Abstract
Purpose
To examine short-term effects of ranibizumab versus bevacizumab on reduction of optical coherence tomography (OCT) central macular thickness (CMT) in patients with macular edema secondary to retinal vein occlusions (RVOs).
Methods
This is a retrospective analysis in which patients with RVOs were injected with either bevacizumab or ranibizumab. At 2 weeks, all patients were injected with a dexamethasone intravitreal implant (Ozurdex®). CMT on OCT and best-corrected visual acuity were obtained at baseline, at 2 weeks (just prior to the dexamethasone intravitreal implant), and 6 weeks.
Results
Sixty-four patients received injections (32 bevacizumab; 32 ranibizumab). At 2 weeks, bevacizumab group had a mean (±standard error of mean [SEM]) CMT reduction of 26.2% ± 3.4% versus 47% ± 3.5% reduction with ranibizumab (P < 0.0001). At 6 weeks, there was a 31.6% ± 3.2% CMT reduction with bevacizumab versus 52% ± 3.2% with ranibizumab (P < 0.0001). At 2 weeks, 15 (9%) of bevacizumab patients versus 25 (78.1%) ranibizumab patients achieved OCT CMT < 300 μm (P = 0.0192). At 6 weeks, 18 (56.3%) of bevacizumab compared to 30 (93.8%) of ranibizumab patients achieved CMT < 300 μm (P = 0.0010). Visual acuity was not significantly different at each time interval between the groups.
Conclusion
Ranibizumab appears to have a greater effect in the short-term of decreasing macular edema on OCT when compared to bevacizumab in patients with RVOs.
Introduction
Affecting an estimated 180,000 eyes per year in the United States, retinal vein occlusions (RVOs) are the second most common type of retinal vascular disorders.Citation1,Citation2 Branch RVOs (BRVOs) comprise approximately 80% of these, but both BRVOs and central retinal vein occlusions (CRVOs) contribute to significant vision loss, mostly as a result of macular edema.Citation1–Citation3 The Branch Vein Occlusion Study (BVOS) group helped to establish grid laser as the treatment standard for appropriate patients with macular edema, with this being the only proven beneficial treatment for many years.Citation4 Following this, the Central Vein Occlusion Study (CVOS) group found that grid laser did in fact decrease macular edema, but did not demonstrate a statistically significant difference in visual acuity (VA) when compared to observation alone.Citation5
In more recent years, studies have demonstrated significantly elevated levels of vascular endothelial growth factor (VEGF) in eyes with RVOs.Citation6–Citation8 These findings, along with the successful use of anti-VEGF medications for neovascular age related macular degeneration (ARMD), led to further studies investigating the use of anti-VEGF agents for the treatment of macular edema secondary to BRVO and CRVO. Ranibizumab (Lucentis®, F. Hoffmann-La Roche Ltd, Basel, Switzerland) is a fragment, antigen binding (Fab) antibody that binds all forms of active VEGF-A, effectively reducing its actions on vascular endothelial cells. Both the BRAVO trial and the CRUISE trial demonstrated the effectiveness of intraocular injections of ranibizumab in improving best-corrected VA (BCVA) and central foveal thickness (CFT) in BRVOs and CRVOs, respectively. This led to US Food and Drug Administration (FDA)-approval for use of ranibizumab for treatment of macular edema following retinal vein occlusions.Citation3,Citation9
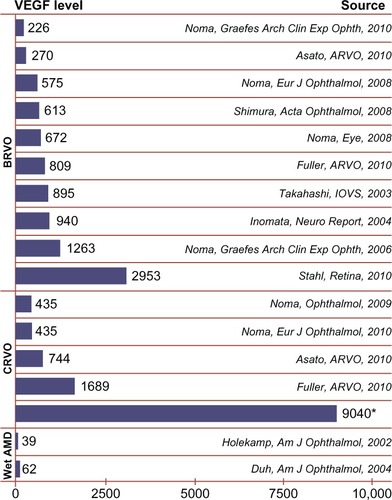
Bevacizumab (Avastin®, F. Hoffmann-La Roche Ltd), a full length monoclonal antibody that also binds all forms of VEGF-A, has been used extensively off-label to treat macular edema secondary to BRVOs and CRVOs, as well as diabetic macular edema and neovascular ARMD. A recent review of several trials indicates that intravitreal bevacizumab improves VA and reduces CFT in macular edema associated with BRVOs.Citation10 The Comparison of Age related Macular Degeneration Treatments Trials (CATT) has demonstrated equal effectiveness of bevacizumab versus ranibizumab for the treatment of neovascular ARMD in terms of VA.Citation11 However, numerous studies have shown that VEGF levels are much higher in eyes with BRVOs, and highest in eyes with CRVOs when compared to eyes with ARMD, indicating a difference in the pathogenesis of the edema (). This also explains why macular edema is more difficult to treat in CRVO patients. Despite their similar actions, bevacizumab and ranibizumab are different molecules, with different behaviors and properties. Case reports in the literature have shown that ranibizumab may have a stronger effect in resolving macular edema in RVOs when compared to bevacizumab.Citation12 Given these differences it is necessary to investigate the use of these drugs in RVOs as a clinical entity separate from neovascular ARMD. The purpose of this study was to evaluate the very short-term effects of intravitreal bevacizumab (Avastin®) versus ranibizumab (Lucentis®) on reducing central macular thickness (CMT) in patients with RVOs.
Figure 1 Meta-analysis of VEGF levels among different diseases as determined by vitreous sampling.
Abbreviations: AMD, age related macular degeneration; BRVO, branch retinal vein occlusions; CRVOM, central retinal vein occlusion; VEGF, vascular endothelial growth factor.
Methods
An institutional review board approved retrospective chart review was performed at a single center in which the charts of patients who underwent combination therapy using an anti-VEGF agent, bevacizumab or ranibizumab, and dexamethasone intravitreal implant (Ozurdex®, Allergan Pharmaceuticals, Irvine, CA, USA) during the period of 2009–2012, were evaluated. The patients were part of a subset analysis of a combination trial in which patients diagnosed with RVOs received an intravitreal injection of 0.50 mg (in 0.05 mL of solution) for ranibizumab, and 1.25 mg (in 0.05 mL of saline) for bevacizumab at baseline, followed by a scheduled Ozurdex® implant 2 weeks later.Citation13 Patients met inclusion criteria for analysis if this was their first RVO, or if the previous anti-VEGF therapy was at least 6 weeks prior, and CMT was greater than 300 μm on spectral domain OCT (SD-OCT). Exclusion criteria included history of vitrectomy, rubeosis, or advanced glaucoma. The anti-VEGF agent injected was mostly determined by insurance coverage.
Patients were initially evaluated using best-corrected Snellen VA and SD-OCT (Zeiss Cirrus, Dublin, CA, USA) at baseline prior to injection of either bevacizumab or ranibizumab. Patients were then reevaluated 2 weeks after initial injection, at which time SD-OCT and VA were repeated. All patients received Ozurdex® at the 2-week visit as well. Six weeks after initial injection, a similar evaluation was repeated. The primary outcome measure was the resolution of initial edema as defined by CMT < 300 μm, at 2 weeks and 6 weeks after intravitreal injection of either ranibizumab or bevacizumab; and to examine if the addition of a second medication increases the number of patients who have resolution of their macular edema. Secondary outcomes included CMT reduction from baseline and VA. A two-tailed t-test was used to compare the outcome measures between the groups at baseline, 2 weeks, and 6 weeks (4 weeks after dexamethasone implant). A Fisher’s exact test was used to compare the number of patients that achieved CMT < 300 μm. A repeated measures analysis of variance (ANOVA) was used to examine each group over the time intervals.
Results
Sixty-four patients were included in the study and followed from baseline to 6 weeks. Thirty-two patients received bevacizumab and 32 patients received ranibizumab. In the bevacizumab group, the mean age of the patient was 72 years ± 2.5 years, with 69% female and 31% male. In the ranibizumab group, the mean age of the patient was 76 years ± 2.1 years, also with 69% female and 31% male. Eleven patients had a CRVO and 21 had a BRVO in the bevacizumab group, versus 9 CRVOs and 23 BRVOs in the ranibizumab group ().
Table 1 Baseline characteristics of the two groups
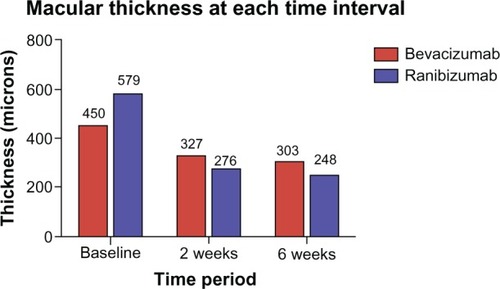
CMT at baseline in the bevacizumab group ranged from 309 μm to 763 μm with a mean of 450.8 μm ± (standard error of the mean [SEM]) 21.3 μm, and in the ranibizumab group 314 μm to 988 μm with a mean of 579.3 μm ± 35.6 μm. In terms of VA, the preinjection bevacizumab group ranged from logMAR of 0.1 to 1.8 with a mean of 0.71 ± 0.07(Snellen 20/100–). The preinjection ranibizumab group ranged from logMAR of 0.2 to 2.3 with a mean of 0.89 ± 0.1 (Snellen 20/160+) ().
Table 2 OCT central macular thickness with breakdown of BRVO and CRVO interval
At 2 weeks postinjection, the mean CMT decreased to 327 μm ± 20.0 μm in the bevacizumab group and 276 μm ± 9.2 μm in the ranibizumab group (). The mean percent change from baseline to 2 weeks was -26.24% ± 3.4% in the bevacizumab group and -47% ± 3.5% in the ranibizumab group (P < 0.0001). The mean 2 week logMAR for the bevacizumab group was 0.53 ± 0.5 (20/60–) compared with 0.58 ± 0.1 (20/80+) in the ranibizumab group (P = 0.6154; ).
Figure 3 Mean logMAR at time intervals.
Abbreviations: VA, visual acuity; logMAR, logarithm of minimal angle of resolution.
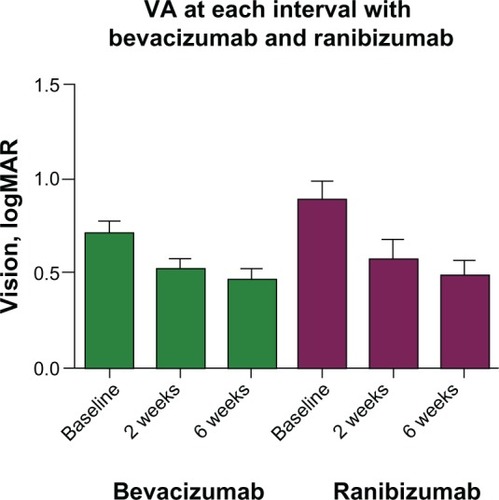
At 6 weeks postinjection, the mean CMT for bevacizumab versus ranibizumab was 303.3 μm ± 18.7 μm and 248.3 μm ± 8.3 μm, respectively. The mean percent change from baseline to 6 weeks was −31.58% ± 3.2% in the bevacizumab group and −52.10% ± 3.2% in the ranibizumab group (P < 0.0001). The mean 6 week logMAR for the bevacizumab group was 0.47 ± 0.05 (20/60+) compared with 0.49 ± 0.07 (20/60–) in the ranibizumab group (P = 0.7767; ).
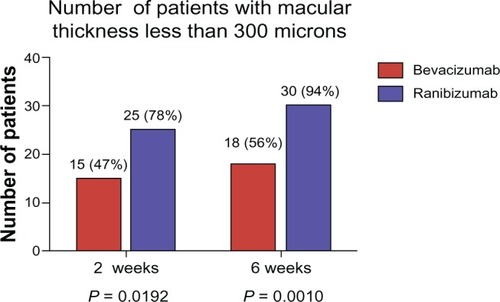
At 2 weeks, 15 patients (46.9%) in the bevacizumab group achieved a CMT < 300 μm versus 25 patients (78.1%) in the ranibizumab group (P = 0.0192). At 6 weeks, 18 patients (56.3%) in the bevacizumab group achieved or maintained CMT < 300 μm compared to 30 patients (93.8%) in the ranibizumab group (P = 0.0010) ( and ).
Figure 4 Scatter plots showing CMT of each patient at baseline, 2 weeks, and 6 weeks.
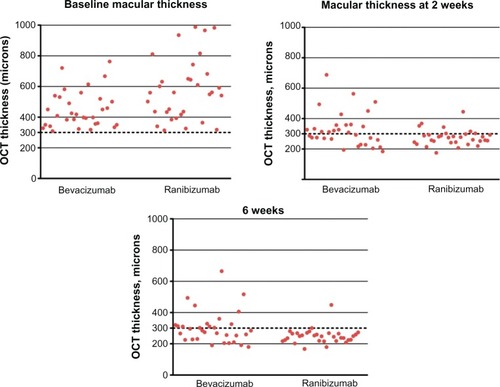
Figure 5 Total number of patients in each group with a CMT < 300 μm.
The bevacizumab and ranibizumab groups both had a statistically significant reduction in CMT (bevacizumab P < 0.0001; ranibizumab P < 0.0001) and improvement in logMAR VA (bevacizumab P < 0.0001; ranibizumab P < 0.0001) over each of the time intervals.
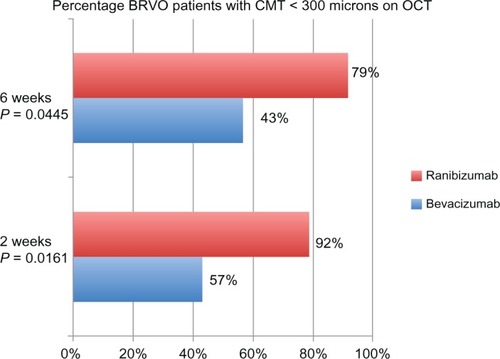
In patients who had BRVOs, at 2 weeks, there was a statistically significant difference in the amount of reduction of CMT with ranibizumab versus bevacizumab (P = 0.0070). In addition, 9/21 (43%) in the bevacizumab group versus 19/24 (79%) in the ranibizumab group achieved a CMT < 300 μm (P = 0.0161). At 6 weeks, there was also a significant difference in the reduction of CMT with ranibizumab versus bevacizumab (P = 0.006). In terms of OCT CMT < 300 μm, 12/21 (57%) patients in the bevacizumab group achieved this goal compared to 22/24 (92%) in the ranibizumab group (P = 0.0132) ().
Figure 6 Percentage of BRVO patients with CMT less than 300 μm at 2 and 6 weeks.
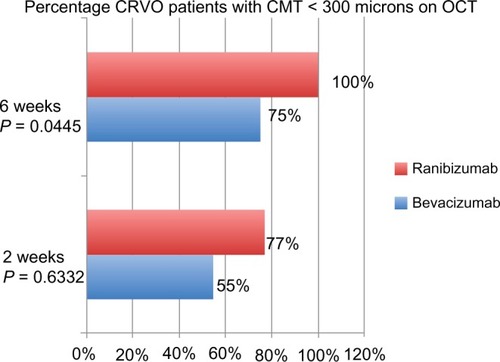
When looking at CRVO patients, at 2 weeks there was a statistically significant difference in the mean percentage of reduction of macular edema with ranibizumab versus bevacizumab (P = 0.0025). In terms of the macula being “dry”, 6 patients in each group (of 11 total bevacizumab and 8 total ranibizumab) had a CMT < 300 μm at 2 weeks (P = 0.6332). At 6 weeks, there was a statistically significant difference in the mean percentage of reduction of macular edema with ranibizumab versus bevacizumab (P = 0.0081). The number of patients in the bevacizumab group < 300 μm remained the same (6), while all of the patients (8) in the ranibizumab group were < 300 μm (P = 0.0445) (). There was no statistically significant difference in the logMAR VA between the bevacizumab and ranibizumab groups with subgroup analysis of BRVO versus CRVO patients.
Figure 7 Percentage of CRVO patients with CMT less than 300 μm at 2 and 6 weeks.
Fourteen of the 32 patients in the bevacizumab group had received previous injection with bevacizumab greater than 6 weeks prior. Of these 14 patients, seven achieved a CMT < 300 μm at 2 weeks ().
Table 3 OCT analyses of previously treated patients with bevacizumab
Discussion
In this study comparing the short-term effects of bevacizumab versus ranibizumab for macular edema in patients with RVOs, ranibizumab appears to be more effective in terms of reducing CMT. The CATT 2-year results demonstrated that bevacizumab and ranibizumab are essentially equal for treatment of neovascular ARMD.Citation11 However, the VEGF levels found in the vitreous in BRVOs and especially CRVOs are significantly higher than in ARMD (). Given these differences, the CATT data cannot be generalized to the macular edema in RVOs. Because of the higher VEGF levels, ranibizumab’s higher affinity for the VEGF molecule may help to explain why it proves to be more effective in reducing CMT in this study.Citation14 In addition, our study only looks at very short-term data (2 weeks and 6 weeks), which may have the advantage of evaluating the anti-VEGF molecules at their time of maximum effectiveness as opposed to the cumulative effects of monthly dosing. Some have proposed that dosing every 2 weeks versus 4 weeks may be necessary for bevacizumab in cases with persistent or rebound macular edema due to overwhelming VEGF in the vitreous.Citation15 This study allows us to compare these two agents at the 2-week interval.
In the BRAVO and CRUISE trialsCitation3,Citation9, ranibizumab was able to decrease baseline retinal edema by a mean of more than 250 μm as early as 7 days after treatment, (the earliest measured time point after injection per protocol) with even more effect at 1 month, continuing to 6 months. In addition, for CRVOs, ranibizumab was able to decrease excess foveal edema from a mean of greater than 300 μm at baseline, to approximately 100 μm at 1 month. In the BRAVO trial, ranibizumab decreased excess foveal edema from a mean of almost 280 μm at baseline, to approximately 150 μm at 1 week, and less than 100 μm at 6 months.Citation3,Citation9 This data is based on Stratus OCT with an assumed mean foveal thickness of 212 μm.Citation16 In clinical practice, the physician wants to know “is the macula dry?” The closest numerical surrogate to this is central field thickness measurement. In Stratus, this is commonly considered 250 μm (which is two standard deviations from the mean), and 300 μm in Cirrus. For the purposes of this study in looking at CMT, 300 μm was used as a cut-off for resolving macular edema. The percentage of patients who reached CMT < 00 μm, was 56.3% in the bevacizumab group versus 93.8% in the ranibizumab group at the 6 week interval (4 weeks post Ozurdex®). This difference in the number of patients who reached CMT < 300 μm between the two groups was statistically significant at both the 2 week and 6 week interval. In addition, when looking at overall percent change in CMT, ranibizumab had a statistically significant (P < 0.0001) greater reduction in CMT at the 2 week and 6 week intervals when compared to bevacizumab. Despite the fact that the ranibizumab group started with a higher overall baseline CMT, more patients in the ranibizumab group (93.8% versus 56.3%) achieved a CMT < 300 μm. In terms of VA, there was no statistically significant difference between the two groups at baseline and each of the intervals. However, both bevacizumab and ranibizumab showed a statistically significant improvement in CMT and logMAR VA. It is interesting to note that despite the significant difference in CMT reduction between the two treatment groups, the VA was not significantly different at any of the intervals. This may be due to other variables not examined in this study such as duration of edema, degree of ischemia, or anterior segment opacities. Given that Snellen VA has less variation in possible data points when compared to OCT thickness measurements, a larger study with more patients may be more appropriately powered to find differences in VA. In addition, this study was designed to mimic clinical practice using Snellen as opposed to Early Treatment Diabetic Retinopathy Study (ETDRS) refractions and patients were not “pushed” to see as many letters as possible.
The differences in reduction of CMT when comparing the patients with CRVO versus BRVO further supports the thought that ranibizumab may be more effective in treating macular edema in disease processes with higher VEGF levels. At 2 weeks, in the patients with CRVO, there was a statistically significant difference between the two groups in terms of percentage reduction of edema but not in number of patients with OCT CMT < 300 μm. However, at 6 weeks, all 8 patients with CRVO in the ranibizumab group had a CMT < 300 μm versus 6/11 patients who received bevacizumab. This may indicate that over time, bevacizumab may not provide as much of a sustained effect in the presence of higher VEGF levels. However, additional studies with larger numbers would be needed to better evaluate these effects.
As research in this area continues to grow, and more medications are developed for treating macular edema, it will become increasingly necessary to tailor treatments to the specific disease process and patient. Applying appropriate agents, alone or in combination, based on levels of chemical mediators involved in the pathogenesis of macular edema will allow us to achieve the best possible results for our patients.
There are some limitations to this study including its retrospective nature, smaller sample size, and the fact that it was carried out at a single center. In addition, patients were not randomized, as the agent injected was mostly determined by insurance coverage. This can lead to some confounding variables that may not be taken into account in this study. One other confounder was that all patients received an Ozurdex® implant at the 2 week visit, which may make the 6 week data more difficult to interpret in terms of comparing bevacizumab and ranibizumab. However, this would not affect the 2 week data. In addition, it is interesting to note that even with the combination therapy, the differences noted for the CMT persisted 4 weeks after the dexamethasone implant. Another limitation is that some of the patients in the bevacizumab group were previously treated ( and ). However, less than half of the bevacizumab patients were pretreated (14 of 32), and of those, 50% achieved a CMT < 300 μm. This percentage is actually slightly higher than the overall percentage of all of the bevacizumab patients that achieved a CMT < 300 μm (47%).
Despite these limitations, we must take into consideration the fact that bevacizumab and ranibizumab are different molecules, and their differences may only become apparent in disease processes with higher levels of VEGF. Based on our study, in RVOs ranibizumab may have a more effective role in reducing CMT when compared to bevacizumab. Further study is needed to clarify longer-term data, and to provide comparison in a prospective, randomized manner. The CRAVE trialCitation17 (bevacizumab versus ranibizumab in treatment of macular edema from vein occlusion) is currently being carried out at other centers with a larger number of patients and will hopefully further answer this question.
Disclosure
MA Singer is a consultant for Genentech, Allergan, Regeneron, Acucela, Santen, and Thrombogenics, and receives research support from Optos, Neovista, Eyegate, Ohr. The other authors report no conflicts of interest in this work.
References
- KleinRMossSEMeuerSMKleinBEThe 15-year cumulative incidence of retinal vein occlusion. The Beaver Dam eye studyArch Ophthalmol200812651351818413521
- US Census BureauAnnual Estimates of the population by sex and five-year age groups for the United States: April 1, 2000 to July 1, 2007 NC-EST2007-01. Release Date: May 1, 2008 Available at: https://www.census.gov/prod/2008pubs/p70-117.pdfAccessed February 16, 2010
- CampochiaroPAHeierJSFeinerLRanibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III studyOphthalmology20101171102111220398941
- [no authors listed]Argon laser photocoagulation for macular edema in branch vein occlusion. Branch Vein Occlusion Study GroupAm J Ophthalmol1984982712826383055
- [no authors listed]Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M reportOphthalmology1995102142514339097788
- NomaHFunatsuHYamasakiMPathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6Am J Ophthalmol200514025626116086947
- NomaHFunatsuHMimuraTAqueous humor levels of vasoactive molecules correlate with vitreous levels and macular edema in central retinal vein occlusionEur J Ophthalmol20102040240919967679
- CampochiaroPAHafizGShahSMRanibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulatorMol Ther20081679179918362932
- BrownDMCampochiaroPASinghRPRanibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III studyOphthalmology20101171124113320381871
- YilmazTCordero-ComaMUse of bevacizumab for macular edema secondary to branch retinal vein occlusion: a systematic reviewGraefes Arch Clin Exp Ophthalmol201225078779322539192
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research GroupRanibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year resultsOphthalmology20121191388139822555112
- LabriolaLTSaddaSRRapid resolution of macular edema associated with central retinal vein occlusion using ranibizumab after failure with multiple bevacizumab injectionsSemin Ophthalmol20112638739122044337
- SingerMABellDJWoodsPEffect of combination therapy with bevacizumab and dexamethasone intravitreal implant in patients with retinal vein occlusionRetina2012321289129422466480
- PieramiciDJRabenaMDAnti-VEGF therapy: comparison of current and future agentsEye (Lond)2008221330133618497829
- StewartMWRosenfieldPJPenhaFMPharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and afibercept (vascular endothelial growth factor Trap-eye)Retina20123243444357
- ChanADukerJSKoTHNormal macular thickness measurements in healthy eyes using Stratus optical coherence tomographyArch Ophthalmol200612419319816476888
- Barnes Retina InstituteBevacizumab Versus Ranibizumab in Treatment of Macular Edema From Vein Occlusion (CRAVE)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 [updated February 9, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT01428388Accessed May 20, 2013
- SingerMABellDJPorbandarwallaSIschemia and VEGF in different retinal diseases and therapies: how does one influence the other?Retinal Physician201292830