Abstract
Purpose
To compare the efficacy and safety of 0.1% dexamethasone/0.3% netilmicin (Netildex), with that of 0.1% dexamethasone/0.3% tobramycin (Tobradex) in the treatment of external ocular inflammation requiring antibiotic therapy.
Methods
In this randomized, double-blind study, 139 subjects with conjunctival inflammation associated with signs of ocular infection were treated with Netildex (n = 71) or Tobradex (n = 68) four times daily for 6 days. The primary efficacy analysis was based on the percentage of patients with at least a 50% decrease in conjunctival hyperemia at the endpoint visit (Day 6 [± 1]) with respect to baseline (responder rate). An equivalence margin of 20% was set for this study. A follow-up visit was performed at Day 14 (± 1). Other efficacy parameters were: conjunctival edema, conjunctival discharge, lid hyperemia, lid edema, presence of ocular infection, and symptoms of ocular discomfort. Safety evaluations included intraocular pressure, visual acuity, and adverse events.
Results
At Day 6, a decrease of conjunctival hyperemia was observed in 87.3% and 90.9% of the patients treated with Netildex and Tobradex, respectively. The 95% confidence interval for the difference between groups (−15.3 ÷ 8.0) satisfied the equivalence hypothesis. Subjects treated with Netildex had a better control of lid hyperemia (P = 0.016), tearing (P = 0.001), burning (P = 0.007), and stinging (P = 0.004). No adverse reactions were observed during the study except one case of keratitis in the Tobradex group.
Conclusion
Netildex was as effective and safe as Tobradex in reducing signs and symptoms in patients with conjunctival inflammation when ocular infection was suspected.
Introduction
Red eye is one of the most common and nonspecific ocular conditions encountered in emergency and outpatient settings, and it may be related to several different pathological conditions.Citation1–Citation4 Conjunctivitis is the most common cause of red eye; this condition is usually benign, and can be managed by primary care physicians.Citation1–Citation5 In the majority of cases, conjunctivitis is of viral origin. A bacterial etiology is normally suggested by the presence of purulent discharge, but the nature of discharge is not clinically useful in determining the cause.Citation1,Citation3 Despite the fact that conjunctivitis is self-limiting, a topical broad-spectrum antibiotic is usually prescribed.Citation5 In addition, since active conjunctivitis is better controlled with preparations containing steroids, topical steroidal/antibiotic combinations are often used.Citation6 Such combinations have several advantages over the use of single components, including better compliance, lower costs, and reduction of the potential wash-out effect.Citation6,Citation7 The choice among the different steroidal/antibiotic fixed combinations available depends on the bacterial resistance pattern to the antibiotic included in the formulation.Citation4 A relatively new steroid/antibiotic fixed combination containing dexamethasone and netilmicin has been available in some European Union (EU) countries since 2006 (Netildex, Società Industria Farmaceutica Italiana [SIFI] SpA, Catania, Italy), and is appropriate for all inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists. The antibiotic component of such combination is netilmicin, a third-generation aminoglycoside characterized by a wide spectrum of activity, including methicillin-resistant strains.Citation8–Citation11 This product is effective in controlling ocular inflammation after cataract surgery.Citation12,Citation13 The aim of the present multicenter, double-blinded equivalence study was to compare the efficacy of dexamethasone/netilmicin with that of Tobradex in the treatment of external ocular inflammation requiring antibiotic treatment.
Materials and methods
Study design
The study was a multicenter, randomized, double-blind equivalence trial. Six clinical sites in Italy and one clinical site in Rumania were involved. The study protocol was approved by the Ethical Committee of the Clinical Trial Commission of Regione Piemonte (Italy, protocol no 5540/28.3) and conducted according to the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all patients. The study included three visits: Day 1, Day 6 (endpoint visit), and Day 14 (follow-up visit).
Patients and treatments
A total of 139 patients with signs of conjunctival inflammation with discharge and chemosis requiring an antibiotic treatment were enrolled in the study. The demographic characteristics of these patients are shown in . Exclusion criteria for entering in the study were intraocular pressure (IOP) >24 mmHg, known or suspected allergy to benzalkonium chloride or aminoglycosides, use of systemic decongestants, and use of anti-inflammatory agents or any ophthalmic medications (other than ocular lubricants) within 7 days prior to study entry. Eligible patients were randomly assigned to receive either Netildex, containing 0.1% dexamethasone plus 0.3% netilmicin (n = 71) or Tobradex (Alcon Laboratories, Inc, Fort Worth, TX, USA) containing 0.1% dexamethasone plus 0.3% tobramycin (n = 68). Both products were preserved with benzalkonium chloride (0.005% in Netildex and 0.01% in Tobradex) and were packaged in an identical fashion to guarantee an appropriate masking for both patients and investigators. In case of bilateral involvement, both eyes were treated with the assigned treatment and the eye with the higher score of conjunctival hyperemia was chosen for statistical evaluation. In the presence of equal scores, the right eye was evaluated.
Table 1 Demographic data (randomized patients)
The presence of ocular infection was assessed by performing a conjunctival swab (Culture Swab® Becton Dickinson, Franklin Lakes, NJ, USA) at Day 1 for all patients; in patients with a positive conjunctival swab at baseline, another swab was taken at the follow-up visit (Day 14 [±1]). The identification of microorganisms grown in culture was performed using the analytical profile index system. Multiple semiautomated systems (Vitek®, Biomerieux, Durham, NC, USA) or Kirby-Bauer disk diffusion method were adopted to perform antimicrobial susceptibility tests. An ocular specimen was considered culture positive if the colony forming units count reached the threshold values described by Cagle and Abshire.Citation14
The treatment with the two steroid/antibiotic combinations started immediately after the swab collection and continued for 6 (±1) days four times daily. The therapy to be adopted during the follow-up period (Day 6 to 14) was established according to the swab culture results. Patients with positive cultures continued the assigned treatment to Day 14; the steroid/antibiotic combination was discontinued in patients with negative cultures.
Efficacy and safety variables
All patients were individually assessed by the same physician. Conjunctival hyperemia was chosen as the primary efficacy parameter of the study and was evaluated after 6 (± 1) days of treatment (endpoint evaluation) and at the follow-up visit at Day 14 (± 1) by using a categorical semiquantitative grading scale: 0 = absence of inflammation; 1 = mild inflammation (some vessel injected); 2 = moderate inflammation (diffuse injection of vessels, but individual vessels are still discernible); 3 = severe inflammation (intense injection of vessels, individual vessels not easily still discernible). Conjunctival edema, conjunctival discharge, lid hyperemia, lid edema, and ocular infection, as well as symptoms of ocular discomfort (pain, photophobia, tearing, burning, stinging, and foreign body sensation), were adopted as secondary efficacy parameters, and were graded using a categorical semiquantitative scale. The overall safety and ocular tolerance were assessed by monitoring intraocular pressure (IOP), visual acuity, and adverse events. IOP was measured by using a calibrated Goldmann tonometer (mean of two measurements). An IOP elevation >6 mmHg was considered clinically significant.
Statistics
All statistical analyses were performed using SAS Business Analytics software (version 9.1; SAS Institute Inc, Cary, NC, USA). The sample size calculation was based on the primary efficacy parameter (ie, decrease in conjunctival hyperemia score ≥50%) considering a two-sided significance level of 5%, a power of 90%, and an equivalence margin of 20%. Assuming that 90% of patients treated with Tobradex would present a decrease in conjunctival hyperemia score ≥50% at Day 6 (±1) and no difference with respect to the test treatment, a sample size of 98 evaluable patients (49 per group) was required.
Patients with valid measurements at visit two (Day 6 [±1]) adhering to all protocol conditions were included in the per-protocol (PP) population, whereas patients with only minor or mild deviations (as fair compliance) were included in the full-analysis (FA) population. Primary analysis was performed on both populations. Secondary efficacy evaluations were conducted only in the FA population. Safety and local tolerance were assessed in the safety population (patients receiving at least one dose of the study drug). The primary efficacy variable was the responder rate, defined as the percentage of patients with at least a 50% decrease in conjunctival hyperemia at Day 6 (±1) (endpoint visit) with respect to baseline. A chi-square test was used to compare the responder rates of the two treatments, and a 95% confidence interval (CI) was used for the difference provided to assess the equivalence. In addition, a Wilcoxon rank-sum test and analysis of variance were used to compare treatments with respect to the score differences and the score percent variation from baseline in conjunctival hyperemia, respectively. The Pratt–Wilcoxon test was performed to compare conjunctival hyperemia scores within each treatment group (baseline versus Day 6 [±1]). Wilcoxon rank-sum test and Pratt–Wilcoxon test were performed, respectively, for between and within group analysis of further efficacy parameters and global subjective tolerance. The incidence of ocular infection was compared between treatments using the Fisher’s Exact test. Differences between treatments for the IOP were evaluated by means of analysis of covariance, including treatment as a fixed effect and the IOP assessed at Day 1 as covariate. Within-group analysis of IOP was performed using the paired t-test to detect significant differences with respect to baseline. Overall, the level of statistical significance α was set at 5%.
Results
Thirty-four of the 139 (24.4%) randomized patients were excluded from the efficacy evaluation due to major protocol violations (missed visit at day 6). The FA and PP datasets consisted of 110 patients (55 in the Netildex group and 55 in the Tobradex group) and 105 patients (51 in the Netildex group and 54 in the Tobradex group), respectively.
The primary efficacy analysis was based on the percentage of patients with at least a 50% decrease in conjunctival hyperemia at the endpoint visit (Day 6 [±1]) with respect to baseline (responder rate). Accordingly, the two products produced a comparable reduction of the conjunctival hyperemia score (>70%); within each group of treatment, this effect was highly statistically significant (P < 0.001) ( and ). A further decrease of the score was observed at the follow-up visit (Day 14), suggesting that there was no rebound effect after cessation of therapy (). The responder rate (FA dataset) was equal to 87.3% in the Netildex group and to 90.9% in the Tobradex group with a difference of −3.6 (95% CI, −15.3 ÷ 8.0) satisfying the equivalence hypothesis (). Similar results were observed also in the PP population ().
Figure 1 Effect of steroid/antibiotic combinations on conjunctival score.
Abbreviation: SD, standard deviation.
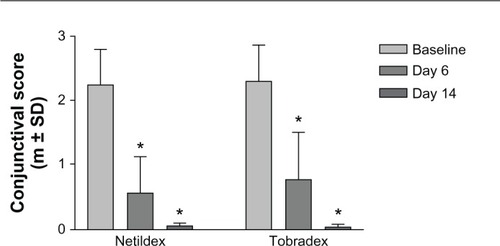
Table 2 Effect on conjunctival hyperemia
Positive cultures at Day 1 were identified in approximately one-third of patients; the most common bacteria isolated were Staphylococcus epidermidis (eleven cases) and Staphylococcus aureus (nine cases). The classification of a swab as culture positive had little influence on the rate of clinical responsiveness. For both treatment groups, the responder rate obtained in the subgroup of patients classified as culture negative was higher than the rate observed in the subgroup of patients classified as culture positive (); however, such differences were not statistically significant. In the majority of patients with positive culture, both treatments produced a complete eradication of infections (eradication rate 91.6% and 87.5% with Netildex and Tobradex, respectively).
Table 3 Effect of the presence of ocular infection on the responder rate (FA dataset)
Further efficacy evaluations were conducted only in the FA dataset. Comparable results between the two groups were observed for all the variables examined (data not shown). However, in the within analysis (change from baseline at Day 6), patients treated with Netildex received lower scores than patients treated with Tobradex for lid hyperemia (P = 0.016), tearing (P = 0.001), burning (P = 0.007), and stinging (P = 0.004) ().
Table 4 Effect on clinical secondary parameters (FA dataset)
Safety was assessed in all 139 randomized patients. Only one patient (treated with Tobradex) experienced keratitis as adverse event.
Pre-surgery IOP values in patients treated with Netildex and Tobradex were 14.9 (± 1.8) mmHg and 14.4 (± 2.0) mmHg (mean ± standard deviation), respectively. At the end of the study, IOP values were 14.7 (± 2.0) mmHg in the Netildex group and 14.3 (± 1.9) mmHg in the Tobradex group.
Discussion
External ocular inflammation is common in adults and children and may have a variety of etiologies, including bacterial infection.Citation5 Effective treatment requires suppression of ocular surface inflammation and, if present, bacterial eradication. Microbiological evaluation is not routinely performed; therefore, a combination of steroid and antibiotic treatment is preferred in most cases.Citation3 The practical advantages of the administration of such fixed combinations are well established and include better compliance, lower cost, and reduction of the potential wash-out effect.Citation6,Citation7
Netildex is a fixed steroid/antibiotic combination containing 0.1% dexamethasone and 0.3% netilmicin. Dexamethasone is one of the most widely used corticosteroids in ophthalmology and has proven highly effective in treating ocular inflammation.Citation15,Citation16 Netilmicin is a third-generation aminoglycoside characterized by a wide spectrum of antimicrobial activity,Citation8–Citation11 a low level of conjunctival and corneal toxicity,Citation17,Citation18 and a high efficacy rate in treating bacterial conjunctivitis.Citation10,Citation19 The combination product of dexamethasone and netilmicin is already available in some EU and non-EU countries in preserved multidose bottles and preservative-free unidoses, and is indicated for steroid-responsive ocular inflammations with or at risk of ocular infection. This combination has already been shown to be safe and effective in controlling ocular inflammation after cataract surgery.Citation12,Citation13
In the present study, we compared the clinical efficacy of Netildex with that of Tobradex in 139 patients with signs of conjunctival inflammation and conjunctival discharge requiring an antibiotic treatment. The results of this randomized, double-blind clinical study demonstrated that the two fixed steroid/antibiotic combinations were equivalent in reducing signs and symptoms. Equivalence between Netildex and Tobradex was demonstrated for the primary efficacy parameter (conjunctival hyperemia) at the endpoint visit (Day 6). The magnitude of the reduction in conjunctival hyperemia (approximately 70%) following treatment was as expected for those treatments, and similar to that previously reported.Citation20,Citation21 The responder rate (the percentage of patients demonstrating a clinical significant improvement) was high for both products (87% and 91% for the Netildex and Tobradex groups, respectively). Patients experienced less burning, stinging, and tearing with Netildex, which may be related to the formulation (solution versus suspension); however, the significance of this finding is not clear, and may not be clinically significant.
Our data confirm that bacterial pathogens are isolated from a low percentage of patients with clinical signs of conjunctivitis (one-third in the present study). This finding has little effect on the clinical effectiveness of a steroid/antibiotic combination. Nevertheless, a high eradication rate was observed in case of infection in all patients regardless of the drug used, confirming the efficacy of aminoglycosides in treating infections of the anterior segment of the eye.
Conclusion
The results of this study confirm that Netildex is as effective and safe as Tobradex in reducing signs and symptoms of external ocular inflammation. The presence of netilmicin guarantees a low prevalence of antibiotic resistanceCitation8–Citation11 and no epithelial toxicity.Citation17,Citation18 In addition, due to the water solubility of the two active ingredients (dexamethasone phosphate and netilmicin sulfate), Netildex is available in a ready-to-use, preservative-free solution, rather than a suspension. This formulation allows dose uniformity, does not require shaking before use, and provides high comfort upon instillation.
Acknowledgments
We thank all the physicians involved in the clinical study (026/SI Study group): P Vaona, F Faraldi, L Palanza, and E Fornara (Torino, Italy); S Azzaro, A Fede, M Parisi, G Stella, and N Fallisi (Ragusa, Italy); G Boccassini, F Rosa, B Kropp, A Balestrazzi, L Rapagnetta, C De Stefano, T Marini Padovan, and M Iossa (Roma, Italy); D Spinelli, MR Curatola, and S Franceschin (Milano, Italy); L Mastropasqua, M Ciancaglini, and G Gambino (Chieti, Italy); L Bauchiero, D Belli, and A Ossella (Ivrea, Italy); and A Nicodin, Dr I Manoliu, and Dr C Iliuta (Bucharest, Romania). We thank also Dr Eileen Collazo for her writing support.
Disclosure
The authors report no conflict of interest in this work. Vincenzo Papa, Daria Rasà, Debora Santoro, and Simona Russo are, or have been, employees of the manufacturer (SIFI SpA) of the product described in this article. Francesco Faraldi has no commercial or proprietary interest in the product described in this article.
References
- MahmoodARNarangATDiagnosis and management of the acute red eyeEmerg Med Clin North Am2008261355518249256
- WirbelauerCManagement of the red eye for the primary care physicianAm J Med2006119430230616564769
- LeibowitzHMThe red eyeN Engl J Med2000343534535110922425
- CronauHKankanalaRMMaugerTDiagnosis and management of red eye in primary careAm Fam Physician201081213714420082509
- EverittHLittlePHow do GPs diagnose and manage acute infective conjunctivitis? A GP surveyFam Pract200219665866012429670
- LeibowitzHMPrattMVFlagstadIJBerrospiARKundsinRHuman conjunctivitis II. TreatmentArch Ophthalmol19769417521756184771
- BarberBLStrahlmanERLaibovitzRGuessHAReinesSAValidation of a questionnaire for comparing the tolerability of ophthalmic medicationsOphthalmology199710423343429052642
- Campoli-RichardsDMChaplinSSayceRHNetilmicin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic useDrugs19893857037562689137
- BonfiglioGScuderiACRussoGNetilmicin: in vitro activity, time-kill evaluation and postantibiotic effect on microorganisms isolated from ocular infectionsChemotherapy200147211712211173813
- PapaVAragonaPScuderiACTreatment of acute bacterial conjunctivitis with topical netilmicinCornea2002211434711805506
- BlancoARSudanoRoccaro ASpotoCGPapaVSusceptibility of methicillin-resistant staphylococci clinical isolate sto netilmicin and other antibiotics commonly used in ophthalmic therapyCurr Eye Res [Epub March 27, 2013]
- RussoSPapaVDi BellaADexamethasone–netilmicin: a new ophthalmic steroid–antibiotic combination. Efficacy and safety after cataract surgeryEye (Lond)2007211586416273088
- PianiniVPassaniARossiGCMPassaniFEfficacy and safety of netilmicin/dexamethasone preservative-free and tobramycin/dexamethasone-preserved fixed combination in patients after cataract surgeryJ Ocul Pharmacol Ther201026661762121034176
- CagleGDAbshireRLQuantitative ocular bacteriology: a method for enumeration and identification of bacteria from the skin-lask margin and conjunctivaInvest Ophthalmol Vis Sci19812067517577239845
- JaanusSDCheethamJKLesherGAAnti-inflammatory drugsBartlettJDJaanusSDClinical Ocular Pharmacology4th edWoburn (MA)Butterworth-Heinemann2001265298
- SherifZPleyerUCorticosteroids in ophthalmology: past-present-futureOphthalmologica2002216530531512424394
- PapaVLeonardiAGetuliCPacelliVRussoPMilazzoGEffect of ofloxacin and netilmicin on human corneal and conjunctival cells in vitroJ Ocul Pharmacol Ther200319653554514733711
- MarinoCPaladinoGMScuderiACTrombettaFMugridgeKEneaVIn vivo toxicity of netilmicin and ofloxacin on intact and mechanically damaged eyes of rabbitCornea200524671071616015091
- MilazzoGPapaVCarstoceaBTopical netilmicin compared with tobramycin in the treatment of external ocular infectionInt J Clin Pharmacol Ther199937524324810363623
- WhiteEMMacyJIBatemanKMComstockTLComparison of the safety and efficacy of loteprednol 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitisCurr Med Res Opin200824128729618062846
- RheeSSMahFSComparison of tobramycin 0.3%/dexamethasone 0.1% and tobramycin 0.3%/loteprednol 0.5% in the management of blepharo-keratoconjunctivitisAdv Ther2007241606717526462