Abstract
Purpose
To evaluate binocular intermediate visual acuity (IVA), depth of focus, and other visual outcomes achieved with a monofocal aspheric intraocular lens (IOL) using pooled data from 2 randomized, double-masked, controlled trials.
Patients and Methods
The studies conducted at 32 sites included patients aged ≥22 years with bilateral cataracts, preoperative corneal astigmatism 1.0 D, and lens power 18.0–25.0 D. Patients received bilateral AcrySof IQ IOLs (SN60WF). Primary endpoint data were collected at month 6. Binocular uncorrected and corrected distance visual acuity (UDVA and CDVA) at 4 m, binocular uncorrected and corrected IVA (UIVA and DCIVA) at 66 cm, manifest refraction spherical equivalent (MRSE), and binocular defocus curve at 4 m were assessed under photopic conditions. Validated questionnaires were used to assess spectacle use and quality of vision.
Results
Of 233 patients who received SN60WF, 228 had visual acuity data at 6 months. Under photopic conditions, 51% of the eyes had pupils >4 mm, 40% had pupils 3–4 mm, and 9% had pupils <3 mm. Mean ± SD UDVA and CDVA were −0.019 ± 0.110 and −0.088 ± 0.082 logMAR, respectively. Mean ± SD UIVA and DCIVA were 0.125 ± 0.145 and 0.196 ± 0.139 logMAR, respectively. UIVA and DCIVA of 20/32 or better were achieved by 83% (188/228) and 71% (162/228) of patients, respectively. Mean ± SD MRSE was −0.007 ± 0.404 D for the first eye and 0.036 ± 0.371 for the second eye. The defocus curve demonstrated binocular vision of 0.24 logMAR or better from +1.2 to −1.5 D. Spectacle independence for distance and intermediate vision was reported by 86% and 41% of the patients, respectively. Based on questionnaires, 61%, 79%, and 65% of the patients did not experience starbursts, halos, or glare.
Conclusion
A monofocal aspheric IOL (SN60WF) assessed in a large, pooled study provided excellent distance vision and clinically functional intermediate vision.
Introduction
The refractive properties of the human eye rely on the combination of its 2 lenses: the cornea, which provides approximately two-thirds of the optical power and average positive spherical aberration of 0.281 ± 0.086 (range, 0.058–0.545) µm,Citation1 and the crystalline lens, which provides the remaining optical power and counteracts the corneal asphericity with negative spherical aberration.Citation2
If a cataract develops and the crystalline lens is removed, vision may be restored by implantation of an artificial intraocular lens (IOL). Monofocal IOLs, which provide only 1 focal point, are the most commonly implanted worldwide. These lenses were originally developed with spherical surfaces (eg, AcrySof SA60AT, Alcon Vision LLC, Fort Worth, TX, USA; CT LUCIA 221P, Zeiss, Jena, Germany); unlike the crystalline lens, these IOLs do not offset the spherical aberration of the cornea and may induce further optical effects. The spherical aberration generated by the aspheric curvature of the cornea elongates the focal point of light in the retina, resulting in a certain depth of focus.Citation3,Citation4 Depth of focus is desirable in monofocal IOLs, as it expands visual capabilities from clarity at distance only into the intermediate range (ie, 66–80 cm). Hence, a spherical IOL should, theoretically, provide the eye with the maximum depth of focus (in the monofocal category) but may not provide optimal image clarity.Citation2
To improve upon the visual quality achieved with spherical lenses, aspheric monofocal IOLs were produced. These IOLs may fully (eg, TECNIS 1 ZCB00 [−0.27 µm asphericity], Johnson & Johnson Vision Care, Inc., Jacksonville, FL, USA) or partially (eg, AcrySof IQ SN60WF [−0.2 µm], Alcon Vision LLC; Clareon SY60WF [−0.2 µm], Alcon Vision LLC; Vivinex XC1-SP [−0.18 µm], Hoya, Shinjuku City, Japan) correct the spherical aberration of the human cornea, balancing the desire for optimal acuity with the benefit of retaining some depth of focus.Citation5,Citation6 Based on a contralateral comparison in 75 patients, contrast sensitivity was significantly better with aspheric AcrySof IQ SN60WF versus spherical SN60AT IOLs.Citation7 Furthermore, AcrySof IQ IOL provided clinically significant improvements for simulated night driving under glare and fog conditions.Citation7
Until recently, monofocal IOLs were not commonly investigated in relation to intermediate visual acuity (IVA). Because new multifocal and extended depth of focus (EDOF) IOLs have been evaluated in randomized controlled studies and compared with monofocal IOLs, the potential for achieving IVA with aspheric monofocal IOLs has been assessed in high-quality clinical studies. Two randomized, double-masked, controlled trials with the same study design investigated binocular IVA, depth of focus, and other visual outcomes with AcrySof IQ (SN60WF) versus Vivity EDOF IOL (DFT015). This analysis presents a combined assessment of the AcrySof IQ data from these 2 studies.
Methods
Design and Patient Population
This analysis used data from 2 prospective, multicenter, randomized, parallel-group, controlled, assessor‑ and patient-masked trials (NCT03010254 and NCT03274986) that used comparable clinical methods and similarly validated questionnaires. The studies were conducted between 21 March 2017 and 26 July 2021 at 32 sites (Australia, Canada, Spain, the United Kingdom, and the United States) and included a comparator (AcrySof IQ Vivity model DFT015, Alcon Vision LLC). Both studies had a 6-month follow-up.
Included in each study were patients aged ≥22 years with bilateral cataracts requiring surgery, preoperative corneal astigmatism <1.0 D, and a calculated lens power between 18.0 and 25.0 D. Exclusion criteria were pregnancy, previous intraocular or corneal surgery, desire for monovision correction, history of anterior or posterior segment pathology, clinically significant corneal pathology that may affect visual outcomes, clinically significant severe dry eye, or ocular surface disease.
Study Procedures
Patients received bilateral AcrySof IQ monofocal aspheric IOLs (model SN60WF) following routine small-incision cataract surgery. All eyes were targeted to emmetropia. A suggested A-constant was included as a guideline on the outer label of SN60WF; however, investigators were recommended to use their personalized A-constant based on clinical experience with SN60WF. Study visits included screening (visit 0), 2 operative visits (for both eye surgeries), and postoperative visits (day 1–2, day 7–14, day 30–60, and day 120–180). Primary endpoint data were collected at month 6 (120–180 days after second eye implantation).
Efficacy Assessments
Binocular corrected and uncorrected visual acuity tests were performed under photopic conditions and reported as logMAR. At 6 months after implantation, binocular uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) were evaluated at 4 m; binocular uncorrected IVA (UIVA) and distance-corrected IVA (DCIVA) were evaluated at 66 cm. Manifest refraction spherical equivalent (MRSE) was also assessed at 6 months after implantation.
Binocular defocus curve was assessed under photopic conditions at 4 m at 6 months after implantation using corrected distance refraction. Visual acuity was assessed between +1.50 D and −2.50 D in 0.5 D defocus steps (except in the region from +0.50 D to −0.50 D, which was assessed in 0.25 D steps).
Photopic pupil sizes of each eye were assessed preoperatively and at 6 months after implantation. Pupil size was measured with the NeurOptics VIP-200 or NeurOptics VIP-300 pupilometer (Irvine, CA, USA).
Patient-Reported Outcomes and Spectacle Use
Validated questionnaires were used to assess spectacle use and quality of vision. The 2 studies used slightly different questionnaires to assess spectacle use; therefore, data were only pooled for responses that were identical among the 2 questionnaires.
In one study, the spectacle use questionnaire included the questions, “How often do you wear eyeglasses for distance tasks (eg, driving)?” and “How often do you wear eyeglasses for intermediate tasks (eg, computer)?” The response scale included “never”, “sometimes”, or “always”. In the other study, the IOL satisfaction (IOLSAT) questionnaire assessed spectacle use and included the questions, “In the past 7 days, how often did you need to wear eyeglasses to see far away?” and “In the past 7 days, how often did you need to wear eyeglasses to see at arm’s length?” The response scale included “never”, “rarely”, “sometimes”, “most of the time”, and “all the time”. Because of the differences in response scales used, only data for patient responses of “never” were pooled. Quality of vision without spectacles was assessed using the validated IOLSAT questionnaire.
Safety
Visual disturbances, adverse device effects (ADEs), and serious adverse events (SAEs) were assessed at month 6. The Questionnaire for Visual Disturbances (QUVID) and Quality of Vision Questionnaire were used to rate frequency, severity, and bother of visual disturbances (eg, glare, halos, starbursts). Data were pooled for responses that were identical among the 2 studies. Because of slight differences in response scales used, only data for responses of “never” (frequency) or “not bothered at all” (bother) were pooled.
Statistical Analysis
The all-implanted analysis set, defined as all randomized eyes that underwent successful IOL implantation, was used to assess effectiveness outcomes, including visual acuity, MRSE, binocular defocus, and patient-reported outcomes. Binocular defocus curve plots were presented across all studies with the amount of defocus on the x-axis and visual acuity on the y-axis; error bars represented 95% CI. The safety analysis set, defined as all eyes with an attempted IOL implantation, was used to assess safety outcomes. All data were summarized descriptively.
Statement of Ethics
The studies were conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Council for Harmonisation E6 Good Clinical Practice consolidated guidelines and were in compliance with the International Organisation for Standardization (ISO) 11,979–7:2014 standards for IOLs and ISO 14155:2011 standards on good clinical practice. The analysis was approved by the following institutional review boards (IRB) and independent ethics committees: CHU de Québec-Université Laval Hospital du St-Sacrement; Bellberry Human Research Ethics Committee; IRB Services; East of England – Cambridge East Research Ethics Committee; El Comité Ético de Investigación Clínica del Centro de Oftalmología Barraquer; Trillium Health Partners – Research Office; and Chesapeake IRB (currently Advarra, Inc). All patients included in these studies signed informed consent.
Results
Patients
Included in the analysis were 233 patients who received the aspheric monofocal SN60WF IOL in both eyes; 228 patients had visual acuity data at the 6 month visit. Mean age was 69.3 years, more than half of the patients were female (55%; 129/233), and most patients were white (91%; 211/233; ). Under photopic conditions, 51% of the eyes had large pupil size >4 mm, 40% of the eyes had medium pupil size 3 to 4 mm, and 9% of the eyes had small pupil size <3 mm at 6 months after implantation.
Table 1 Patient Demographics and Baseline Characteristics (Safety Analysis Set)
Visual Outcomes
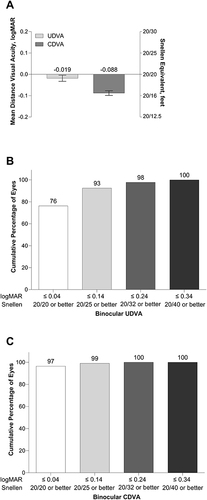
At 6 months after implantation, mean ± SD binocular UDVA and CDVA were −0.019 ± 0.110 and −0.088 ± 0.082 logMAR, respectively (). At 6 months, 76% of the patients (174/228) achieved UDVA of 20/20 or better (≤0.04 logMAR), and all patients achieved a UDVA of 20/40 or better (≤0.34 logMAR; ). Additionally, 97% of the patients (220/228) achieved CDVA of 20/20 or better (≤0.04 logMAR), and all patients achieved CDVA of 20/32 or better (≤0.24 logMAR; ).
Figure 1 Mean photopic binocular UDVA and CDVA at 6 months after implantation of the aspheric monofocal lens (A), cumulative distribution of binocular UDVA (B), and cumulative distribution of binocular CDVA (C).
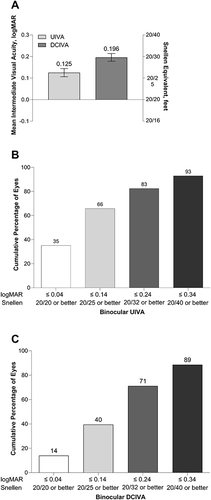
Mean ± SD binocular UIVA and DCIVA were 0.125 ± 0.145 and 0.196 ± 0.139 logMAR, respectively, at 6 months after implantation (). In terms of Snellen's equivalent, at 6 months, 83% of the patients (188/228) achieved binocular UIVA of 20/32 or better ) and 93% of the patients (212/228) achieved binocular UIVA of 20/40 or better. At 6 months after implantation, 71% of the patients (162/228) achieved binocular DCIVA of 20/32 or better, and 89% (202/228) achieved binocular DCIVA of 20/40 or better ().
Figure 2 Mean photopic binocular UIVA and DCIVA (66 cm) at 6 months after implantation of the aspheric monofocal lens (A), mean cumulative distribution of binocular UIVA (B), and cumulative distribution of binocular DCIVA (C).
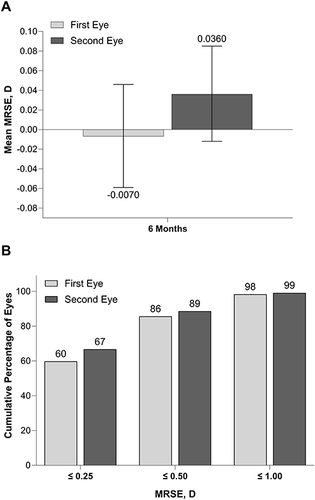
At 6 months, mean ± SD MRSE was −0.007 ± 0.404 D for the first eye and 0.036 ± 0.371 for the second eye (). Most of the first (196/229; 86%) and second eyes (202/228; 89%) were within 0.50 D of emmetropia (). MRSE > 1.00 D was reported in 4/229 first eyes (2%) and 2/228 second eyes (1%).
Figure 3 Mean MRSE in first and second eyes at 6 months (A) and cumulative distribution of MRSE in eyes with the aspheric monofocal lenses at 6 months (B). MRSE, manifest refraction spherical equivalent. Error bars represent 95% CIs.
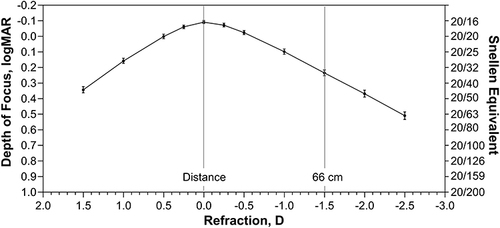
Pooled binocular defocus curve data at 6 months after implantation showed that the aspheric monofocal SN60WF IOL achieved binocular visual acuity of 0.24 logMAR or better from +1.2 to −1.5 D ().
Patient-Reported Outcomes
When using pooled data from the 2 studies, 86% (165/191) of patients reported never needing to wear spectacles for distance vision, and 41% (79/191) reported never needing to wear spectacles for intermediate vision. In one of the studies used in this pooled analysis, 95% (105/111) and 59% (66/111) of patients “never” or “rarely” needed to wear spectacles for distance vision and intermediate vision, respectively. In the other study, 95% (76/80) and 85% (68/80) of patients “never” or “sometimes” wore eyeglasses for distance and intermediate tasks, respectively.
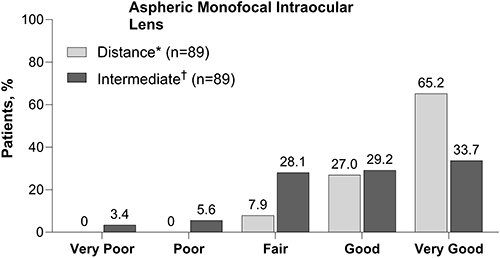
In one study, spectacle-free distance vision under bright conditions was reported as “very good” or “good” by 92% (82/89) of patients at 6 months after implantation. Spectacle-free vision at arm’s length (corresponding to intermediate vision) under bright conditions was reported as “very good” or “good” by 63% (56/89) of patients at 6 months after implantation ().
Safety
At 6 months after implantation, visual disturbances data pooled from QUVID and Quality of Vision Questionnaires from the 2 studies showed that 61%, 79%, and 65% of the patients did not experience starbursts, halos, or glare. Data pooled from both studies showed that 68% of the patients were “not bothered at all” by starbursts or glare and 84% were “not bothered at all” by halos. In the study that used the QUVID questionnaire, severe starbursts, halos, and glare were experienced by 3%, 1%, and 0% of the patients.
One SAE of severe photopsia (negative dysphotopsia) was reported and resulted in IOL explantation. One patient had bilateral negative photopsia that was a nonserious ADE and resolved without treatment.
Discussion
This analysis assessed visual and other outcomes in patients implanted with AcrySof IQ aspheric monofocal IOLs (model SN60WF) targeted for emmetropia. In the cohort of 288 patients, there was excellent distance vision with UDVA of −0.019 ± 0.110 logMAR, as expected with a monofocal IOL, and UIVA of 0.125 ± 0.145 logMAR at 66 cm. These data were generated by combining control IOL results from 2 similar randomized controlled trials.
Intermediate vision has become increasingly important for patient satisfaction due to the rise in reliance on tasks that require functional vision at approximately 66–80 cm. In addition, patients who achieve a high degree of spectacle-free distance vision may prefer not to wear glasses for intermediate vision, as this provides increased freedom from visual aids throughout their day. Multifocal IOLs have been specifically designed to provide good distance, intermediate, and near vision; however, this type of IOL may be associated with visual artifacts including glare, halos, and dysphotopsia.Citation8 EDOF IOLs that achieve ANSI standards provide good distance, intermediate, and functional near visual acuity without dysphotopsia. However, the additional costs associated with these premium lenses may be prohibitive, and they are not appropriate for patients with certain comorbidities. Therefore, there is a need for IOLs that can be accessible globally, with lower costs and less photic phenomena, providing excellent distance visual acuity with some depth of focus into the intermediate range.
SN60WF IOL is a monofocal lens that does not contain EDOF or multifocal optical design features required to provide vision to meet every visual demand at intermediate and near ranges. However, the asphericity of SN60WF IOL provides −0.20 µm spherical aberration, partially correcting the average human cornea with a mean spherical aberration of 0.281 ± 0.086 µm.Citation1,Citation9 Spherical aberration can extend the depth of focus; furthermore, negative spherical aberration has been shown to improve intermediate visual acuity.Citation4,Citation10 The asphericity of SN60WF IOL is designed to maximize the quality of distance visual acuity while retaining depth of focus capable of providing IVA that meets many of the patient’s visual demands at intermediate distances.Citation11
Our results show that on average, the SN60WF IOL can provide patients with either uncorrected or distance-corrected IVA at a level that is sufficient to meet most intermediate visual demands. If achieved, this level of IVA approximately corresponds to “M0.64” or “6” American-point type reading at 66 cm,Citation12 and enables the patient to perform most intermediate tasks, such as viewing a vehicle dashboard, reading from computer screens, and more easily distinguishing details in a store. The data from this pooled analysis show that 83% of the patients receiving binocular aspheric monofocal SN60WF lenses (targeted for emmetropia) achieved uncorrected intermediate vision of 20/32 Snellen or better at 66 cm with minimal dissatisfaction from visual disturbances.
Visual outcomes with aspheric monofocal IOLs may depend, in part, on the individual’s corneal curvature, pupil size, and other physical aspects. The effect of these parameters was not assessed in this pooled analysis; however, one of the studies (the US FDA trial of Vivity versus AcrySof IQ) reported the SN60WF depth of focus in the small and medium pupil size groups to be slightly better than the larger pupil size group.Citation13 Further work should be done to investigate which patients would receive benefit from aspheric monofocal IOLs and which may not, take into consideration the impact of pupil size and other physical correlates.
Although previous studies compared SN60WF and SN60AT, only distance visual acuity was assessed, and IVA data were not generated. Spherical IOLs may provide a greater aberration-based depth of focus than aspheric IOLs; therefore, future studies should compare the IVA achieved with spherical and aspheric IOLs across similar material platforms.
Recently, modified aspheric monofocal IOLs such as TECNIS Eyhance (model ICB00, Johnson & Johnson Vision Care)Citation14,Citation15 and IsoPure (model 123; PhysIOL S.A., Liege, Belgium)Citation16 have become available, and clinical results have been published. These IOLs may be marketed as EDOF IOLs in certain countries; however, they have not demonstrated that they have achieved the combination of IVA and defocus curve criteria stipulated by the ANSI standards for EDOF IOLs.Citation17,Citation18 Similar to SN60WF, these IOLs have aspheric designs that assist with maintaining or modifying the natural corneal spherical aberration and, consequently, the depth of focus achieved. However, the asphericity of these IOLs is either graduated or zonal in their design across the lens.Citation19,Citation20
The reports of IVA with ICB00 vary in published clinical studies. For those studies that included a sample of at least 20 patients (40 eyes) targeted for emmetropia, mean monocular UIVA at 66 cm ranged from 0.16 to 0.29 logMAR at ≥3 months post-implantation, and mean binocular UIVA was 0.07 to 0.17 logMAR.Citation14,Citation17,Citation21–23 In contrast, the intermediate vision achieved with standard aspheric monofocal IOLs (ZCB00 and PCB00, Johnson & Johnson Vision Care) was significantly worse compared with ICB00 (P<0.001), with mean monocular UIVA of 0.27 to 0.40 logMAR and mean binocular UIVA of 0.17 to 0.30 logMAR.Citation14,Citation17,Citation21–23
Published clinical data on IsoPure were more limited at the time of writing; one study (n = 21 patients, 42 eyes) that assessed the IsoPure IOL reported a mean monocular UIVA (66 cm) of 0.24 logMAR and a mean binocular UIVA of 0.22 logMAR at 12 months after implantation.Citation24 A standard monofocal PCB00 had significantly worse monocular and binocular UIVA compared with IsoPure (P<0.001), with mean monocular and binocular UIVA of 0.38 and 0.33 logMAR, respectively.Citation24 Overall, uncorrected intermediate visual acuity at 66 cm achieved under photopic conditions with the aspheric monofocal SN60WF when targeting emmetropia was clinically comparable to the results for Eyhance and IsoPure. Although differences in methodologies make comparison difficult, SN60WF may have achieved better binocular intermediate vision compared with other standard monofocal aspheric IOLs.
The 2 studies analyzed in this pooled analysis had slight variations in the questionnaires; only questions that were identical were combined in the analysis. The combined data found a substantial proportion of patients who achieved spectacle independence for distance (86%; 165/191) and intermediate (41%; 79/191) vision. In one of the studies, a high proportion of AcrySof IQ patients reported “very good” or ‘good’ uncorrected distance (92%; 82/89)) and intermediate (63%; 56/89) vision.
AcrySof monofocal IOLs have been on the market for over 20 years.Citation5,Citation6 This combined analysis of 2 randomized controlled studies found that the AcrySof IQ lens provided 83% of the patients with uncorrected intermediate visual acuity of better than 0.2 logMAR under photopic conditions at 66 cm. This aspheric optical design of AcrySof IQ has since been applied to the Clareon IOL material (Alcon Vision LLC),Citation25 where similar visual outcomes would be expected.
Strengths of this pooled analysis include multicenter design, randomized, controlled, double-masked visual acuity assessments, large sample size, and the use of validated patient-reported outcome measures to assess spectacle use and patient satisfaction. The main limitation of this analysis was the minor differences between the 2 studies, even though the same protocols were applied. However, the 2 studies generally provided similar individual results. The current study did not assess spherical aberration variations in the study population and its contribution to IVA; analyze visual acuity data stratified by pupil size, or compare outcomes with data from spherical IOLs. Future studies should include additional head-to-head comparisons to evaluate the relative benefits of different lens designs and assess potential correlations between corneal spherical aberration and pupil size with the level of intermediate vision achieved. Furthermore, future studies should evaluate whether there is a correlation between postoperative cylinder and sphere power with IVA.
In conclusion, data from a large pool of bilaterally implanted patients from multiple regions indicate that the AcrySof IQ aspheric IOL provides excellent distance vision (mean UDVA, −0.019 ± 0.110 logMAR) and clinically functional intermediate vision for a substantial proportion of patients (83% of the patients achieved binocular UIVA of 20/32). Most patients were not bothered by halos, starbursts, or glare, and a substantial percentage of patients reported spectacle independence for distance (86%) and intermediate vision (41%).
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Disclosure
C. McCabe is a shareholder of Engage Technologies; has received grant/research support from Alcon Vision LLC, Allergan, Bausch & Lomb, EyePoint Pharmaceuticals, Glaukos, Ivantis, Johnson & Johnson Vision, Ocular Therapeutix, and Ora; and is a consultant for Alcon Vision LLC, Allergan, Bausch & Lomb, BVI, Atia Vision, Dompe, Easee, Engage Technologies, EyePoint Pharmaceuticals, Imprimis, Ivantis, Novartis, Ocular Therapeutix, Omeros, Orasis, RxSight, Science Based Health, Sight Sciences, Tarsus, and Zeiss. C. Bala has received support from Alcon Vision LLC and Johnson & Johnson. He is also a consultant for Johnson & Johnson. R. Peterson and J. Hull are employees of Alcon Vision LLC. The authors report no other conflicts of interest in this work.
Acknowledgments
Medical writing assistance was provided by Natalia Zhukovskaya, PhD, of ICON plc (Blue Bell, PA), and was funded by Alcon.
Data Sharing Statement
The data used to support the primary findings of this study are available upon reasonable request from the study sponsor, Alcon Research LLC.
Additional information
Funding
References
- Wang L, Dai E, Koch DD, Nathoo A. Optical aberrations of the human anterior cornea. J Cataract Refract Surg. 2003;29(8):1514–1521. doi:10.1016/S0886-3350(03)00467-X
- Holladay JT, Piers PA, Koranyi G, van der Mooren M, Norrby NE. A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. J Refract Surg. 2002;18(6):683–691. doi:10.3928/1081-597X-20021101-04
- Mikš A, Pokorný P. Spherical aberration of an optical system and its influence on depth of focus. Applied Optics. 2017;56(17):5099–5105. doi:10.1364/AO.56.005099
- Bakaraju RC, Ehrmann K, Papas EB, Ho A. Depth-of-focus and its association with the spherical aberration sign. A ray-tracing analysis. J Optom. 2010;3(1):51–59. doi:10.3921/joptom.2010.51
- US Food and Drug Administration. Clareon Aspheric IOL, Clareon Toric Aspheric IOL, Clareon Aspheric IOL, AutonoMe System, Clareon Toric IOL, AutonoMe System – P190018. Available From: https://www.fda.gov/medical-devices/recently-approved-devices/clareon-aspheric-iol-clareon-toric-aspheric-iol-clareon-aspheric-iol-autonome-system-clareon-toric. Accessed February 8, 2023.
- Karakelle M. The science behind the AcrySof IQ IOL. Cata Ref Surg. 2018;1:1.
- AcrySof® IQ Aspheric Natural IOL. Model: SN60WF. Fort Worth, TX: Product Information. Alcon Laboratories, Inc.; 2017.
- Tchah H, Nam K, Yoo A. Predictive factors for photic phenomena after refractive, rotationally asymmetric, multifocal intraocular lens implantation. Int J Ophthalmol. 2017;10(2):241–245. doi:10.18240/ijo.2017.02.10
- McCabe C, Berdahl J, Reiser H, et al. Clinical outcomes in a U.S. registration study of a new EDOF intraocular lens with a nondiffractive design. J Cataract Refract Surg. 2022;48(11):1297–1304. doi:10.1097/j.jcrs.0000000000000978
- Hervella L, Villegas EA, Robles C, Artal P. Spherical aberration customization to extend the depth of focus with a clinical adaptive optics visual simulator. J Refract Surg. 2020;36(4):223–229. doi:10.3928/1081597X-20200212-02
- Fernández J, Rodríguez-Vallejo M, Burguera N, Rocha-de-Lossada C, Piñero DP. Spherical aberration for expanding depth of focus. J Cataract Refract Surg. 2021;47(12):1587–1595. doi:10.1097/j.jcrs.0000000000000713
- Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi:10.1016/j.jcrs.2004.01.014
- Summary of safety and effectiveness data (SSED). Food and drug administration. 2023; Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf/P930014S126B.pdf. Accessed May 20, 2024.
- Mencucci R, Cennamo M, Venturi D, Vignapiano R, Favuzza E. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: preliminary results. J Cataract Refract Surg. 2020;46(3):378–387. doi:10.1097/j.jcrs.0000000000000061
- Yangzes S, Kamble N, Grewal S, Grewal SPS. Comparison of an aspheric monofocal intraocular lens with the new generation monofocal lens using defocus curve. Indian J Ophthalmol. 2020;68(12):3025–3029. doi:10.4103/ijo.IJO_985_20
- McGlacken-Byrne A, Morris J, Loane E. Clinical results obtained with the new PhysIOL Isopure 123: an isofocal optical design to achieve functional intermediate vision. Investigative Ophthalmology & Visual Science. 2022;63(7):4044–F0008.
- Fernandez J, Rocha-de-Lossada C, Zamorano-Martin F, Rodriguez-Calvo-de-Mora M, Rodriguez-Vallejo M. Positioning of enhanced monofocal intraocular lenses between conventional monofocal and extended depth of focus lenses: a scoping review. BMC Ophthalmol. 2023;23(1):101. doi:10.1186/s12886-023-02844-1
- American National Standard for Ophthalmics -. Extended Depth of Focus Intraocular Lenses. ANSI Z80.35-2018. American National Standards Institute, Inc.. 2018
- TECNIS Eyhance IOL (ICB00). Full Prescribing Information, Johnson & Johnson Surgical Vision. Santa Ana, CA: Inc; 2021.
- ISOPURE 123. Summary of Product Characteristics; BVI Waltham, MA, USA. Liege, Belgium: PhysIOL s.a; 2022.
- Lopes D, Loureiro T, Carreira R, et al. Comparative evaluation of visual outcomes after bilateral implantation of an advanced or conventional monofocal intraocular lens. Eur J Ophthalmol. 2022;32(1):229–234. doi:10.1177/1120672121995343
- Auffarth GU, Gerl M, Tsai L, et al. Clinical evaluation of a new monofocal IOL with enhanced intermediate function in patients with cataract. J Cataract Refract Surg. 2021;47(2):184–191. doi:10.1097/j.jcrs.0000000000000399
- Kang KH, Song MY, Kim KY, et al. Visual performance and optical quality after implantation of a new generation monofocal intraocular lens. Korean J Ophthalmol. 2021;35(2):112–119. doi:10.3341/kjo.2020.1115
- Bova A, Vita S. Clinical and aberrometric evaluation of a new monofocal IOL with intermediate vision improvement. J Ophthalmol. 2022;2022:1. doi:10.1155/2022/4119698
- Lehmann R, Maxwell A, Lubeck DM, et al. Effectiveness and safety of the clareon monofocal intraocular lens: outcomes from a 12-month single-arm clinical study in a large sample. Clin Ophthalmol. 2021;15:1647–1657. doi:10.2147/OPTH.S295008