Abstract
Background
Retinal detachment is a major postsurgical threat in pediatric cataract surgery; however, the effect of axial length remains unclear. This study aimed to assess the relationship between axial length and detachment risk in vulnerable patients.
Methods
This retrospective cohort study analyzed 132 eyes of 84 pediatric cataract surgery patients aged <20 years old. Axial length was measured preoperatively, and the incidence of retinal detachment was recorded over a median follow-up of 4 years. Logistic regression analysis was used to examine the axial length-detachment relationship.
Results
Twenty eyes had postoperative retinal detachments. The median axial length was longer in the detachment group (23.6 mm) than in the non-detachment group (21.6 mm). Eyes with axial length ≤23.4 mm had 0.55-fold decreased odds of detachment compared to longer eyes. Preexisting myopia and glaucoma confer heightened risk. Approximately half of the patients retained some detachment risk eight years postoperatively.
Conclusion
Shorter eyes (axial length ≤23.4 mm) appear to be protected against pediatric retinal detachment after cataract surgery, whereas myopia, glaucoma, and axial elongation > 23.4 mm elevate the postoperative risk. Understanding these anatomical risk profiles requires surgical planning and follow-up care of children undergoing lensectomy.
Plain language Summary
This study investigated the protective role of a shorter axial length in preventing retinal detachment after pediatric cataract surgery. This highlights the correlation between smaller eye sizes and reduced detachment risk, emphasizing the need for careful consideration of anatomical factors in surgical planning and patient monitoring, particularly for patients with preexisting myopia or postoperative glaucoma.
Introduction
Congenital cataract surgery within the first year of life is imperative for preventing permanent visual impairment due to amblyopia. Despite advances in surgical techniques, postoperative complications such as retinal detachment (RD) remain a threat to long-term visual prognosis.Citation1,Citation2 Previous pediatric studies have identified young age at surgery, surgical complexity, and postoperative inflammation as potential risk factors for RD.Citation3–5 However, research on the role of the underlying ocular anatomy and axial elongation is lacking.
In adults, increased axial length (AL) is an established contributor to RD development after cataract procedures.Citation6–9 Biomechanical changes due to axial elongation are hypothesized to cause vitreous liquefaction and alter vitreoretinal adhesion, producing an environment conducive to retinal tears and subsequent detachment.
Multiple studies have examined the association between AL and RD risk after pediatric cataract surgery. Agarkar et al demonstrated that myopic AL confers significantly increased postoperative RD susceptibility in this population.Citation3 This is consistent with the findings of Al Muammar, who also identified high AL as a significant risk factor for RD after cataract surgery in highly myopic patients.Citation10 However, Malukiewicz found that RD surgery can cause an increase in AL, which may further complicate the relationship between AL and RD.Citation11 A small case series found that most children who developed detachment after congenital cataract surgery had AL of 22 to 24 mm.Citation3 Despite the substantial evidence linking axial elongation with RD after adult cataract surgery, few studies have specifically investigated the role of ocular anatomy and AL as contributing factors to detachment risk profiles after congenital cataract removal in children. However, the relationship between AL and detachment rates in this unique subpopulation remains unclear.
Understanding anatomical risk factors, such as AL, is critical, as detachment threatens vision rehabilitation and quality of life in pediatric patients. For example, a study found that 7% of children developed RD within 20 years after congenital cataract surgery, emphasizing its long-term implications.Citation12 Preoperative assessment of the detachment risk profiles based on biometry can optimize surgical plans and follow-up protocols.
This retrospective cohort study aimed to delineate the association between preoperative AL and RD rates after congenital pediatric cataract surgery.
We hypothesized that increased preoperative AL would predict an elevated detachment risk postoperatively, providing a target for risk-reduction interventions. The results delineate anatomical risk profiles to optimize surgical planning and follow-up care for children undergoing cataract extraction. Understanding axial elongation helps ophthalmologists tailor early intervention and myopia control strategies, such as atropine drops and specialized lenses, and recommend lifestyle changes to slow progression and prevent complications.
Methods
Study Design
This retrospective cohort study used a two-arm design to assess the relationship between AL and the incidence of RD following congenital cataract surgery in pediatric patients. This study was conducted from June 2014 to December 2022 and aimed to compare children who developed RD post-surgery with those who did not.
Participants
The participants in this study were patients under 20 years of age who had been diagnosed with congenital cataracts as well as those who had undergone cataract surgery before the age of 6 years and had been followed up for at least 2 years. To be included in the study, participants had to have a confirmed diagnosis of congenital cataracts and receive surgical treatment within the specified time frame. Those who were excluded from the study had a history of previous ocular surgery, additional congenital anomalies affecting the eye, systemic diseases that affected the eye, or incomplete data during the follow-up period.
Study Setting
This study was conducted at the Pediatric Ophthalmology and Strabismus Division of the King Khaled Eye Specialist Hospital (KKESH), a high-volume tertiary eye care center equipped with advanced pediatric ophthalmology facilities.
Interventions
Preoperative AL measurements were obtained by an experienced ophthalmology technician under general anesthesia using standardized ophthalmic ultrasound equipment. The equipment used for non-immersion ultrasound biometry typically consists of an A-scan ultrasound device with a probe. The patients were positioned supine with their eyes facing directly upwards. Topical anesthesia was administered, and the eyelids were gently held open with an eyelid speculum. The ultrasound probe tip was coated with a small amount of coupling gel. The probe was carefully positioned close to the cornea, without any contact. The ultrasound device was activated, and sound waves were emitted from the probe. Alignment of the probe with the optical axis of the eye is critical. Multiple measurements were taken to ensure accuracy and consistency. Specific formulas were then used to calculate the appropriate IOL power based on the biometric data obtained. Following the measurements, the eye was cleaned to remove any remaining gel and the patient was prepared for surgery.
The eye was prepared and antisepticized. An operating microscope was positioned to enhance visibility, and a small incision was made at the limbus or clear cornea to access the anterior chamber. A circular, curvilinear, continuous capsulorhexis opening was created, and the cataractous lens was carefully removed by aspirating soft lens material. To ensure favorable phacoemulsification results and reduce the risk of complications, it was essential to maintain a stable surgical chamber. The machine’s configuration enabled precise adjustments of the settings to control intraocular pressure (IOP) during lens aspiration. By providing a hermetically sealed environment, the procedure for lens removal was facilitated, thereby ensuring the safety and predictability of the surgery. Following the removal of the lens, a posterior capsulotomy and an anterior vitrectomy were conducted on all subjects in the study to extract a portion of the vitreous body.
Depending on factors such as patient age, eye anatomy, and surgical outcomes, the surgeon determines whether to implant an IOL. In KKESH, patients must be at least 15 months old to receive IOL in the same setting. If the capsular bag is intact, IOL is typically placed within the capsular bag. If the capsular bag is not suitable, IOL may be placed in the ciliary sulcus.
The incisions were then sealed either by self-sealing or using absorbable sutures. After surgery, patients received instructions for postoperative care, including topical antibiotics and steroids, as well as follow-up appointments to monitor healing and visual rehabilitation.
Data Collection
AL taken at the time of cataract surgery was used as the variable to be studied, along with demographic data, presence, or absence of myopia, which is a refractive state characterized by an elongated eye with a longer refractive power, resulting in light being focused in front of the retina when the eye is relaxed. We defined it as a spherical equivalent equal to or higher than −1 diopter, determined by retinoscopy when lens transparency allows it. Retinal details, surgical information, and follow-up observations were also obtained. The data were retrieved from the electronic medical record system and transferred to a preestablished dataset formatted in Microsoft Excel (Microsoft Corp, Redmond, WA, USA) before being imported into Stata version 17 (StataCorp LLC, College Station, TX) for statistical analysis. Pertinent clinical variables, such as medical comorbidities, additional ocular conditions, and surgical factors, were also examined in relation to outcomes. Postoperative retinal assessments with dilated fundus examination were conducted using indirect ophthalmoscopy at regular intervals at each visit.
Sample Size
The sample size was calculated to have the capacity to discern a substantial distinction in the frequency of RD, with a significance level of 0.05, power of 80%, effect size of 0.5, and ratio of sample size unexposed/exposed to 4. Based on preliminary data, it was estimated that the probability of an event occurring in the exposed group was 0.2, whereas it was assumed to be 0 in the unexposed group. Hence, the theoretical sample size comprised of 15 eyes in the RD group and 60 eyes in the non-RD group, resulting in a total of 75 eyes.
Statistical Analysis
Descriptive statistics were employed to succinctly summarize the characteristics of the collected variables. Frequencies and percentages constituted the primary measures for detailing the distribution of the categorical variables. For continuous variables, central tendency and dispersion were articulated using median and interquartile range (IQR). Inferential statistical techniques were meticulously applied to ascertain the relationship between AL and RD incidence while controlling for potential confounding variables. Fisher’s exact test was used for the analysis of categorical data, and the Wilcoxon rank test was employed for the examination of nonparametric distributions. Furthermore, logistic regression was employed to evaluate the relationship between AL and RD. This methodology offers a comprehensive risk assessment for all subjects, as opposed to solely focusing on the subset of individuals with RD and thereby provides a comprehensive perspective on post-surgical risks.
The Kaplan-Meier method was used for survival analysis.
Ethical Considerations
Ethical approval was obtained from the Institutional Review Board of King Khaled Eye Specialist Hospital (Protocol 23093-R). As the study was conducted retrospectively, the requirement for informed consent was waived. The principles outlined in the Declaration of Helsinki were adhered to throughout the study, and measures were taken to ensure confidentiality and protection of participant data.
Results
This study assessed 132 eyes from 84 pediatric patients who underwent cataract surgery. Among the 132 eyes, 112 belonged to patients who did not experience RD during the follow-up period. The remaining 20 eyes were of patients who developed RD after surgery (). The overall median duration of follow-up for all patients was 4 years, with an IQR of 2–7 years. Specifically, for those who experienced RD, the median duration of follow-up from cataract surgery until the detachment event was 7 years, with an IQR of 1 to 13 years. The maximum follow-up period was 20 years.
Table 1 Associations Between Clinical Characteristics and Retinal Detachment After Cataract Surgery in Children: A Statistical Analysis
Relevant baseline features were compared between the RD and non-RD groups (). Patients who ended up with detachment were significantly older at baseline, with a median age of 11 years versus 8 years in the non-detachment group (p=0.003). No other baseline demographic or clinical characteristics showed any significant between-group differences. Specifically, there were no statistically significant differences found in the median age at initial cataract diagnosis/surgery or in the surgical procedure duration between the groups.
Table 2 Comparative Analysis of Age and Timelines in Pediatric Patients with and without Retinal Detachment Following Cataract Surgery
The multivariable logistic regression analysis yielded findings that demonstrated age and AL were not statistically significant predictors of RD. Specifically, age showed an odds ratio of 0.97, with a 95% confidence interval ranging from 0.6 to 1.5 (p =0.889), while AL showed an odds ratio of 1.06, with a 95% confidence interval between 0.8 and 1.3 (p = 0.662). Additionally, the interaction term between age and AL, with an odds ratio of 1.00 (p = 0.552), did not significantly contribute to the model.
The primary outcome measure was AL obtained before surgery. Patients who developed postsurgical RD had notably longer AL at baseline than those who did not develop RD. The median AL in the detachment group was 23.6 mm (IQR 20.7 to 26.7 mm) versus 21.6 mm (IQR 20.7 to 23.2 mm) in the non-detachment group, a statistically significant difference (p=0.027).
Further analysis demonstrated that AL ≤ 23.4 mm were associated with significantly decreased risks of RD, with odds ratios ranging from 0.47 to 0.72 (p<0.05) (). The relationship between AL and the odds of RD was quantified by odds ratios starting at 0.47 for an AL of 22 millimeters, indicating a lower initial probability of RD. This probability undergoes a marginal increase, with odds ratios fluctuating slightly between 0.54 and 0.55 up to 22.7 millimeters. A noticeable upsurge in the likelihood of RD is observed from 22.8 millimeters, with odds ratios ascending to 0.68 and sustaining until 23.2 millimeters. The upward trend continues, as demonstrated by the rise in odds ratios to 0.83 at an AL of 24 millimeters, suggesting a progressively increasing risk of RD. The orange line served as a means of illustrating the p-values associated with the odds ratios for AL ranging from 22 to 24 millimeters. This line represents a statistical significance metric, with fluctuations in its position indicating the variability of the odds ratios at different AL. It is worth noting that when the orange line dips below the 0.05 threshold (represented by the red dot line), it indicates a statistically significant divergence from the null hypothesis for AL equal to or less than 23.4 millimeters.
Figure 1 Trend and Statistical Significance of Odds Ratios for Axial Length Based on p-values in Unadjusted Logistic Regression Analysis. The graph displays a dual-line representation of the relationship between the AL and the odds ratio in an unadjusted logistic regression analysis. The X-axis measures the AL in millimeters, while the first Y-axis (blue line with diamond spots) represents the odds ratios associated with each AL. This line demonstrates how the odds ratio changes as the AL increases, permitting the identification of trends such as increasing or decreasing probabilities related to different AL. The second Y-axis (Orange line with square spots) displays the corresponding p-values for each AL. The red dot line indicates the statistical significance of the odds ratios at various points, representing a p-value of ≤ 0.05.
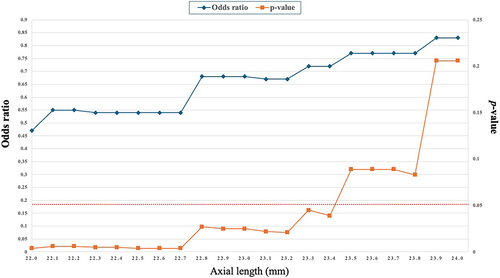
Patients who developed postsurgical RD had notably longer AL at baseline than those who did not develop RD. The median AL in the detachment group was 23.6 mm (IQR 20.7 to 26.7 mm) versus 21.6 mm (IQR 20.7 to 23.2 mm) in the non-detachment group, a statistically significant difference (p=0.027). Further analysis demonstrated that AL ≤ 23.4 mm were associated with significantly decreased risks of RD, with odds ratios ranging from 0.47 to 0.72 (p<0.05).
The median (IQR) of myopic patients’ diopters in the non-RD group was 6 diopters (3, 7), while in the RD group it was 6.5 diopters (5, 8), with a p-value of 0.387.
The analysis of the relationship between cataract morphology and the incidence of RD revealed a p-value of 0.041 through the application of Fisher’s exact test, indicating a significant association considering the limited sample sizes present in certain categories.
offers a comparative examination of the connection between various morphologies of cataracts and the prevalence of RD. The results indicate that nuclear cataracts were the most observed type in patients without RD, while no cases of RD were observed in patients with posterior polar cataracts. Statistically significant associations with RD were found for nuclear and cortical cataracts, with the latter showing a markedly high odds ratio of 31.0, suggesting a substantial increase in risk. It is important to note that while lamellar cataracts showed an elevated odds ratio, this did not reach statistical significance, and there was insufficient data to draw meaningful conclusions about other cataract types.
Table 3 Association Between Cataract Types and the Incidence of Retinal Detachment: Odds Ratios and Statistical Significance
In one case, suturing of the corneal wound was not performed. This patient did not exhibit any complications or RD. The absence of shallowing of the anterior chamber was observed across the entire cohort.
Our research revealed that 112 eyes did not show signs of RD, while 34 eyes from this group encountered complications either during or following the procedure. It is worth mentioning that 18 out of the 20 eyes that exhibited RD also experienced complications.
The study revealed that no statistically significant association (p= 0.933) was found between the various methods of IOL placement, such as in the sulcus, in the bag, or scleral fixation, and the occurrence of RD. The Fisher’s exact test conducted on the IOL implantation at the same setting and its association with RD yielded a p-value of 0.045, indicating a potential decrease in the risk of RD with concurrent IOL implantation. The odds ratio for simultaneous IOL insertion and RD was 0.331 (95% CI: 0.121–0.904, p=0.031).
It was observed that a higher incidence of RD was noted in surgeries performed by the consultant, however this difference was not statistically significant (p=0.089).
The results of the logistic regression indicated a strong and statistically significant association between the occurrence of complications and the likelihood of RD in the studied population. The odds ratio for complications and RD was 20.65, 95% CI [4.5, 93.9] and (p=<0.001). The complications evaluated were strabismus, amblyopia, glaucoma, inflammatory membrane, capsular phimosis, posterior capsule opacification, phthisis bulbi, endophthalmitis and subluxated intraocular lens. The risk ratio for glaucoma and RD was 11.52, with a 95% confidence interval of [3.19, 41.61], and a p-value less than 0.001.
Among the 112 patients without RD, 4 had intraocular inflammatory membranes, while 4 of the 20 patients with RD did. The odds ratio was 6.75 95% CI [1.53, 29.71] (p-value=0.02). The relationship between RD and other complications was not found to be statistically significant.
Additional significant risk factors for postsurgical RD were preexisting myopia (p=0.002) and postoperative glaucoma (p<0.001) (). No statistically significant differences emerged between the groups in terms of affected eye, sex distribution, presence of bilateral cataracts, or operating surgeon experience level.
As a supplementary analysis, multivariate logistic regression analysis corroborated the finding that greater AL was associated with a higher risk of RD (). Specifically, having an AL ≤ 23.4 mm was linked with 0.55-fold decreased odds of detachment relative to longer AL (p=0.009). While myopia conferred an elevated odds ratio of 12.04, this was deemed statistically non-significant (p=0.086). In contrast, glaucoma remained a substantial risk factor in the regression analysis and was associated with a 49-fold increase in the odds of detachment (p<0.001).
Table 4 Impact of Axial Length Less or Equal to 23.4 mm, Myopia, IOL Insertion and Glaucoma on the Risk of Retinal Detachment: A Logistic Regression Analysis
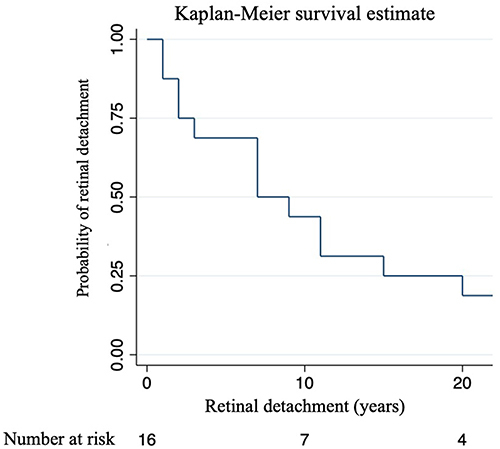
A Kaplan-Meier analysis () showed that approximately half of the patients retained some detachment risk 8 years after cataract surgery. Twenty years post-surgery, the risk was considered negligible, implying that nearly all observed RD occurred within two decades of surgery.
Figure 2 Retinal Detachment Risk Over Time: A Survival Analysis. The Kaplan-Meier plot illustrates the probability of RD in relation to the time of cataract surgery. The x-axis shows the time of cataract surgery in years, whereas the y-axis shows the probability of RD. Each mark on the graph signifies the likelihood of individuals being at risk after cataract surgery. The plot depicts the progression of the probability over time.
Discussion
The analysis demonstrated that AL ≤ 23.4 mm, IOL implantation, and glaucoma were significantly associated with RD risk following pediatric cataract surgery. Specifically, shorter AL conferred a protective effect, whereas IOL implantation in the same setting and glaucoma elevated the risk of detachment. Surprisingly, myopia itself did not emerge as a significant risk modifier.
The rise in RD among older children, as indicated in the research, might be attributed to a variety of factors. Research has indicated that the risk of RD increases with age due to the natural changes in the vitreous body, which can lead to vitreoretinal traction and subsequent detachment.Citation13 Additionally, the likelihood of RD may be greater in cases of myopic eyes, where the elongation of the eyeball can lead to retinal tears and detachments. These may become more apparent as children grow and myopia progresses.
The occurrence of RD in children and adolescents is relatively rare, and studies indicate a higher likelihood in those with certain ocular conditions, such as high myopia, prior ocular surgery, and trauma.Citation3,Citation4 Daien et al conducted a comprehensive examination of the influence of age on RD after cataract surgery, emphasizing a greater risk in younger age groups, which may align with similar patterns in the pediatric population.Citation4
The logistic regression model indicates a weak and inverse relationship between age and the likelihood of RD, suggesting that for every additional year of age, the risk of RD decreases by approximately 3. The p-value of 0.889 suggests that this relationship is likely due to random variation rather than a true inverse association in the population. The interaction between age and AL has a negligible synergistic effect, as indicated by the odds ratio of approximately 1.0. Although this suggests that the influence of age on the likelihood of RD is slightly modified by AL, the interaction does not reach statistical significance with a p-value of 0.552. This implies that there is insufficient evidence to establish that the combined effect of age and AL has a substantial impact on the risk of RD. It is crucial to recognize the limitations of the study, particularly the sample size and other potential confounding variables not included in the model.
The relationship between AL and the RD () is depicted by the observed increase in odds ratios, which indicates a positive correlation. This association is significant in ophthalmologic research as it is believed to influence a range of ocular conditions. Understanding this relationship is critical for predicting clinical outcomes and assessing associated risks, thereby enabling evidence-based decision-making among healthcare practitioners. The blue line in the graph illustrated the impact of AL on event probability, as determined by logistic regression, and is essential for identifying these relationships in the absence of confounder adjustments. The orange line provided a measure of the statistical strength of the relationship, as demonstrated by the odds ratios. Together, these lines offer a comprehensive understanding of the connection between AL and RD, as well as the confidence in these findings across various AL. This dual representation is vital for interpreting the data’s validity and applicability in a scientific or clinical context. Compared with previous studies that showed that an increase in AL is associated with a higher likelihood of RD, our findings indicate that a shorter preoperative AL reduces the risk of RD.Citation14–16 This aligns with the established link between longer eyes and increased risk of detachment.Citation17 While several reports have identified longer AL as a risk factor for detachment in children after surgery, most have only briefly touched upon this subject.Citation3,Citation10,Citation18 A study by Zhao et al suggested that shorter AL offer greater protection by minimizing vitreoretinal traction compared to longer myopic eyes.Citation19 Additionally, Yang et al highlighted that a longer AL poses a risk of complications during cataract surgery, including postoperative retinal tears and capsular contraction syndrome.Citation20
Under normal conditions, the vitreous body is firmly attached to the retinal internal limiting membrane. Nevertheless, increased AL typically results in biomechanical strain on the sclera and subsequent vitreoretinal interface changes, liquefaction of the vitreous, and anomalous posterior vitreous detachment (PVD).Citation21 Retinal tissue is subjected to dynamic forces as a result of these changes, which can lead to tears and subsequent detachment.Citation22 The level of traction increases with longer AL as the eye wall stretches to a larger radius, causing the vitreous base to expand and cover more area, resulting in a stronger pull on the retina. People with highly myopic eyes larger than 26 mm may experience additional complications such as tilted optic discs or staphylomas, which can worsen traction. The findings of Mimura et al and Chen et al provide evidence for this claim.Citation14,Citation17 It is possible that the shorter AL (≤ 23.4 mm in our patient group helped to protect against increased vitreoretinal traction), which may have contributed to the development of retinal tears or detachment after pediatric cataract surgery. This protective mechanism warrants further investigation given its potential clinical implications.
The myopic shift that occurs after cataract surgery in children and its possible association with RD are pressing issues in the field of pediatric ophthalmology. Several studies have examined myopic shift that occurs after IOLs implantation in children who have undergone cataract surgery. Weakley et al reported a myopic shift at the age of five years in infants who had undergone cataract surgery with IOL implantation.Citation23 Additionally, studies by Ganesh et al and Valeina et al highlighted the high rate of myopic shift in children after IOL implantation, emphasizing the need to investigate the factors that contribute to this shift.Citation24,Citation25 While these studies offer valuable insights, to fully comprehend whether myopic shift interplays with RD risk after pediatric cataract surgery, further inquiry in larger populations is still required to conclusively delineate whether postoperative myopic shift interrelates with or potentiates detachment. However, when developing surgical strategies and postoperative protocols for myopic children, considering their potentially heightened vulnerability remains essential.
Understanding the connection between axial elongation and the risk of RD after lens removal in high myopia is a topic of focus in ophthalmology.
The impact of axial elongation on myopia progression has been the basis of research, with studies demonstrating a significant association between axial elongation and the progression of myopia.Citation26,Citation27 In the context of myopia, research has suggested that individuals with shorter AL in their eyes may possess a certain resistance to RD. This is because shorter AL result in reduced biomechanical stretching and stress on both the sclera and retinal tissue. Consequently, the vitreoretinal interface may remain more intact, and the vitreous body is less likely to apply traction on the retina.Citation27 It is well-documented that axial elongation, commonly associated with myopia, increases the risk for RD. However, myopia, as a clinical condition, involves variable refractive errors and AL, which may not uniformly confer an increased risk of RD. This distinction is crucial in understanding the specific pathophysiological factors that contribute to the development of RD. Several studies have highlighted the association between increased axial elongation, the development of RD, and other sight-threatening conditions in high myopia.Citation28,Citation29 High myopia, which leads to significant axial elongation, is associated with an increased risk of myopic maculopathy, RD, glaucoma, and blindness.Citation28 Furthermore, studies have shown a strong positive correlation between axial elongation and myopia progression.Citation27,Citation30 Additionally, the incidence of RD following lens removal has been found to be higher in patients with pathological myopia and axial elongation.Citation31 Further analysis of high myopia revealed that axial elongation significantly predicted the incidence of detachment following lens removal. This is consistent with studies in adults demonstrating that pseudophakic RD in patients who have undergone phacoemulsification increases with the AL. Taken together, these findings suggest that increased eye size may play a role in RD development.
In contrast to previous research linking high myopia with an increased risk of RD, our results do not demonstrate a statistically significant connection between myopia and RD. This finding highlights the intricate interplay of factors that contribute to RD and implies that axial elongation in myopia may not directly predict RD risk as previously thought. Consequently, our research adds to the ongoing discussion by investigating axial elongation and myopia progression in a more comprehensive manner, incorporating ocular anatomy, refractive status, and risk factors.Citation32,Citation33 However, an odds ratio of 12 indicates that myopia may still be a noteworthy risk factor. Nevertheless, a wide confidence interval suggests an uncertainty in this estimate. It is essential to carefully interpret these results, considering the study design, sample size, and potential confounders affecting the relationship between myopia and detachment. Although not statistically significant, a considerably elevated odds ratio implied a clinically meaningful association between myopia and postoperative RD after pediatric cataract surgery. Additional studies are necessary to fully understand the relationship between myopia and detachment after pediatric cataract surgery.
The practice of pediatric cataract surgery at our teaching hospital is a sophisticated procedure, intricately tied to the surgeon’s level of expertise. Remarkably, nearly half of the procedures in our study cohort were carried out by fellows under the stringent supervision of consultants. Despite the varying levels of experience, the outcomes remained consistent, with no significant disparity in the incidence of RD postoperatively, which suggests that, alongside AL and other identified factors, the structure of our surgical training program successfully mitigates the risk of complications.
With respect to the greater frequency of RD procedures performed by consultants, we propose that this may be due to their involvement with more intricate cases or those presenting higher preexisting risks, a phenomenon that is not rare in specialized surgical practices.
The probability of RD when an IOL was implanted during the same surgical procedure was approximately one-third the likelihood of RD when an IOL was not implanted. This suggests that the presence of the IOL at the time of surgery may offer some protection against RD. The relationship between primary IOL implantation after cataract surgery in children and the risk of RD has been controversial. Some authors found that primary IOL implantation after pediatric cataract surgery is associated with a higher risk of postoperative RD.Citation18,Citation33,Citation34 However, Inoue et al reported that IOL implantation after cataract surgery decreased the incidence of postoperative RD.Citation35 The relationship between IOL implantation following pediatric cataract surgery and the risk of RD appears to be influenced by a variety of factors, including the surgical approach and individual patient characteristics, such as axial myopia. Although some evidence suggests that IOL implantation can increase the risk of RD in certain cases, it may also reduce the risk under certain conditions. Additionally, it is worth noting that PVD occurs more frequently following congenital cataract surgery with IOL implantation.Citation18 It is crucial to closely surveil postoperative cataract patients with IOL implantation for the emergence of RD and other ocular disorders. It is critical to monitor for PVD to prevent the development of further ocular pathologies. Further studies are required to identify other possible risk factors for RD in this group.
Our analysis demonstrates a clear connection between certain types of cataracts and RD, with cortical cataracts exhibiting a particularly high risk, as indicated by an OR of 31.0, a 95% CI of [1.90; 506.77], and a p-value of 0.016. While nuclear cataracts displayed a moderate yet statistically significant OR of 1.4, suggesting a slight increase in RD risk, the data for lamellar and posterior subcapsular cataracts were variable, with an OR of 10.3 for the latter. This highlights the importance of assessing individual patient risk profiles when evaluating RD risk.
The odds of having RD are over 20 times higher for patients with complications than for those without. This is statistically significant, as shown by a p-value < 0.001. This wide interval suggests that while the exact effect of complications on RD is imprecise, there is a strong association between the two.
The odds of having RD are over 11 times higher in patients with glaucoma than in those without. The statistical tests indicate a strong and significant association between glaucoma and the occurrence of RD. The presence of glaucoma increases the likelihood of RD substantially, which is statistically significant and suggests clinical importance in the management and monitoring of patients with glaucoma who undergo cataract surgery. The wide confidence interval for the odds ratio implies that while the association is strong, the exact strength of the relationship could vary across different patient populations or surgical contexts. The presence of glaucoma should be considered a significant risk factor for RD in patients who have undergone cataract surgery. The data indicates a connection between RD and inflammatory membranes in this patient group, as shown by the significant association found.
The high odds ratio implies that the presence of an inflammatory membrane significantly increases the likelihood of RD, however, the wide confidence interval emphasizes the need for additional research to refine this conclusion. The results should be interpreted with caution due to the limited sample size and potential for unaccounted confounding variables.
According to our findings, glaucoma had the strongest significant association with the risk of RD after pediatric cataract surgery. Glaucoma was associated with a 49-fold increase in the odds of developing RD (OR 49.01, p<0.001). The wide 95% confidence interval of 5.53 to 434.64 indicates some uncertainty in the precision of this odds ratio estimate. However, given the large effect size and highly statistically significant p-value, glaucoma clearly confers a substantially elevated detachment risk. This condition may have led to anatomical and vascular changes, which could potentially result in the observed outcome.Citation36 Additionally, the significant influence of the disease on the optic nerve and structural damage may play a role in the development of rhegmatogenous RD, which primarily arises from a retinal break and leads to the accumulation of fluid in the subretinal space.Citation37 Additionally, alterations in ocular blood flow commonly observed in glaucoma could compromise wound healing and increase inflammation following cataract surgery, further increasing the risk of RD.Citation38,Citation39 The medications used to control intraocular pressure may also interfere with these processes. Therefore, the findings suggest that children with glaucomatous disease require extra careful preoperative counseling about RD odds, controlled surgical manipulation to protect ocular structures, and very close postoperative monitoring for any signs of detachment so that cases can be repaired early.
The long-term risk of RD following cataract surgery in pediatric patients has been extensively studied. Our investigation revealed an RD risk of approximately 20% after 20 years. A study reported a 5.5% risk of RD within 10 years after cataract surgery in children with no known ocular or systemic anomalies, with an increased risk in male, myopic, and intellectually disabled children.Citation40 According to Haargaard et al the overall risk of RD after pediatric cataract surgery was 7% after 20 years.Citation12 Another study reported that the cumulative incidence of RD repair within five years of pediatric cataract surgery was approximately 2%.Citation41 Risk factors associated with RD repair include a history of prematurity, persistent fetal vasculature (PFV), and the absence of IOL placement.Citation3
In a study of adults, the risk of RD after cataract surgery was higher in younger patients, eyes with longer AL, and eyes with posterior capsular rupture (PCR) with vitreous loss during surgery.Citation40 Our research findings differ from the typically reported results in the literature, which suggest that younger patients are more likely to experience RD after cataract surgery. Conversely, our pediatric cohort demonstrated an inverse relationship, with patients who developed RD being significantly older at the time of their cataract diagnosis (p=0.003). This discrepancy could be attributed to the unique characteristics of pediatric cataract surgery and postoperative anatomical changes. Most studies, focus on adults and may not account for pediatric-specific factors such as ocular growth or developmental issues.Citation3,Citation12 Our findings suggest that the risk factors for RD may evolve differently in a developing eye, where the interplay between ocular growth, vitreoretinal interface changes, and biomechanical properties of the pediatric eye may contribute to an increased RD risk as the child ages. Such nuances highlight the need for age-appropriate surveillance strategies post-cataract surgery in the pediatric population and raise questions about the optimal timing of surgery and follow-up intervals for older children. Further investigation into the pathophysiological differences between pediatric and adult post-cataract surgery eyes is warranted to clarify these findings. Our study contributes to the ongoing discussion about postoperative RD in pediatric cataract patients and underscores the importance of considering age-specific risks when planning cataract surgery and postoperative care in children.
Therefore, the higher risk of RD after cataract surgery in children found in our study compared to other studies may be a result of differences in study populations, variations in surgical techniques and follow-up protocols, length of follow-up, advancements in surgical techniques, and management of RD. Additionally, it is important to consider the inherent limitations of survival analysis and potential for bias in our study. These combined factors could have contributed to the higher risk of RD observed in our study than in other studies.
These findings underscore several imperatives of pediatric cataract surgery to address the considerable risk of retinal complications, especially in vulnerable subgroups such as children with glaucoma, eyes larger than 23.4 mm and IOL implantation during cataract surgery. Recognizing the significance of confounding variables, particularly patient age, in pediatric cataract surgery outcomes is essential. Younger patients may exhibit unique anatomical and physiological responses to surgery, which may result in intraoperative complications such as vitreous loss or capsular tear, irrespective of the surgeon’s skill. These complications, together with comorbid ocular conditions such as uveitis or glaucoma, intraoperative findings, postoperative care, presence of systemic diseases, genetic predispositions, and the impact of preexisting ocular conditions like myopia, may predispose the ocular environment to postoperative complications, thereby impacting the surgical outcome. Confounding variables interact within the surgical narrative as interconnected elements that can potentially confound the incidence rates and patterns of RD following surgery.
A thorough preoperative assessment of the child’s health status, cataract severity, ocular conditions, and risk factors is crucial for planning appropriate interventions. Meticulous surgical techniques and vigilant postoperative monitoring, including routine and dilated fundus examinations, are also critical to ensure proper healing and enable early detection of complications. Moreover, a collaborative approach with a team of experts, including ophthalmologists, pediatricians, anesthesiologists, and nurses, is essential to managing the multifaceted challenges children face perioperatively. Following these guidelines for careful evaluation, precision surgery, attentive aftercare, and integrated specialty treatment can help mitigate risks and ultimately improve outcomes by reducing adverse events after pediatric cataract extraction such as RD. This study employed a retrospective approach, drawing conclusions from existing medical records. The limitations of this study included a small, single-center cohort, a short follow-up period, and potential biases that may affect the validity of our findings, such as selection and information biases, uncontrolled confounding factors, and the inability to establish causality. Additionally, it is possible that unrecorded late-onset RD cases were excluded from our analysis. The limitations of these findings indicate the necessity for additional research in larger, multi-center cohorts to validate these results and further investigate the underlying mechanisms.
Conclusions
In summary, this study suggests that AL > 23.4 mm, preexisting myopia, and postoperative glaucoma may be notable risk factors for RD following pediatric cataract surgery, with nearly half still demonstrating some detachment risk at 8 years postoperatively. Greater AL was associated with statistically increased detachment odds, whereas very long eyes (> 26 mm) conferred 10-fold higher odds. Although myopia did not reach statistical significance, the elevated odds ratio suggests a clinically meaningful relationship, which warrants further investigation. The advanced age of 11 years in the detachment group highlights that adolescent eyes may require extra vigilance. Overall, these findings highlight the importance of personalized preoperative counseling, surgical precautions, and postoperative monitoring in pediatric patients undergoing lensectomy, based on identifiable vulnerability factors. Limitations, such as sample size, retrospective design, and short follow-up, echo the need for additional research with larger prospective cohorts and long-term data to conclusively characterize pediatric RD incidence and risk factors. These results provide initial evidence for guiding the management of high-risk children. Additional studies are essential to optimize outcomes in this special population.
Acknowledgement
Dr. Mawaddah Sabr and Dr. Gorka Sesma made significant contributions to the manuscript, earning them the status of the co-first authors. We extend special gratitude to Dr. Ahmed Mousa from the Research Department of KKESH for his invaluable support and guidance in statistical analysis.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Lagrèze WA. Treatment of congenital and early childhood cataract. Ophthalmologe. 2021;118(S2):135–144. doi:10.1007/s00347-021-01370-z
- Foster A, Gilbert C, Rahi J. Epidemiology of cataract in childhood: a global perspective. J Cataract Refract Surg. 1997;23(Supplement 1):601–604. doi:10.1016/S0886-3350(97)80040-5
- Agarkar S, Gokhale VV, Raman R, Bhende M, Swaminathan G, Jain M. Incidence, risk factors, and outcomes of retinal detachment after pediatric cataract surgery. Ophthalmology. 2018;125(1):36–42. doi:10.1016/j.ophtha.2017.07.003
- Daien V, Korobelnik JF, Delcourt C, et al. French Medical-Administrative Database for Epidemiology and Safety in Ophthalmology (EPISAFE): the EPISAFE Collaboration Program in Cataract Surgery. Ophthalmic Res. 2017;58(2):67–73. doi:10.1159/000456721
- Lenhart PD, Lambert SR. Current management of infantile cataracts. Survey Ophthalmol. 2022;67(5):1476–1505. doi:10.1016/j.survophthal.2022.03.005
- Huang YC, Chu YC, Wang NK, et al. Impact of etiology on the outcome of pediatric rhegmatogenous retinal detachment. Retina. 2019;39(1):118. doi:10.1097/IAE.0000000000001908
- Yorston D, Yang YF, Sullivan PM. Retinal detachment following surgery for congenital cataract: presentation and outcomes. Eye. 2005;19(3):317–321. doi:10.1038/sj.eye.6701463
- Qureshi MH, Steel DHW. Retinal detachment following cataract phacoemulsification—a review of the literature. Eye. 2020;34(4):616–631. doi:10.1038/s41433-019-0575-z
- Rabiah PK, Du H, Hahn EA. Frequency and predictors of retinal detachment after pediatric cataract surgery without primary intraocular lens implantation. J AAPOS. 2005;9(2):152–159. doi:10.1016/j.jaapos.2004.12.013
- Al Muammar AR, Al-Harkan D, Al-Rashidy S, Al-Suliman S, Mousa A. Frequency of retinal detachment after cataract surgery in highly myopic patients. Saudi Med J. 2013;34(5):511–517.
- Malukiewicz-Wiśniewska G, Stafiej J. Changes in axial length after retinal detachment surgery. Eur j Ophthalmolo. 1999;9(2):115–119.
- Haargaard B, Andersen EW, Oudin A, et al. Risk of retinal detachment after pediatric cataract surgery. Invest Ophthalmol Visual Sci. 2014;55(5):2947–2951. doi:10.1167/iovs.14-13996
- Rosner M, Treister G, Belkin M. Epidemiology of retinal detachment in childhood and adolescence. J Pediatr Ophthalmol Strabismus. 1987;24(1):42–44. doi:10.3928/0191-3913-19870101-09
- Chen J, Chen S, Zhao X, et al. Characteristics and management of myopic traction maculopathy in myopic eyes with axial length less than 26.5 mm. Retina. 2022;42(3):540–547. doi:10.1097/IAE.0000000000003351
- Lake S, Bottema M, Williams K, Reynolds K. The correlation between optical coherence tomography retinal shape irregularity and axial length. PLoS One. 2019;14(12):e0227207.
- Thylefors J, Jakobsson G, Zetterberg M, Sheikh R. Retinal detachment after cataract surgery: a population‐based study. Acta Ophthalmologica. 2022;100(8). doi:10.1111/aos.15142
- Mimura R, Mori K, Torii H, et al. Ultra-widefield retinal imaging for analyzing the association between types of pathological myopia and posterior staphyloma. JCM. 2019;8(10):1505. doi:10.3390/jcm8101505
- Zhang JS, Wang JD, Yusufu M, et al. The effect of retaining intact posterior capsule in congenital cataract surgery in children aged 4–8 years. BMC Ophthalmol. 2021;21(1):332. doi:10.1186/s12886-021-02098-9
- Zhao YE, Jiang Y, Zhao Y, et al. Cataract surgery: eyes with Microphthalmos Compared to a Comparison Group; 2023. doi:10.21203/rs.3.rs-3398936/v1.
- Yang Y, Chen H, An J, Fan W. Long-term effects of phacoemulsification and intraocular lens implantation in a patient with pathologic myopia and extremely long axial length: a case report. Medicine. 2020;99(37):e22081. doi:10.1097/MD.0000000000022081
- Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefe’s Arch Clin Exp Ophthalmol. 2004;242(8):690–698. doi:10.1007/s00417-004-0980-1
- Schulz A, Szurman P. Vitreous substitutes as drug release systems. Trans Vision Sci Technol. 2022;11(9):14. doi:10.1167/tvst.11.9.14
- Weakley DR, Lynn MJ, Dubois L, et al. Myopic shift 5 years after intraocular lens implantation in the infant aphakia treatment study. Ophthalmology. 2017;124(6):822–827. doi:10.1016/j.ophtha.2016.12.040
- Ganesh S, Gupta R, Sethi S, Gurung C, Mehta R. Myopic shift after intraocular lens implantation in children less than two years of age. Nep J Oph. 2018;10(1):11–15. doi:10.3126/nepjoph.v10i1.21662
- Valeina S, Heede S, Erts R, et al. Factors influencing myopic shift in children after intraocular lens implantation. Eur J Ophthalmol. 2020;30(5):933–940. doi:10.1177/1120672119845228
- Hyman L. Relationship of Age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123(7):977. doi:10.1001/archopht.123.7.977
- Hou W, Norton TT, Hyman L, Gwiazda J; the COMET Group. Axial elongation in myopic children and its association with myopia progression in the correction of myopia evaluation trial. Eye Contact Lens. 2018;44(4):248–259. doi:10.1097/ICL.0000000000000505
- Kinoshita N, Konno Y, Hamada N, et al. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep. 2020;10(1):12750. doi:10.1038/s41598-020-69710-8
- Li SM, Kang MT, Wu SS, et al. Efficacy, safety and acceptability of orthokeratology on slowing axial elongation in myopic children by meta-analysis. Curr Eye Res. 2016;41(5):600–608. doi:10.3109/02713683.2015.1050743
- McBrien NA, Adams DW. A longitudinal investigation of adult-onset and adult-progression of myopia in an occupational group. Refractive and biometric findings. Invest Ophthalmol Vis Sci. 1997;38(2):321–333.
- Barraquer C. Incidence of retinal detachment following clear-lens extraction in myopic patients: retrospective analysis. Arch Ophthalmol. 1994;112(3):336. doi:10.1001/archopht.1994.01090150066025
- Celorio J, Pruett RC. Prevalence of lattice degeneration and its relation to axial length in severe myopia. Am J Ophthalmol. 1991;111(1):20–23. doi:10.1016/S0002-9394(14)76891-6
- De la Huerta I, Williams GA. Rhegmatogenous retinal detachment after pediatric cataract surgery. Ophthalmology. 2018;125(1):4–5. doi:10.1016/j.ophtha.2017.08.032
- Jin S, Zhang J, Wang J, et al. Advisability of primary intraocular lens implantation for infants under 2: a systematic review and meta‐analysis. Int J Clin Pract. 2021;75(9). doi:10.1111/ijcp.14143
- Inoue M, Shinoda K, Ishida S, et al. Intraocular lens implantation after atopic cataract surgery decreases incidence of postoperative retinal detachment. Ophthalmology. 2005;112(10):1719–1724. doi:10.1016/j.ophtha.2005.04.021
- Starr MR, Huang D, Wong JC, et al. Prevalence, characteristics, and outcomes of rhegmatogenous retinal detachment in eyes with trabeculectomy or glaucoma drainage devices. Retina. 2022;42(11):2039–2045. doi:10.1097/IAE.0000000000003587
- Amer R, Nalcı H, Yalçındağ N. Exudative retinal detachment. Survey Ophthalmol. 2017;62(6):723–769. doi:10.1016/j.survophthal.2017.05.001
- Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retinal Eye Res. 2002;21(4):359–393. doi:10.1016/S1350-9462(02)00008-3
- Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B. Dysfunctional regulation of ocular blood flow: a risk factor for glaucoma? Clin Ophthalmol. 2008;2(4):849–861. doi:10.2147/opth.s2774
- Petousis V, Sallam AA, Haynes RJ, et al. Risk factors for retinal detachment following cataract surgery: the impact of posterior capsular rupture. Br J Ophthalmol. 2016;100(11):1461–1465. doi:10.1136/bjophthalmol-2015-307729
- Oke I, Hwang B, Heo H, Nguyen A, Lambert SR. Risk factors for retinal detachment repair after pediatric cataract surgery in the United States. Ophthalmol Sci. 2022;2(4):100203. doi:10.1016/j.xops.2022.100203

