Abstract
Background
The aim of this study was to evaluate the influence of topical bevacizumab on the formation and function of filtering blebs in eyes with early bleb failure after antiglaucoma surgery.
Methods
Of all patients who underwent mitomycin-augmented trabeculectomy for glaucoma in the Department of Ophthalmology at the Medical University in Lublin, Poland, between March 2009 and March 2010, a total of 21 eyes from 20 patients with injected filtration bleb 9.8 ± 4.7 days after surgery were included in this observational case series. All patients were treated with standard steroid therapy and topical bevacizumab 5 mg/mL five times a day for 20.9 ± 9.8 days. Patients were followed up every other day, and a full eye examination was performed 14, 30, 60, and 180 days after initiation of treatment. Blebs were evaluated for vascularity by slit-lamp examination with concomitant photographic documentation and intraocular pressure measurement.
Results
Elevated functional bleb with significantly reduced vascularity was present in 16 eyes, and was flat and nonfunctional in five eyes. Intraocular pressure in all eyes decreased from a mean of 26.6 ± 9.6 mmHg before surgery to 14.6 ± 7.7 mmHg and 15.8 ± 8.3 mmHg at 2 and 6 months after surgery, respectively. Filtration bleb leak was noted in three eyes while on treatment with bevacizumab.
Conclusion
Topical application of bevacizumab might favor functional bleb formation after trabeculectomy in eyes with a high risk of failure.
Introduction
Trabeculectomy remains a standard filtration procedure in glaucoma surgery. It aims to decrease intraocular pressure (IOP) by constant shunting of aqueous fluid from the anterior chamber into the subconjunctival space, with subsequent creation of a filtering bleb.Citation1 Filtering bleb insufficiency as a result of excessive scarring attributable to proliferation of fibroblasts and subconjunctival fibrosis is one of the main reasons for treatment failure.Citation2 In this respect, trabeculectomy differs from other surgical procedures because its success relies on inhibition of wound healing.Citation1
One of the crucial steps in physiologic wound healing is angiogenesis.Citation3 Therefore, antiangiogenic agents might be a promising therapeutic strategy aimed at inhibition of postoperative wound healing after trabeculectomy. One of the most important proangiogenic substances involved in both physiologic and pathologic angiogenesis is vascular endothelial growth factor (VEGF). VEGF is one member of a group of substances among which VEGF-A is considered a prototype for a whole family.Citation4
Bevacizumab (Avastin®, Roche, Basel, Switzerland) is a humanized monoclonal antibody against all isoforms of VEGF-A, and in 2004 was the first antiangiogenic substance approved by the US Food and Drug Administration for therapeutic use in oncology.Citation4–Citation6 In addition to being used in the treatment of cancer, bevacizumab is currently being used off-label in ophthalmology to treat various pathologies involving neovascularization. Intravitreal injections are proposed as a part of the therapeutic protocols for age-related macular degeneration, myopia, premature retinopathy, central retinal vein thrombosis, and retinal vascular malformations.Citation5,Citation7
Anterior chamber injections are recommended for iris neovascularization, with topical application or subconjunctival injections being advocated for corneal neovascularization or recurrent pterygium.Citation6,Citation8,Citation9 Attempts to treat glaucoma with subconjunctival injections of bevacizumab have also been described.Citation10 Importantly, no significant side effects in the posteriorCitation11 or anteriorCitation12 segments of the eye have been reported to date. Based on the available data, we elected to use topical bevacizumab as a therapeutic modality after filtration procedures in order to elucidate its potential influence on wound healing and the morphology of the filtering bleb.
Materials and methods
The study protocol was approved by the local ethics committee and adhered to the tenets of the Declaration of Helsinki. Of all patients who were treated surgically for glaucoma in the department of ophthalmology, Medical University in Lublin, between March 2009 and March 2010, a total of 21 eyes (from 20 patients) with primary open angle glaucoma, primary closure angle glaucoma, or capsular glaucoma were included in the study. Twenty eyes underwent trabeculectomy with mitomycin C, and the remaining eye underwent a filtration procedure with implantation of a drainage device (Express™, Atrium Medical Corporation, Hudson, NH, USA) under a scleral flap. Inclusion criteria were the presence of an injected filtration bleb regardless of IOP levels and a poor prognosis, ie, adequate to grades H0, H1, E0, E1, and V3, and V4 of the Indiana Bleb Appearance Grading Scale.Citation13 Our cohort consisted of 12 females and nine males of mean age 67.8 ± 11.2 years. Detailed demographic data are shown in .
Table 1 Detailed demographic and treatment data for the study cohort
Surgical technique
After peribulbar anesthesia, a fornix-based conjunctival flap was prepared. Hemostasis was achieved using wet field cautery. A rectangular scleral flap measuring 4 × 3 mm was then dissected to a depth of one half the scleral thickness until the entire corneoscleral limbus was exposed. Two small 4 mm × 3 mm rectangular fragments of surgical sponge were then saturated with a 0.3 mg/dL solution of mitomycin C in saline; one was placed under the scleral flap and the other over the scleral flap under Tenon’s capsule for 3 minutes. The exposure site was irrigated copiously with saline. Trabeculectomy was performed using 1–2 bites with a Pierce punch, after which peripheral iridectomy was performed. The scleral flap was closed with two fixed and two releasable 10–0 nylon sutures in the standard manner. Next, Tenon’s capsule and the conjunctiva were each tightly closed with single 10–0 nylon sutures.
In addition to standard postoperative therapy of dexamethasone five times a day in decreasing doses for up to 6 weeks, an antibiotic (Oftaquix®, Santen GmbH, Munchen, Germany) five times a day, and 1% topical atropine (Atropinum Sulfuricum 1%, Polfa Warsaw, Poland) twice a day for 10 days, all study patients received topical bevacizumab five times a day. Topical drops were titrated to 5 mg/mL with sterile saline from a commercially available bevacizumab solution and were subsequently stored at 4°C. Patients were followed up every other day, and a full eye examination was performed on days 14, 30, 60, and 180 after initialization of treatment. Standard examination included IOP measurement, assessment of best corrected visual acuity, slit-lamp examination of the anterior and posterior segments of the eye, and visual fields at 30 and 180 days after surgery. Visits were scheduled more often in some cases. Blebs were evaluated for vascularity by the same surgeon using the slit-lamp with concomitant photographic documentation.
Results
Patients were enrolled for the study if they met inclusion criteria, with 21 eyes qualifying at a mean of 9.8 ± 4.7 (4–20) days after surgery. Bevacizumab was administered for up to 15 days in 11 eyes, and in 10 eyes we extended therapy because of poor bleb morphology or increased IOP. The mean duration of treatment for the whole group was 20.9 ± 9.8 days, with the shortest treatment period being 11 days and the longest being 43 days. By the end of treatment, a pale, elevated and functional filtration bleb was present in 16 eyes (). In five cases (JB, FS, JC, ZS, KW) deemed to be failures at the end of treatment with bevacizumab, a pale yet circumscribed flat, and nonfiltering bleb was present (). Because of elevated IOP (38 mmHg) ten weeks after surgery, one of the patients eventually underwent postoperative wound revision, and the other patient is still under surveillance. However, it should be noted that all of the patients in our cohort had pale avascular blebs, whether functional or not, during long-term follow-up.
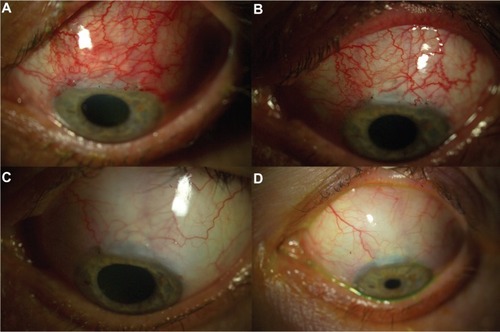
Figure 1 Successful treatment of early bleb failure with topical bevacizumab in patient 3. (A) Flat and injected bleb prior to bevacizumab application, (B) 7 days after topical administration of bevacizumab, (C) 2 months after topical administration of bevacizumab, and (D) 6 months after topical administration of bevacizumab, revealing a pale, elevated, and functional bleb.
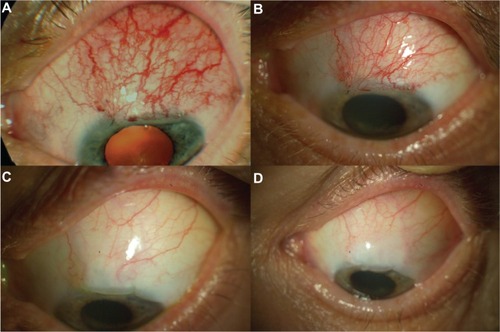
Figure 2 Unsuccessful treatment of early bleb failure with topical bevacizumab in patient 7. (A) Flat and injected bleb prior to bevacizumab application, (B) 7 days after topical administration of bevacizumab, (C) 2 months after topical administration of bevacizumab, and (D) 6 months after topical administration of bevacizumab, revealing a pale, flat, and nonfunctional bleb.
The mean IOP in all of the eyes was 26.6 ± 9.6 mmHg prior to surgery, 16.9 ± 8.4 mmHg at the beginning of treatment with bevacizumab, and 14.6 ± 7.7 mmHg and 15.8 ± 8.3 mmHg at 2 and 6 months after surgery, respectively. Detailed IOP values are summarized in .
Table 2 Intraocular pressures (mmHg) in all of the eyes studied prior to surgery, at the beginning of treatment with bevacizumab, and at 2 and 6 months later
Filtration bleb leak occurred in three eyes treated with bevacizumab. Treatment with bevacizumab was terminated on day 14 in one eye because of a positive Seidel test despite poor bleb morphology. Bleb leak was still present on subsequent evaluations, and successful reconstruction of filtration bleb was performed six weeks postoperatively. After meticulous analysis of the literature on the possible side effects of intraocular administration of bevacizumab, along with careful assessment of the potential benefits of this agent, we subsequently elected to continue treatment despite the positive Seidel test. Accordingly, in the next case, despite a positive Seidel test being present between days 16 and 23 after surgery, topical bevacizumab was started on postoperative day 9. In the remaining case, despite a positive Seidel test being present on the first day after surgery, topical bevacizumab was started on postoperative day 8. The leak persisted over 18 days of treatment with bevacizumab, and secondary sutures were placed for persistent bleb leak 7 days after cessation of bevacizumab. No other side effects were noted during follow-up.
Discussion
The first several weeks after a filtration procedure are crucial for the ultimate success of treatment. Essential for efficacy is preservation of the patency of the filtration fistula created during surgery. Gentle intraoperative tissue handling and use of steroids and antifibrotic agents have been suggested to maintain the patency of this conduit, but this goal is not always attained, and postoperative inflammation and scar formation may lead to early bleb failure during the first few months after surgery. Increased vascularity and reduction in bleb height and area, with or without an increase in IOP, constitute the early signs of bleb failure.Citation14 Prompt measures need to be taken to prevent development of permanent adhesions between the conjunctiva and episclera. Frequent use of topical or systemic corticosteroids is presently considered to be standard of care in such cases. Further, an adjunctive antimetabolite, such as 5-fluorouracil or mitomycin C, is usually used perioperatively for further reduction of subconjunctival fibrosis. Measures such as ocular massage and/or removal of releasable sutures or laser suture lysis are further initial measures for management of this situation. However, despite all the aforementioned maneuvers, the failure rate is as high as 30%–40% in long-term follow-up.Citation15,Citation16 In the face of such a high failure rate, further treatment modalities are needed.
In our series, 16 of 21 patients (approximately 75%) with developing bleb failure recovered, which seems to be a very high success rate, especially when compared with standard treatment (reported success rate 26%) or subconjunctival injections of 5-fluorouracil (reported 51% success rate) in eyes with a poor prognosis.Citation17 Further, the remaining 25% of blebs blanched relatively quickly, but the fistulae did not remain patent. The natural history of bleb healing involves reduction of vascularity,Citation18 regardless of the final filtration efficacy. Importantly, nonfunctional blebs should undergo revision no later than 3 months because the healing process has ended by this time and no further improvement can be expected.Citation19 Therefore, it seems that topical bevacizumab 5 mg/mL drops should either be started immediately after surgery or immediately at the first signs of bleb failure.
Wound healing in scleral tissue is physiologically similar to that in other tissues, with the exception of the blood vessels; the sclera contains very few of these, with most originating from the adjacent episclera.Citation20 Hampered access of the vessels to the forming scar might increase the effectiveness of antiangiogenic treatment in the early postoperative period, not only immediately but also within the first week after surgery. On the other hand, most of the available data show that the neovascularization process at the surgical wound site is most vigorous during the first 2 weeks after surgery.Citation21 Based on the aforementioned data, our therapeutic protocol of starting bevacizumab within the first 3 weeks after surgery seems to be valid. Subsequently, we administered bevacizumab for up to 6 weeks based on individual patient requirements, judged primarily by clinical signs of neovascularization. Importantly, the given time frame for the use of bevacizumab is the time when proangiogenic processes occur, in contrast with other eye-surface diseases, such as corneal neovascularization after transplant when angiogenesis is continuously perpetuated. In cases with poor bleb formation where, on top of neovascularization, one can expect extensive fibrinogen formation, additional rationale for administration of bevacizumab arises from recent publications, which provide some evidence for its anti-inflammatory and antifibroblastic effects.Citation6,Citation22 However, recent developments support the notion that treatment with anti-VEGF factors might cause excessive sclerosis in patients with proliferative diabetic retinopathy.Citation23,Citation24 Bevacizumab has been used previously in glaucoma surgery in the form of postoperative subconjunctival injections. Both animal and human studies have demonstrated this to be effective.Citation21,Citation25 Similarly, intraoperative injections during revision surgeries for nonfunctional filtration blebs have been shown to increase the chances of a successful outcome.Citation26
To the best of our knowledge, this is the first report to date of topical administration of bevacizumab for threatening bleb failure, and is a follow-up of our own findings reported in a letter published in 2011.Citation27 In our opinion, this modality has several advantages, in that it is less traumatic, allows extended administration on an outpatient basis, and reduces the costs of therapy. Further, given the limited systemic absorption of bevacizumab in comparison with subconjunctival injections, the topical route of administration might avoid serious side effects. Oncology patients treated with systemic injections of bevacizumab suffer potentially dangerous side effects, including hypertension, emboli, hematologic disturbances, and ulceration of the mucous membranes.Citation28 Importantly, subconjunctival and intravitreal injections result in accumulation of bevacizumab in the contralateral eye, while topical administration results in low levels of bevacizumab in the iris and retina of the ipsilateral eye.Citation29 Moreover, no significant side effects of topical administration on the surface of the eye have been reported.Citation30 Simultaneously, one can expect that the bevacizumab concentration in the vicinity of the scleral flap is adequate, based on existing reports of the affinity of bevacizumab for sites of neovascularization. Additionally, inflammation surrounding the postoperative wound facilitates drug bioavailablility.Citation31 This has been demonstrated by successful use of bevacizumab drops in the treatment of corneal neovascularization.Citation6 Accordingly, we elected to implement a similar protocol in our study.
Another important aspect of bevacizumab administration is the likelihood of impairment of conjunctival healing that might increase the risk of early bleb leaks, especially when used in conjunction with mitomycin C, which itself has been reported to be an antiangiogenic agent.Citation32 Importantly, in our group, we noticed some proportion of leaks (14%), indicating that these should be treated as a potential side effect. However, our data show a significantly lower proportion of leaks when compared with other reports.Citation32,Citation33 Moreover, one should keep in mind that one of the reported leaks occurred before treatment with bevacizumab, while two others occurred after treatment.
Taken together, our data support the hypothesis that topical administration of bevacizumab might be beneficial in the treatment of early filtering bleb insufficiency due to increased vascularity. An important aspect of its implementation in the treatment protocol is the fact that topical application of bevacizumab does not preclude use of other standard preventive measures. However, long-term observation in a randomized, double-blind, placebo-controlled prospective study is needed to assess the safety and efficacy of this strategy.
Disclosure
The authors report no conflicts of interest in this work.
References
- SpaethGLOphthalmic Surgery: Principles and Practice3rd edPhiladelphia, PAWB Saunders Co2003
- SkutaGLParrishRK2ndWound healing in glaucoma filtering surgerySurv Ophthalmol1987321491703328315
- LiJChenJKirsnerRPathophysiology of acute wound healingClin Dermatol20072591817276196
- MaJWaxmanDJCombination of antiangiogenesis with chemotherapy for more effective cancer treatmentMol Cancer Ther200873670368419074844
- GrisantiSZiemssenFBevacizumab: off-label use in ophthalmologyIndian J Ophthalmol20075541742017951896
- BockFKonigYKruseFBaierMCursiefenCBevacizumab (Avastin) eye drops inhibit corneal neovascularizationGraefes Arch Clin Exp Ophthalmol200824628128417934753
- LalwaniGABerrocalAMMurrayTGOff-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurityRetina200828S13S1818317338
- GrisantiSBiesterSPetersSTatarOZiemssenFBartz-SchmidtKUIntracameral bevacizumab for iris rubeosisAm J Ophthalmol200614215816016815268
- FallahMRKhosraviKHashemianMNBeheshtnezhadAHRajabiMTGohariMEfficacy of topical bevacizumab for inhibiting growth of impending recurrent pterygiumCurr Eye Res201035172220021250
- Jurkowska-DudzinskaJKosior-JareckaEZarnowskiTComparison of the use of 5-FU and bevacizumab in primary trabeculectomy: results at one yearClin Experiment Ophthalmol201240135142
- BakriSJCameronJDMcCannelCAPulidoJSMarlerRJAbsence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit modelAm J Ophthalmol200614216216416815270
- BockFKonigYDietrichTZimmermannPBaierMCursiefenCInhibition of angiogenesis in the anterior chamber of the eyeOphthalmologe2007104336344 German.17372736
- CantorLBMantravadiAWuDunnDSwamynathanKCortesAMorphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana Bleb Appearance Grading ScaleJ Glaucoma20031226627112782847
- Azuara-BlancoAKatzLJDysfunctional filtering blebsSurv Ophthalmol199843931269763136
- JampelHDSolusJFTraceyPAOutcomes and bleb-related complications of trabeculectomyOphthalmology201211971272222244944
- LandersJMartinKSarkiesNBourneRWatsonPA twenty-year follow-up study of trabeculectomy: risk factors and outcomesOphthalmology201211969470222196977
- [No authors listed]Three-year follow-up of the Fluorouracil Filtering Surgery StudyAm J Ophthalmol199311582928420383
- HolloGWound healing and glaucoma surgery: modulating the scarring process with conventional antimetabolites and new moleculesDev Ophthalmol201250798922517175
- FeldmanRMTabetRRNeedle revision of filtering blebsJ Glaucoma20081759460018854740
- American Academy of OphthalmologyFundamentals and Principles of Ophthalmology, 2000–2001San Francisco, CAAmerican Academy of Ophthalmology2000
- CooteMARuddleJBQinQCrowstonJGVascular changes after intra-bleb injection of bevacizumabJ Glaucoma20081751751818854726
- KimTIChungJLHongJPMinKSeoKYKimEKBevacizumab application delays epithelial healing in rabbit corneaInvest Ophthalmol Vis Sci2009504653465919458331
- BatmanCOzdamarYThe effect of bevacizumab for anterior segment neovascularization after silicone oil removal in eyes with previous vitreoretinal surgeryEye (Lond)2010241243124620019762
- KuiperEJVan NieuwenhovenFAde SmetMDThe angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathyPLoS One20083e267518628999
- MemarzadehFVarmaRLinLTPostoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbitInvest Ophthalmol Vis Sci2009503233323719182254
- KahookMYSchumanJSNoeckerRJNeedle bleb revision of encapsulated filtering bleb with bevacizumabOphthalmic Surg Lasers Imaging20063714815016583638
- ZarnowskiTTulidowicz-BielakMTopical bevacizumab is efficacious in the early bleb failure after trabeculectomyActa Ophthalmol201189605606
- EskensFAVerweijJThe clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a reviewEur J Cancer2006423127313917098419
- NomotoHShiragaFKunoNPharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbitsInvest Ophthalmol Vis Sci2009504807481319324856
- BockFOnderkaJRummeltCSafety profile of topical VEGF neutralization at the corneaInvest Ophthalmol Vis Sci2009502095210219151400
- FeinerLBarrEEShuiYBHolekampNMBrantleyMAJrSafety of intravitreal injection of bevacizumab in rabbit eyesRetina20062688288817031287
- AnandNAroraSClowesMMitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaksBr J Ophthalmol20069017518016424529
- AlwitryARotchfordAPatelVAbedinAMoodieJKingAJEarly bleb leak after trabeculectomy and prognosis for bleb failureEye (Lond)20092385886318497838