Abstract
Purpose
To describe a case of non-arteritic ischemic optic neuropathy (NAION) secondary to acute primary-angle closure (APAC).
Methods
Case report.
Results
A 50-year-old woman with painful visual loss in the right eye was found to be in APAC with a right afferent pupillary defect. Laser peripheral iridotomy relieved pain but did not improve vision. Diffuse optic disc edema in the right eye and a small cup-to-disc ratio in the left eye were evident. Magnetic resonance imaging was normal. The patient was diagnosed with non-arteritic ischemic optic neuropathy (NAION) secondary to APAC, a rare clinical entity which can result in markedly decreased visual acuity.
Conclusion
NAION secondary to APAC is a rare clinical entity that can result in severe vision loss.
Introduction
The prevalence of acute primary-angle closure (APAC) is 0.04 to 0.6 per thousand in the United States in patients older than 40.Citation1,Citation2 The incidence of non-arteritic ischemic optic neuropathy (NAION) is two to ten per 100,000 in the population older than 50.Citation3,Citation4 NAION secondary to APAC was described by Sonty and Schwartz in 1981.Citation5 There have been four subsequent case reports (five eyes) describing NAION secondary to APAC.Citation6–Citation9 Patients present with typical APAC symptoms and signs, which include pain, headache, nausea and vomiting, conjunctival injection, corneal edema, elevated intraocular pressure (IOP), closed angles, and a shallow anterior chamber. NAION was present at the time of presentation for APAC in four of six eyes and developed one week after APAC in two eyes.Citation5–Citation9
Case report
A 50-year-old woman with a history of hypertension and dyslipidemia presented with redness, pain, decreased vision, headache, and nausea, for approximately 10 hours. She reported no similar prior episodes. The patient was taking metoprolol, 50 mg, at bedtime; hydrochlorothiazide, 25 mg, daily; amlodipine, 25 mg, daily; lisinopril, 20 mg, daily for hypertension; simvastatin, 40 mg, at bedtime for dyslipidemia; and aspirin, 81 mg, daily for cardiovascular risk factors.
Best-corrected visual acuity (VA) was hand motion at 4 feet right eye and 20/20 left eye (manifest refraction: plano + 0.75 × 135). Her pupil was 5 mm in the right eye and 4 mm in the left, and she was noted to have a moderate relative afferent pupillary defect (RAPD) in the right eye. Her IOP were 41 mmHg right eye and 15 mmHg left eye. The right eye had moderately severe ciliary injection, corneal edema, a shallow anterior chamber (closed peripherally, ½ corneal thickness centrally), and a moderate nuclear sclerotic cataract. There was no posterior synechiae, and her pupil was minimally reactive. The left eye was quiet, had a shallow anterior segment (¼ corneal thickness peripherally, ½ corneal thickness centrally), and a mild nuclear sclerotic cataract. The view of the posterior segment in the right eye was obscured by the corneal edema. The left optic nerve head appeared normal with a cup-to-disc ratio of 0.1. Gonioscopy demonstrated that her angle was closed 360° in the right eye and was Shaffer Grade II (anterior trabecular meshwork) 360° in the left.
The patient was treated for APAC in the right eye with timolol, dorzolamide, and brimonidine drops every 30 minutes and acetazolamide 500 mg orally. One hour later, the IOP was 34 mmHg and she was still experiencing severe pain and decreased vision. After instillation of pilocarpine 2%, a laser peripheral iridotomy (LPI) was performed in the right eye without complications and the anterior chamber deepened, the IOP decreased to 14 mmHg, and the patient felt a marked improvement in pain. However, she reported that her vision did not improve. Due to the right RAPD, the patient was asked to return the following morning for a dilated fundus exam.
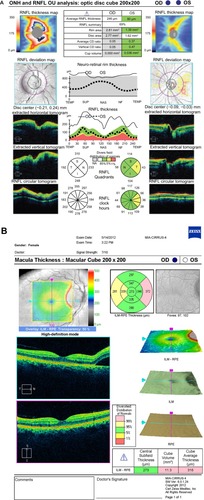
Upon the patient’s return, she reported no pain in her right eye but noted no change in vision. VA remained at hand motion at 4 feet with a moderate RAPD and an IOP of 12 mmHg. Her conjunctival injection and corneal edema had resolved. Her LPI was patent, and her anterior chamber was shallow (¼ corneal thickness peripherally), but significantly deeper than the previous day. Gonioscopy demonstrated her angle was Shaffer Grade II 360° without peripheral anterior synechiae. The right optic nerve head had 360° edema and an area of subretinal fluid extending into the nasal aspect of macula from the optic disc temporally (). The examination of the left eye was unchanged (). Optical coherence tomography (OCT) demonstrated optic nerve edema and subretinal fluid extending to the macula in the right eye ( and ).
Figure 1 Fundus photo of the right and left eye.
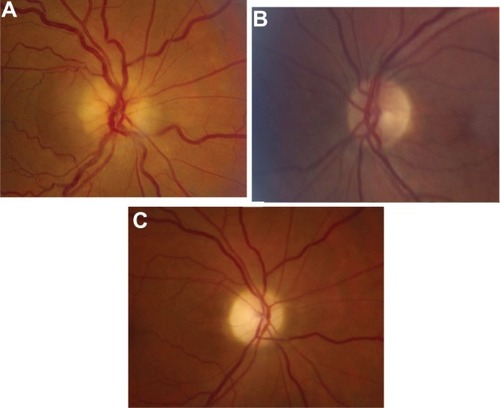
Figure 2 OCT of the optic nerve and macula. (A) Retinal nerve fiber layer analysis demonstrates 360° optic nerve edema in the right eye and no optic nerve edema in the left. (B) Macula OCT demonstrates a small area of subretinal fluid extending from the temporal portion of the optic disc into the nasal macula in the right eye.
A brain magnetic resonance imaging (MRI) with contrast to rule out infiltrative or compressive causes of the optic nerve edema was within normal limits. The patient had no giant cell arteritis symptoms and no headaches prior to this acute event, and the headaches resolved completely after the LPI. The patient was diagnosed with NAION related to angle closure.
The patient was counseled on the importance of stopping use of her antihypertensive medications in the evening and of improved blood pressure and dyslipidemia control. An LPI was performed in her left eye without complications. Two days after presentation, the patient was started on oral prednisone taper (60 mg prednisone daily for 2 weeks, followed by 50 mg for 5 days and then 40 mg for 5 days, and then cutting down by 5 mg every 3 days to 5 mg, followed by 2.5 mg for 3 days and then 1 mg for 3 days) and instructed to follow-up in one month. The decision to use steroids in this patient was based on a retrospective study by Hayreh and Zimmerman, which demonstrated improvement in VA and visual field in NAION patients who presented within two weeks of onset.Citation10 A larger improvement was seen in patients with poorer presenting VA.Citation10
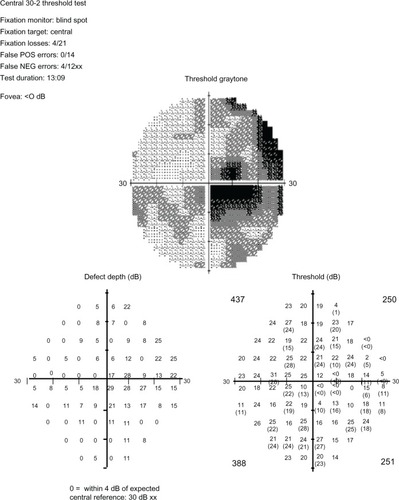
One month later, VA was count fingers at two feet in the right eye and 20/20 in the left. The IOP was 12, right eye, and 14, left eye. A Humphrey visual field of the right eye demonstrated a cecocentral scotoma (). The optic nerve edema of the right eye resolved and the disc had diffuse atrophy (). The patient was prescribed new polycarbonate lenses and counseled about monocular precautions.
Discussion
NAION secondary to APAC is a rare clinical entity that can result in severe vision loss. NAION is caused by ischemia to the optic nerve head, mainly from the posterior ciliary artery and its branches.Citation11 Blood flow to the optic nerve head is determined by a ratio of perfusion pressure and resistance to flow.Citation12 Resistance to flow is determined by efficiency of autoregulation, vascular integrity, and the rheological properties of blood.Citation12 These factors can be impaired in patients with diabetes, hypertension, and atherosclerosis.Citation12 Perfusion pressure is determined by the difference in mean arterial pressure (MAP) and IOP.Citation12 The MAP is reduced with relative nocturnal hypotension and antihypertensive medications.Citation12 High IOPs during phacoemulsification may contribute to the increased incidence of NAION after cataract surgery.Citation13–Citation15 Elevated IOP has been identified as a cause of decreased blood flow to the optic nerve head in five cases (six eyes) previously reported by other authors and our case of NAION after APAC ().Citation5–Citation9
Table 1 Summary of the five previously reported patients with NAION-associated APAC (six eyes) and our additional patient
Taken together with our case, of all seven eyes with APAC-associated NAION, the duration of APAC symptoms ranged from 1 day to 1 month. Five of the seven eyes had NAION at presentation, while the remainder developed NAION one week after presentation for APAC. The IOP at presentation ranged from 40–56 mmHg. Five of seven eyes underwent LPI in the NAION eye and contralateral eye.Citation5–Citation9 Slavin and Margulis described one patient who developed an NAION one week after an APAC attack in the left eye and refused an LPI in the right eye.Citation8 One month later, the patient had an APAC attack in the right eye and developed a right NAION a week later.Citation8 Sequential NAION has not been reported in the other APAC-associated NAION cases.Citation5–Citation7,Citation9 One possible hypothesis for the 1 week delay in presentation with NAION after presentation with APAC in this patient is that the initial APAC event may have started the cycle of NAION, but it was not clinically significant for 1 week. Hayreh described the cycle of NAION as ischemia of axons resulting in axoplasmic flow stasis, which in turn cause axoplasmic accumulation and subsequent axonal swelling, which in turn compresses the capillaries, leading to more ischemia.Citation12
Including our patient, only two of the seven eyes (29%) with APAC-associated NAION attained acuity of 20/30 or better,Citation5–Citation9 which is worse than the 49% of NAION patients who attained 20/30 or better in a study by Hayreh et al.Citation16 However, the low sample size of APAC-associated NAION patients is a barrier for meaningful comparisons with other NAION patients. It is also possible that the 2 cases of the delayed onset NAION after APAC previously reported may be spontaneous NAION.
Patients with APAC can also develop mild optic nerve edema that is not associated with NAION.Citation17 Tsai et al found a statistically significant difference in the retinal nerve fiber layer thickness (RNFL) of the APAC eye and the contralateral eye measured by OCT 1 week after the APAC event.Citation17 However, a statistically significant difference was not observed at the 4- and 12-week follow-ups.Citation17 These patients can be differentiated from NAION secondary to APAC patients by the lack of subsequent optic nerve atrophy and preserved visual acuity and visual field.
NAION secondary to APAC is a rare but potentially blinding clinical entity. In addition to counseling patients about NAION risk factors (eg, evening antihypertensive medications and control of hypertension, diabetes, and dyslipidemia), patients must be counseled on the importance of performing an LPI in the contralateral eye. In APAC patients with an RAPD, a large amount of optic nerve edema, persistent decreased visual acuity, and a small cup-to-disc ratio in the contralateral eye should raise clinical suspicion for NAION.
Disclosure
The authors report no conflicts of interest in this work.
References
- KleinBEKleinRSponselWEPrevalence of glaucoma. The Beaver Dam Eye StudyOphthalmology19929910149915041454314
- TielschJMKatzJSinghKA population-based evaluation of glaucoma screening: the Baltimore Eye SurveyAm J Epidemiol199113410110211101746520
- HattenhauerMGLeavittJAHodgeDOGrillRGrayDTIncidence of nonarteritic anterior ischemic optic neuropathyAm J Ophthalmol199712311031079186104
- JohnsonLNArnoldACIncidence of nonarteritic and arteritic anterior ischemic optic neuropathy. Population-based study in the state of Missouri and Los Angeles County, CaliforniaJ Neuroophthalmol199414138448032479
- SontySSchwartzBVascular accidents in acute angle closure glaucomaOphthalmology19818832252287231910
- YipLWYongVKHohSTWongHTOptical coherence tomography of optic disc swelling in acute primary angle-closure glaucomaArch Ophthalmol2005123456756915824237
- NahumYNewmanHKurtzSRachmielRNonarteritic anterior ischemic optic neuropathy in a patient with primary acute angle-closure glaucomaCan J Ophthalmol200843672372419020646
- SlavinMLMargulisMAnterior ischemic optic neuropathy following acute angle-closure glaucomaArch Ophthalmol20011198121511483097
- ChoudhariNSGeorgeRKankariaVSunilGTAnterior ischemic optic neuropathy precipitated by acute primary-angle closureIndian J Ophthalmol201058543744020689205
- HayrehSSZimmermanMBNon-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapyGraefes Arch Clin Exp Ophthalmol200824671029104618404273
- HayrehSSManagement of ischemic optic neuropathiesIndian J Ophthalmol201159212313621350282
- HayrehSSIschemic optic neuropathyProg Retin Eye Res2009281346219063989
- LamBLJabaly-HabibHAl-SheikhNRisk of non-arteritic anterior ischaemic optic neuropathy (NAION) after cataract extraction in the fellow eye of patients with prior unilateral NAIONBr J Ophthalmol200791558558717446504
- McCulleyTJLamBLFeuerWJIncidence of nonarteritic anterior ischemic optic neuropathy associated with cataract extractionOphthalmology200110871275127811425687
- ZhaoYLiXTaoAWangJLuFIntraocular pressure and calculated diastolic ocular perfusion pressure during three simulated steps of phacoemulsification in vivoInvest Ophthalmol Vis Sci20095062927293119168897
- HayrehSSZimmermanMBNonarteritic anterior ischemic optic neuropathy: natural history of visual outcomeOphthalmology2008115229830517698200
- TsaiJCLinPWTengMCLaiICLongitudinal changes in retinal nerve fiber layer thickness after acute primary angle closure measured with optical coherence tomographyInvest Ophthalmol Vis Sci20074841659166417389497