Abstract
Purpose
To report a case of corneal graft failure due to epithelial ingrowth after an uneventful combined Descemet stripping automated endothelial keratoplasty (DSAEK) and phacoemulsification cataract surgery with intraocular lens implant treated successfully with a repeat DSAEK.
Methods
A 77-year-old male patient underwent combined DSAEK and phacoemulsification with intraocular lens implant implantation for Fuchs’ endothelial dystrophy plus cataract in the right eye. The donor cornea was cut on the Moria ALTK system and introduced using a suture pull-through technique. After an episode of endothelial rejection, the graft failed, with signs suggesting epithelial ingrowth. It was stripped from the host cornea using a Descemet’s membrane stripper, and a Simcoe irrigation-aspiration cannula was used to remove all traces of interface material. The excised lenticule was examined histologically using a hematoxylin and eosin stain.
Result
The patient regained and maintained excellent visual acuity with no sign of recurrence of epithelial ingrowth. Histopathological evaluation of the donor tissue of the first graft showed epithelial ingrowth on the stromal surface of the graft and very few endothelial cells, in keeping with the diagnosis of graft failure.
Conclusion
Epithelial ingrowth is a possible cause of endothelial graft failure, but histologically proven cases are rare. Surgical intervention can achieve successful clearance, with the potential for cure and an excellent outcome.
Introduction
Endothelial transplantation has overtaken penetrating keratoplasty (PK) in popularity for the treatment of endothelial dysfunction.Citation1 The technique was originally described by Melles et al,Citation2 who named it “posterior lamellar keratoplasty,” and later modified by Terry and OusleyCitation3 and renamed “deep lamellar endothelial keratoplasty.” The technique of stripping Descemet’s membrane (descemetorhexis) was again described by Melles et alCitation4 and termed “Descemet’s stripping endothelial keratoplasty.” Further modification of the procedure used an automated blade microkeratome to create a lamellar dissection of the donor cornea, as described by Gorovoy,Citation5 and was termed “Descemet stripping automated endothelial keratoplasty” (DSAEK). This technique is now widely used by corneal surgeons for treatment of a variety of corneal disorders characterized by compromised endothelial function.
Epithelial ingrowth is a rare but well-documented complication of anterior segment surgery or ocular trauma, and has been documented after corneal graft surgery.Citation6–Citation10 Corneal graft failure attributed to histologically proven epithelial ingrowth or downgrowth after DSAEK has very rarely been reported in the literature.Citation11–Citation18 We report a case of histologically proven epithelial ingrowth at the interface of the graft and host, leading to graft failure after uneventful DSAEK, treated successfully with stripping and careful aspiration of interface material, followed by repeat DSAEK.
Case report
A 77-year-old male with Fuchs’ endothelial dystrophy presented with decreased vision in the right eye. Best-corrected visual acuity (BCVA) was 20/30. He had a past history of phacoemulsification cataract surgery with intraocular lens (IOL) implantation in the left eye. Following this he had developed corneal edema for which he had undergone DSAEK with an excellent outcome (BCVA 20/25). Combined DSAEK with phacoemulsification and IOL insertion was therefore planned for the right eye.
After routine phacoemulsification and implantation of a posterior chamber IOL under a cohesive viscoelastic (Microvisc Plus™ [sodium hyaluronate 1.4%], Bohus BioTech, Strömstad, Sweden), a descemetorhexis was performed. The donor lenticule was cut using the Moria ALTK system (Moria, Antony, France) and an 8.5 mm Barron corneal punch (Katena Products, Inc, NJ, USA). A slight irregularity and lip at one side of the donor lenticule was noted. The donor lenticule was introduced through an incision enlarged to 5 mm, using a 10-O-polypropylene double-armed suture and a pull-through technique. The wound was closed with interrupted 10/0 nylon sutures, all viscoelastic was carefully removed, and the donor lenticule tamponaded in position with an air bubble, which was reduced before leaving theater, to avoid pupil block. No venting incisions were made. The immediate postoperative period was uneventful. His BCVA improved to 20/30 at 5 months after the operation. Ten months after the procedure he had an episode of endothelial rejection, which was treated with intensive topical corticosteroid eye drops. Though this settled, a white linear interface opacity was noted just within the superior margin of the DSAEK lenticule immediately after the rejection episode (–). Slit-lamp examination failed to find any relation to the region of irregular lenticule, as noted preimplantation, and the area of white opacity. This gradually enlarged over the ensuing 12 months with increasing upper corneal edema, and BCVA dropped to 20/200. Intraocular pressure remained normal. Unfortunately, an endothelial cell count attempt after the rejection episode was unsuccessful. A diagnosis of graft failure secondary to possible epithelial ingrowth was made, and the patient underwent a repeat DSAEK procedure, using a tissue-matched donor lenticule. The original surgical wound was carefully re-entered, the failed donor lenticule was stripped using a Descemet’s membrane stripper, and the interface meticulously cleared of any foreign material using the Descemet’s membrane stripper followed by thorough aspiration using a Simcoe manual irrigation/aspiration cannula. The replacement lenticule was introduced using the suture pull-through technique and the operation concluded as previously described.
Figure 1 (A) Photograph after Descemet stripping automated endothelial keratoplasty showing a white opacity (arrow) at the interface at the 12 o’clock position away from the temporal host corneal incision site. (B) Magnified view of the area of white opacity (arrow) at the interface. (C) Slit-lamp image showing epithelial ingrowth (arrow) between the graft and the host cornea. (D) Postoperative photograph after repeat Descemet stripping automated endothelial keratoplasty showing a clear cornea.
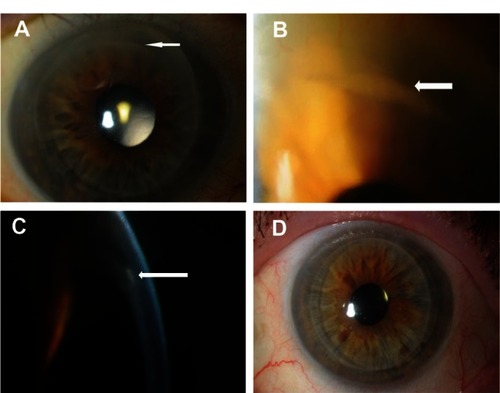
Postoperatively, BCVA improved to 20/25 and there was no further rejection. At 18 months postoperatively there was no sign of recurrence (). BCVA remained at 20/25 and was possibly limited by mild dry age-related macular degeneration.
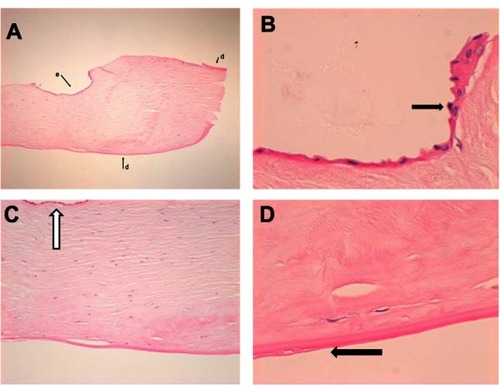
Histolopathological examination of the explanted failed donor lenticule showed a thin layer of epithelium overlying the stroma. The anterior portion of the stroma in this area also showed a portion of thickened, warty Descemet’s membrane. The appearances suggested that there had been entrapment of host Descemet membrane at the graft host interface and ingrowth of epithelium at this point. Lack of endothelial cells on the donor lenticule was consistent with graft failure (–).
Figure 2 (A) Low power shot of the removed donor button to show full thickness of specimen at one side. Here there is epithelium (e) on part of the anterior face of the specimen. Also, Descemet’s membrane (d), is present on the back of the specimen, as well as, focally, at the front on this edge (hematoxylin and eosin, original magnification ×4). (B) Hematoxylin and eosin, original magnification ×40 to show epithelium along the anterior aspect of the specimen (arrow). Note that there is no Descemet’s membrane. (C) Hematoxylin and eosin, original magnification ×10 of the removed donor button shows view of area beneath epithelium. Arrow shows epithelium on the anterior face of the specimen. Posteriorly there is no endothelium. (D) High power of the back of the removed donor button specimen (hematoxylin and eosin, original magnification ×40). Shows graft Descemet membrane with some thickening and incipient guttae (arrow). There is no endothelium, suggestive of graft failure. Note the normal relationship of Descemet membrane to stroma.
Discussion
Epithelial proliferation in the anterior segment of the eye is a rare but serious complication of any ocular trauma or surgery. Although many authors use the term “downgrowth”Citation10,Citation12–Citation15 to describe this phenomenon, others, including the authors, prefer the term “ingrowth.”Citation11,Citation16–Citation19 Whichever term is used, in cases involving corneal transplantation the origin of the ectopic epithelium may be conjectured to be from either the host or the donor. Epithelial migration into the anterior chamber post DSAEK has rarely been reported.Citation9–Citation20 Our results of a literature search on epithelial downgrowth, epithelial interface implantation, or epithelial ingrowth after DSAEK are given in . Not all such cases lead to graft failure. To the best of our knowledge, there are only four reported cases of histologically proven epithelial ingrowth at the interface after DSAEKCitation11,Citation13,Citation16,Citation17 leading to graft failure, only two of which were successfully treated by a repeat DSAEK,Citation11,Citation17 with the other two cases treated by PKCitation13 and posterior mushroom keratoplasty,Citation16 respectively. These cases were different from ours as they were complicated by graft dislocation or attempted suturing and rebubble, which increase the risk of epithelial cells being dislodged into the anterior chamber or at the interface of the graft and host. In our case, the postoperative period was entirely uneventful, but suspicion falls on the slightly irregular cutting of the donor lenticule as the source of epithelial implantation, which contributed to the eventual failure of the graft.
Table 1 Literature review of epithelial migration into anterior chamber after DSAEK
Reviewing the other reported cases, Suh et al,Citation9 in a series of 118 DSAEK eyes, found one case of presumed epithelial implantation in the interface documented clinically and by anterior segment optical coherence tomography. The graft was clear and no histological evidence was documented. In another case series, the same authors described five further cases of epithelial ingrowth after DSAEK, but none of their cases developed graft failure.Citation18 CulbertsonCitation10 documented a case that the author thought was of epithelial downgrowth, but no histological evidence was provided. Walker et alCitation12 described a case as epithelial downgrowth after DSAEK with multiple white opacities at the interface. Confocal microscopy showed the cells to be epithelial and at the interface. Though the patient did not progress to graft failure, that case was treated with a PK.
Prasher et alCitation13 reported two cases of epithelial ingrowth after DSAEK. In the first, the ingrowth was only on the endothelial surface of the donor cornea and was treated with a repeat DSAEK. In the second, interface epithelial ingrowth was histologically confirmed as causing graft failure in a patient treated with PK. Phillips et alCitation14 reported a case of two failed DSAEKs where histology of the removed failed graft showed conjunctival epithelial cells over the surgical margin and on to the posterior surface. It was not clear whether the downgrowth of epithelium was the cause or the result of the repeat graft failure. Gorovoy and RatanasitCitation15 documented one case of epithelial ingrowth that was not at the interface in a patient who had DSAEK. They treated the patient with a repeat DSAEK. Saelens et alCitation16 documented epithelial lined cysts at the interface after DSAEK, which was of donor origin, as revealed by X-Y karyotyping, which they treated with a penetrating posterior mushroom keratoplasty. Lee et al,Citation17 in a retrospective histopathologic study of eight corneas in seven patients who had DSAEK graft failure, found one case of epithelial ingrowth at the interface. They documented that this case had donor graft dislocation during the first DSAEK procedure, which failed to reattach despite repeat rebubble and attempted transcorneal suturing of the graft. Bansal et alCitation19 reported a case of intracorneal epithelial ingrowth after Descemet stripping endothelial keratoplasty with stromal puncture for phakic bullous keratopathy, which they treated conservatively.
We used tissue-matched graft only for the repeat DSAEK procedure and not for the original graft. The benefit of human leukocyte antigen (HLA) matching in keratoplasty is controversial and repeatedly questioned. There are certain studies that support the role of HLA matching by suggesting that it has a role in extending the graft survival, although there are other studies that question its benefit, especially as there can be a considerable delay in obtaining such a matched graft.Citation21–Citation26 Although the Collaborative Corneal Transplantation Studies (CCTS) Research GroupCitation5 and the Corneal Transplant Follow-up Study (CTFS)Citation1 failed to show a beneficial effect of HLA matching, there have been questions raised as to the result of these studies, due to the aggressive immunosuppressant regimen used, which might alter the beneficial effect of HLA matching. Others, like Reinhard et alCitation24 and Ross et al,Citation26 have shown, respectively, that in patients with pseudophakic bullous keratopathy and in normal-risk patients there is a beneficial effect of an HLA-matched graft.
Given the controversy around HLA matching, and because of the fact that we were not planning to use aggressive systemic immunosuppressant therapy postoperatively, we opted for a tissue-matched graft in the hope of a better chance of graft survival in this case of repeat DSAEK.
Our case was unique because graft failure occurred after histologically proven epithelial ingrowth in an otherwise uneventful DSAEK procedure. One may argue that the combination of cataract extraction and IOL implantation along with the DSAEK increases the amount of surgical manipulation and provides a portal of entry for host epithelial cells to enter the anterior chamber. In our suture pull-through method we pass the straight needle of the polypropylene suture from the stroma/Descemet side and come out of the endothelium of the donor lenticule. If there is an eccentric punch of the donor graft, there can be a possibility that the remaining epithelial cells on the donor lenticule may be embedded in suture track in the graft during the process of suture passage. This may increase the risk of epithelial ingrowth. However, if such a case of eccentric trephination occurs, we do not pass the needle through that area of the donor lenticule, in order to avoid this possibility.
In our case, both the donor and the recipient were male patients, and hence X-Y karyotyping of the implanted epithelium was not possible to determine whether it was of donor or host origin as documented in certain cases.Citation15,Citation16 However, the lack of continuity between the host corneal DSAEK wound and the epithelial interface possibly argues against invasion by the host epithelium. The DSAEK wound was closed with interrupted 10-O nylon sutures, and there was no evidence of wound leak or tissue incarceration, both of which are considered risk factors for epithelial ingrowth.Citation20 We did not use any venting incision, which can sometimes lead to epithelial cells of the host origin being implanted in the interface. We made a side port incision at 11 o’clock, which was hydrated at the end of the operation. We did not suture the side port. The original site of the epithelial ingrowth was noted to be from the 2 o’clock position and spread superiorly, which was not continuous with the side port incision.
We suspect that irregular trephination at the point of punching the lenticule to size may have resulted in the implantation of a small amount of donor epithelium, but we acknowledge that in the circumstances we have no proof of this. Our case therefore also serves as a reminder of the extreme importance of meticulous technique at all stages of the DSAEK procedure. It is possible that the epithelial implantation occurred as a result of irregular trephination and was therefore potentially avoidable.
Our case indicates that repeat DSAEK may be a successful surgical solution for this very rare but serious complication. We emphasize, however, the importance of very careful stripping and cleaning of the interface, as any epithelial cell rests remaining would risk a recurrence of the problem. We found the combination of manual scraping with a Descemet’s membrane stripper plus the Simcoe manual irrigation/aspiration cannula to be highly effective in this regard. However, not every suspected epithelial implantation leads to graft failure, and we have at least one patient (unpublished observation) in whom apparent interface epithelial inclusions have remained static or even regressed over time. Our literature review suggests that other surgeons have shared this experience.Citation9,Citation10,Citation12,Citation18,Citation19
In summary, we have demonstrated that graft failure due to epithelial ingrowth can occur after an apparently uneventful DSAEK. Early recognition of the condition, careful removal of the implanted epithelium, and repeat DSAEK may help to achieve a successful outcome without the need for more invasive treatments, such as PK.
Disclosure
The co-authors have been equally involved in the management of the cases. None of the authors has any proprietary interest. Previously presented in part as a poster at the UKISCRS annual meeting, 2011.
References
- GhaznawiNChenESDescemet’s stripping automated endothelial keratoplasty: innovations in surgical techniqueCurr Opin Ophthalmol201021428328720467318
- MellesGREgginkFALanderFA surgical technique for posterior lamellar keratoplastyCornea1998176186269820943
- TerryMAOusleyPJDeep lamellar endothelial keratoplasty in the first United States patients: early clinical resultsCornea20012023924311322409
- MellesGRWijdhRHNieuwendaalCPA technique to excise the Descemet membrane from a recipient cornea (descemetorhexis)Cornea200423328628815084862
- GorovoyMSDescemet-stripping automated endothelial keratoplastyCornea20062588688917102661
- MaumeneeAEShannonCREpithelial invasion of the anterior chamberAm J Ophthalmol19564192994213326999
- WeinerMJTrentacosteJPonDMEpithelial downgrowth: a 30-year clinicopathological reviewBr J Ophthalmol1989736112920156
- FederRSKrachmerJHThe diagnosis of epithelial downgrowth after keratoplastyAm J Ophthalmol1985996977033893142
- SuhLHYooSHDeobhaktaAComplications of Descemet’s stripping with automated endothelial keratoplasty: survey of 118 eyes at one instituteOphthalmology20081151517152418378315
- CulbertsonWWDescemet stripping endothelial keratoplastyInt Ophthalmol Clin200646315516816929232
- KoenigSBCovertDJEpithelial ingrowth after Descemet stripping automated endothelial keratoplastyCornea20082772772918580268
- WalkerBMHindmanHBEbrahimiKBEpithelial downgrowth following Descemet’s stripping automated endothelial keratoplasty [letter]Arch Ophthalmol200812627828018268229
- PrasherPMuftuogluOHsiaoMLEpithelial downgrowth after Descemet-stripping automated endothelial keratoplastyCornea200928670871119512895
- PhillipsPMTerryMAKaufmanSCEpithelial downgrowth after Descemet-stripping automated endothelial keratoplastyJ Cataract Refract Surg20093519319619101445
- GorovoyMSRatanasitAEpithelial downgrowth after Descemet stripping automated endothelial keratoplastyCornea2010291192119420628298
- SaelensIEYBartelsMCVan RijGIntroduction of epithelial cells in the flap-graft interface during Descemet stripping automated endothelial keratoplastyArch Ophthalmol2009127793693719597119
- LeeJADjalilianARRiazKMClinical and histopathologic features of failed Descemet stripping automated endothelial keratoplasty graftsCornea200928553053519421044
- SuhLHShoushaMAVenturaRUEpithelial ingrowth after Descemet stripping automated endothelial keratoplasty: description of cases and assessment with anterior segment optical coherence tomographyCornea20113052853421107249
- BansalRRamasubramanianADasPIntracorneal epithelial ingrowth after Descemet stripping endothelial keratoplasty and stromal punctureCornea200928333433719387237
- ChenSHPinedaRJrEpithelial and fibrous downgrowth: mechanisms of diseaseOphthalmol Clin North Am2002151414812064080
- VailAGoreSMBradleyBACollaborating surgeons. Conclusions of the Corneal Transplant Follow-up StudyBr J Ophthalmol1997816316369349147
- GoreSMVailABradleyBACorneal Transplant Follow-up Study Collaborators. HLA-DR matching in corneal transplantation: systematic review of published evidenceTransplantation199560103310397491677
- VailAGoreSMBradleyBAInfluence of donor and histocompatibility factors on corneal graft outcomeTransplantation199458121012167992365
- ReinhardTBöhringerDEnczmannJImprovement of graft prognosis in penetrating normal-risk keratoplasty by HLA class I and II matchingEye20041826927715004576
- Collaborative Corneal Transplantation Studies Research GroupThe Collaborative Corneal Transplantation Studies (CCTS): effectiveness of histocompatibility matching in high-risk corneal transplantationArch Ophthalmol1992110139214031417537
- RossAHJonesMNANguyenDQLong-term topical steroid treatment after penetrating keratoplasty in patients with pseudophakic bullous keratopathyOphthalmology20091162369237219815284