrants. The quality of input of selected scans was superior or equal to seven. In the case of a narrow pupil, the OCT was realized after instillation of a drop of tropicamide.
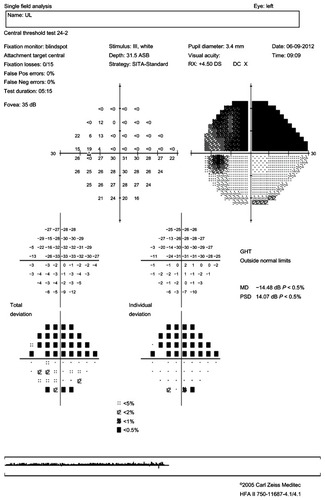
Standard automated perimetry (SAP) was done using the Humphrey Field Analyzer 750 II (Carl Zeiss Meditec AG). We used the test of central threshold 24-2 (54 points tested) with FASTPAC strategy. The VF was considered reliable if false positives, false negatives, and fixation losses were below 33%. A VF was definitely normal if all locations were within normal limits on the total deviation plot. A VF was definitely abnormal if any of the following conditions were met: (1) the Glaucoma Hemifield Test VF index was abnormal; (2) the corrected pattern standard deviation VF index was abnormal (P < 5%); (3) a single point was worse than the 0.5% probability level on the total and/or pattern deviation plot; (4) two clustered points are beyond the reference range (P < 5%), and at least one point was worse than the P < 1% on the total and/or pattern deviation plot (a cluster was defined as ≥two horizontally or vertically, not diagonally, contiguous abnormal points with P < 5%); and/or (5) three or more clustered points were worse than P < 5% on the total and/or pattern deviation plot.
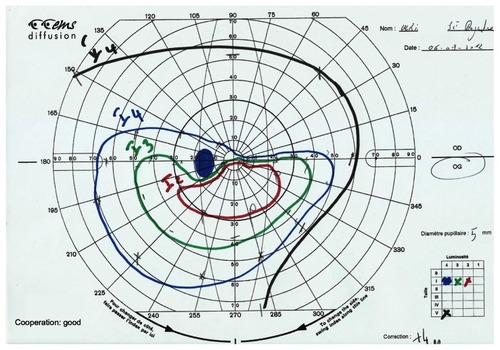
In order to determine the abnormality classification for the entire VF, we used the most predominant defect. The pattern of abnormal points on the deviation plot (total or pattern) showing the greater number of abnormal points, was used to determine the appropriate abnormality classification. When necessary, Goldmann’s VF was also conducted in order to specify the deficit’s form.
The VF abnormalities were ranked in 21 categories based on the Optic Neuritis Treatment Trial (ONTT) classification and reorganized into three principal groups: neurologic, optic nerve, and artifactual abnormalities ().Citation16
Table 1 Classification of the visual field abnormalities
The data was computerized and analyzed in a strictly anonymous manner using MS Excel (Microsoft Corporation, Redmond, WA, USA) and Statview (SAS Institute Inc, Cary, NC, USA). The following tests were used for statistical analysis: Chi-square test for frequency comparisons, Chi-square Yates correction for small populations, and Student’s t-test for average comparisons. This study was approved by the Consulting Committee for the Protection of Individuals of Biomedical Research sponsored by the French Ministry of Health.
Results
Demographic characteristics of the two groups are displayed in . NMO patients were on average 47 years old and 92% were females. The average age at the NMO onset was 37.1 ± 12.0 years. NMO and MS had been evolving for at least 10 years. The optic-medullary period was 25.3 ± 38.9 months. 55% of the patients were positive to AQP4-Ab. There was no difference between the two groups regarding average age, sex ratio, age of onset, and length of the disease. The number of attacks per patient, number of attacks the first 2 years, and the yearly rate of attacks were all higher in the NMO group.
Table 2 Demographic comparison between the NMO and MS groups
Ten NMO and 30 MS eyes had no previous history of ON. Thirty-three NMO and 19 MS eyes had one previous ON attack. Eight NMO and three MS eyes had two previous attacks. Four NMO and two MS eyes had more than two previous attacks.
The results of the visual function and of optic nerve fibers thickness in absence of and post-ON episode are presented in . For the eyes with no ON history, there was no difference in visual acuity (Snellen) and optic nerve fibers thickness between the two groups. Nevertheless, ETDRS visual acuity, contrasts vision (Sloan chart 1.25%), mean deviation SAP, and foveal threshold were not as good in the MS group.
Table 3 Visual function and optic nerve fibers thickness in the absence of prior history and after an optic neuritis episode
After one ON attack, Snellen visual acuity, ETDRS, and the optic nerve fibers thickness were smaller in the NMO group than in the MS group (20/50 versus 20/30, P = 0.02; 31.4 versus 44.1, P = 0.04; 64.4 μm versus 77 μm, P = 0.06, respectively). The proportion of eyes with a visual acuity ≤20/200 was 42% in the NMO group versus 21% in the MS group (P = 0.03).
shows the results of the VF morphological analysis in absence of and post-ON episode. In the absence of ON, the foveal threshold was 34 for the NMO group and 32.5 for the MS group (P = 0.07). Half of the NMO patients’ VFs were normal, a third of the MS patients’ VFs were normal, and one third of the MS patients’ VFs showed diffuse lesions. In the absence of ON there was no difference in the distribution of VF deficits between the two groups.
Table 4 Visual field abnormalities in absence of and after one optic neuritis episode
Post-ON attack, the foveal threshold was 16.2 in the NMO group and 27.4 in the MS group (P = 0.01), and almost 40% of the NMO group’s eyes showed total VF loss versus only 5.3% for MS group’s eyes (P = 0.01). In the case of total VF loss, the positive predictive value in favor of NMO was 92.8% and the negative predictive value was 47.3%. In the NMO group, neurological abnormalities were found in 21.3% of cases and fascicular abnormalities in 27.3%, of which 12.1% were altitudinal deficits ( and ). No altitudinal deficit was observed in the MS group. Diffuse abnormalities were found in 42.1% of the MS eyes versus 6% of the NMO eyes (P = 0.005). In the presence of a diffuse deficit, the positive predictive value in favor of MS was 80% and the negative predictive value 73.8%.
Discussion
Our study showed that after a first ON attack, 40% of the NMO patients’ eyes presented total VF loss and that the ON complicated with total VF loss was, over nine out of ten times, an NMO. In Fernandes et al’s study, after a single ON episode, a mean deviation lower than −20 dB was associated with a 6.0 odds ratio for NMO.Citation17 In two out of three cases in another study, NMO started with ON, sometimes bilaterally.Citation6 It was rare to see the ON associated with myelitis or appearing after the discovery of medullar lesions. Lennon et alCitation3 identified a biological marker (AQP4-Ab) to differentiate NMO from MS. However, this antibody directed against aquaporin-4 has a sensitivity of 60%–70% and specificity of 91%.Citation4,Citation18 Further, in many cases, MRI of isolated ON is normal; ON is then considered idiopathic or due to MS. In contrast with the idiopathic or MS-related ON for which vision is usually recovered in a few weeks, ON due to NMO has a very poor prognosis.Citation19
Thus, the relation to MS or NMO must be identified as soon as possible in order to start the proper treatment for the acute phase as well as a thorough immunosuppressant treatment. Besides MS, blinding ONs are exceptional. They only represent 3.6% of observed ON throughout the course of MS, even course though 36.9% of patients show total vision loss at the acute phase.Citation16 In Slamovits et al’s study, among 12 ON cases without light perception, eight recovered with a visual acuity superior to 20/40 and four with a visual acuity less than 20/40 but with periphery vision conservation. None stayed blind.Citation20
A first ON attack complicated by total vision loss in an AQP4-Ab negative patient, female and of African, Indian, or Asian decent must be considered as the first NMO sign. Indeed, the ocular start and ON frequency are more frequent among these populations while the onset and frequency of medullar attacks is higher in the white population.Citation21,Citation22 Optical coherence tomography can also contribute to orient the NMO diagnosis when RNFL is inferior to 50 μm and when mostly superior and inferior quadrants are concerned.Citation23,Citation24
Given the deficit’s seriousness and fast onset of irreversible optic nerve lesions, the treatment of NMO due to ON is an emergency. Because of the good results obtained through plasma exchanges, we propose their systematic use in association with intravenous corticotherapy, rather than waiting to conclude the inefficacy of corticotherapy alone.Citation8,Citation10,Citation25
Localized anomalies (neurologic anomalies and nerve fiber bundle anomalies) concern about 50% of the deficits in our series. Fernandes et al, whose series’ numbers were similar to ours, also found half of the deficits to be localized.Citation17 Nakajima et al collected VFs for 15 NMO patients with Goldmann perimetry.Citation26 He found, as we did, about 25% of deficits with a neurological aspect.Citation26 In Sadda et al’s study, after a posterior ischemic ON, one third of the eyes kept a visual acuity inferior to 20/640 and when measurable, 30% of the VF showed altitudinal deficit.Citation27 The rarity of diffuse lesions (6% in our series), the localized deficits depth, high proportion of neurological aspect deficits, as well as RNFL significant decrease of the four quadrants, show a severe axonal lesion compared to MS. These severe axonal lesions recall those observed throughout the course of vascular related neuropathies such as the ischemic or glaucomatous ON.Citation23,Citation24 In Fernandes et al’s and our series, the proportion of localized deficits among MS patients was smaller, one third, and appropriate for the ONTT results at 1 year.Citation17 Consequently, it is very common throughout the course of MS to observe a selective reach of the interpapillomacular bundle.
Four eyes (12.1%) of NMO patients showed altitudinal deficits. This type of deficit was not found among the 27 eyes of Fernandes et al’s series, but was noted as the most frequent of the non-central deficits in Nakajima et al’s series.Citation17,Citation26 These altitudinal deficits had all been observed at the time of the first ON attack; they were superior in three cases and inferior in two cases. Altitudinal deficit is usually characteristic of ischemic ON linked to an occlusion of the posterior ciliary arteries. Encountered throughout the course of NMO, such a deficit brings to mind a vascular contribution in ON occurrence. Further, throughout the NMO, the optic nerve appears grayish and softened. The demyelinization predominates at the center of the nerve and is sometimes at the origin of a central cavitation.Citation28 As a consequence of the AQP4-Ab presence, they are associated to perivascular immunoglobulin deposits (Ig M) and complement (C9), axonal necrosis lesions, and atrophy that are never observed throughout the MS.Citation29 Other components in favor of the vascular contribution to ON hypothesis include the revelation of retinal vascular anomalies of attenuation type on the peripapillary arterioles and frosty or occluded aspect of the arterioles located at more than two papillary diameters of the papilla.Citation30 All of these vascular anomalies would be the result of an inflammatory reaction caused by the AQP4-Ab directed against aquaporin-4, strongly expressed at the level of the optic nerve astrocytes and the arterioles of the retina’s deep layer.Citation31
Identifying NMO as soon as possible and putting in place the most adapted treatment is critical for a lesion with poor ocular prognosis and for which vital prognosis is very unfavorable. The VF lesions observed after one episode of ON in NMO are different from those usually observed in MS. Given a total VF loss, the positive predictive value in favor of an NMO was 92.8% and the negative predictive value was 47.3%. Obtained with an ambispective study, these values should be confirmed with a prospective study. The VF analysis helps to invoke the NMO diagnosis when faced with severe or altitudinal lesions that appeared during the first ON attack.
Acknowledgments
We thank Agathe Merle, MPA, MPH (University of Wisconsin-Madison, Wisconsin), Karen Thérèse (CHU Fort de France), and Eric Ventura (CHU Fort de France).
Disclosure
The authors report no conflicts of interest in this work.
References
- DevicEMyélite subaiguë compliquée de névrite optiqueBull Méd1894810331034
- WingerchukDMLennonVALucchinettiCFPittockSJWeinshenkerBGThe spectrum of neuromyelitis opticaLancet Neurol20076980581517706564
- LennonVAKryzerTJPittockSJVerkmanASHinsonSRIgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channelJ Exp Med2005202447347716087714
- LennonVAWingerchukDMKryserTJA serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosisLancet200436494512106211215589308
- LucchinettiCFMandlerRNMcGavernDA role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis opticaBrain2002125Pt 71450146112076996
- MerleHOlindoSBonnanMNatural history of the visual impairment of relapsing neuromyelitis opticaOphthalmology2007114481081517141316
- HickmanSJDaltonCMMillerDHPlantGTManagement of acute optic neuritisLancet200236093491953196212493277
- WatanabeSNakashimaIMisuTTherapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis opticaMult Scler200713112813217294622
- YoshidaHAndoAShoKAnti-aquaporin-4 antibody-positive optic neuritis treated with double-filtration plasmapheresisJ Ocul Pharmacol Ther201026438138520698801
- MerleHOlindoSJeanninSTreatment of optic neuritis by plasma exchange (add-on) in neuromyelitis opticaArch Ophthalmol2012130785886222776923
- WingerchukDMHogancampWFO’BrienPCWeinshenkerBGThe clinical course of neuromyelitis optica (Devic’s syndrome)Neurology19995351107111410496275
- WingerchukDMLennonVAPittockSJRevised diagnostic criteria for neuromyelitis opticaNeurology200666101485148916717206
- KurtzkeJFRating neurologic impairment in multiple sclerosis: a expanded disability status scale (EDSS)Neurology19833311144414526685237
- McDonaldWICompstonAEdanGRecommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnoses of multiples sclerosisAnn Neurol200150112112711456302
- TrobeJDBeckRWMokePSClearyPAContrast sensitivity and other vision tests in the optic neuritis treatment trialAm J Ophthalmol199612155475538610798
- KeltnerJLJohnsonCACelloKEDontchevMGalRLBeckRWOptic Neuritis Study GroupVisual field profile of optic neuritis. A final follow-up report from the optic neuritis treatment trial from baseline through 15 yearsArch Ophthalmol2010128333033720212204
- FernandesDBRamos RdeIFalcochioCApóstolos-PereiraSCallegaroDMonteiroMLComparison of visual acuity and automated perimetry findings in patients with neuromyelitis optica or multiple sclerosis after single or multiple attacks of optic neuritisJ Neuroophthalmol201232210210622157535
- WatersPJariusSLittletonEAquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitisArch Neurol200865791391918625857
- McDonaldWIBarnesDThe ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual systemJ Neurol Neurosurg Psychiatry19925597477521402963
- SlamovitsTLRosenCEChengKPStriphGGVisual recovery in patients with optic neuritis and visual loss to no light perceptionAm J Ophthalmol199111122092141992742
- OsuntokunBOThe pattern of neurological illness in tropical Africa. Experience at Ibadan, NigeriaJ Neurol Sci19711244174424324654
- KiraJMultiple sclerosis in the Japanese populationLancet Neurol20032211712712849268
- NaismithRTTutlamNTXuJOptical coherence tomography differs in neuromyelitis optica compared with multiple sclerosisNeurology200972121077108219307541
- MerleHOlindoSDonnioARicherRSmadjaDCabrePRetinal peripapillary nerve fiber layer thickness in neuromyelitis opticaInvest Ophthalmol Vis Sci200849104412441718614811
- WangKCWangSJLeeCLChenSYTsaiCPThe rescue effect of plasma exchange for neuromyelitis opticaJ Clin Neurosci2011181434620888238
- NakajimaHHosokawaTSuginoMVisual field defects of optic neuritis in neuromyelitis optica compared with multiple sclerosisBMC Neurol2010104520565857
- SaddaSRNeeMMillerNRBiousseVNewmanNKouzisAClinical spectrum of posterior ischemic optic neuropathyAm J Ophthalmol2001132574375011704036
- MandlerRNDavisLEJefferyDRKornfeldMDevic’s neuromyelitis optica: a clinicopathological study of 8 patientsAnn Neurol19933421621688338340
- FardetLGénéreauTMikaeloffYFontaineBSeilheanDCabaneJDevic’s neuromyelitis optica: study of nine casesActa Neurol Scand2003108319320012911463
- GreenAJCreeBADistinctive retinal nerve fibre layer and vascular changes in neuromyelitis optica following optic neuritisJ Neurol Neurosurg Psychiatry20098091002100519465415
- RoemerSFParisiJELennonVAPattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosisBrain2007130Pt 51194120517282996